Deck 1: Remembering General Chemistry: Electronic Structure and Bonding
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/74
Play
Full screen (f)
Deck 1: Remembering General Chemistry: Electronic Structure and Bonding
1
How many degenerate p orbitals are in the second shell?
A) 0
B) 1
C) 2
D) 3
E) 4
A) 0
B) 1
C) 2
D) 3
E) 4
3
2
Which of the following statements correctly describes the third shell that surrounds the nucleus of an atom?
A) The third shell contains only s and p atomic orbitals.
B) The maximum number of electrons that can occupy the third shell is 18.
C) The total number of atomic orbitals present in the third shell is 16.
D) The third shell can contain f orbitals.
E) All third shell elements must have d electrons.
A) The third shell contains only s and p atomic orbitals.
B) The maximum number of electrons that can occupy the third shell is 18.
C) The total number of atomic orbitals present in the third shell is 16.
D) The third shell can contain f orbitals.
E) All third shell elements must have d electrons.
The maximum number of electrons that can occupy the third shell is 18.
3
What is the electronic configuration of N3-?
A)
B)
C)
D)
E)
A)
B)
C)
D)
E)
4
What are the formal charges on nitrogen and on the starred oxygen in the following species? 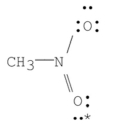
A)
B)
C)
D)
E)

A)
B)
C)
D)
E)

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
5
The formal charge on nitrogen in the following compound is ________.
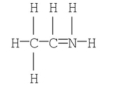
A) +2
B) +1
C) 0
D) -1
E) -2

A) +2
B) +1
C) 0
D) -1
E) -2

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
6
What is the hybridization of the indicated atom ? 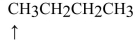
A) sp
B) sp2
C) sp3
D) none of the above
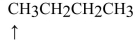
A) sp
B) sp2
C) sp3
D) none of the above

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
7
Which of the following is the electronic configuration of the element Fe?
A)
B)
C)
D)
E)
A)
B)
C)
D)
E)

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
8
Which of the following contains polar covalent bonds?
A) NH3
B) Na2O
C) H2
D) KF
E) both A and C
A) NH3
B) Na2O
C) H2
D) KF
E) both A and C

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
9
What element is represented by the following electronic configuration?
A) F
B) C
C) N
D) Al
E) O
A) F
B) C
C) N
D) Al
E) O

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
10
Which of the following Lewis structures is correct for ?
A)
B)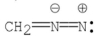
C)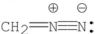
D)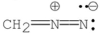
E)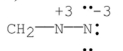
A)
B)
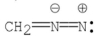
C)
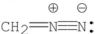
D)

E)
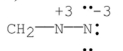

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
11
Which of the following atoms is the least electronegative?
A) P
B) Na
C) I
D) B
E) O
A) P
B) Na
C) I
D) B
E) O

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
12
Which of the following compounds have bonds that are predominantly ionic?
A) KCl
B) CF4
C) NH3
D) both A and B
E) both B and C
A) KCl
B) CF4
C) NH3
D) both A and B
E) both B and C

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
13
How many nonbonding pairs of electrons are H2NOH
A) 0
B) 1
C) 2
D) 3
E) 4
A) 0
B) 1
C) 2
D) 3
E) 4

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
14
What type of bonding is found ?
A) ionic
B) hydrogen
C) covalent
D) polar
A) ionic
B) hydrogen
C) covalent
D) polar

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
15
Which of the following covalent bonds has the largest dipole moment?
A) C-C
B) C-H
C) C-O
D) H-N
E) H-F
A) C-C
B) C-H
C) C-O
D) H-N
E) H-F

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
16
The atomic number of boron is 5. The correct electronic configuration of boron is ________.
A)
B)
C)
D)
E)
A)
B)
C)
D)
E)

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
17
What is the formal charge on the nitrogen in the ammonium ion?
A) -2
B) -1
C) 0
D) +1
E) +2
A) -2
B) -1
C) 0
D) +1
E) +2

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
18
Which of the following is the most likely Lewis structure for C2H2?
A)
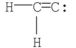
B)
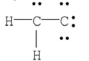
C)
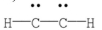
D)
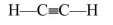
E)
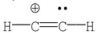
A)

B)

C)

D)
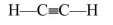
E)
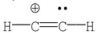

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
19
The compound CH3NH2, contains a C-N bond. Which of the following best describes the charge on the nitrogen atom in this compound?
A)
B) slightly positive
C) uncharged
D) slightly negative
E)
A)
B) slightly positive
C) uncharged
D) slightly negative
E)

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
20
How many unpaired electrons are present in the electronic configuation of carbon (atomic number =6)?
A) none
B) one
C) two
D) three
E) four
A) none
B) one
C) two
D) three
E) four

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
21
The N , is formed by the overlap of what two orbitals?
A) sp3-sp3
B) sp3-sp2
C) sp2-sp2
D) sp2-s
E) sp3-s
A) sp3-sp3
B) sp3-sp2
C) sp2-sp2
D) sp2-s
E) sp3-s

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
22
Are all the carbons in this structure in the same plane? 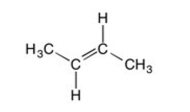
A) Yes
B) No

A) Yes
B) No

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
23
How many sp carbons are in the following compound?
A) Zero
B) One
C) Two
D) Three
A) Zero
B) One
C) Two
D) Three

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
24
What is the bond angle and hybridization of the carbon in +CH3 ?
A)120°, sp2
B) 120°, sp3
C) 109.5°, sp2
D) 120°, sp2
E) 109.5°, sp3
A)120°, sp2
B) 120°, sp3
C) 109.5°, sp2
D) 120°, sp2
E) 109.5°, sp3

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
25
Which of the following has an sp2 carbon?
A)
B)
C)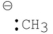
D) A and B
E) A, B and C
A)
B)
C)
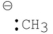
D) A and B
E) A, B and C

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
26
How many sp2 hybridized atoms are in the following compound? 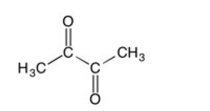
A) Two
B) Three
C) Four
D) Five
E) Six

A) Two
B) Three
C) Four
D) Five
E) Six

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
27
Which carbons in the following compound are sp hybridized? 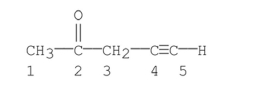
A) carbon 1
B) carbon 2
C) carbons 1, 3
D) carbons 4
E) carbons 4, 5

A) carbon 1
B) carbon 2
C) carbons 1, 3
D) carbons 4
E) carbons 4, 5

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
28
How many carbon-carbon sigma bonds are in the following compound? 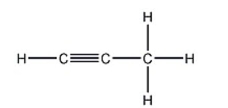
A) 1
B) 2
C) 3
D) 4

A) 1
B) 2
C) 3
D) 4

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
29
Each lone pair on the CH3OH occupies a(n) ________ orbital.
A) s
B) p
C) sp
D) sp2
E) sp3
A) s
B) p
C) sp
D) sp2
E) sp3

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
30
Of the hydrogen halides, the strongest bond is found in ________ and the longest bond is found in ________.
A) HF; HF
B) HF; HI
C) HI; HF
D) HI; HI
E) HCl; HBr
A) HF; HF
B) HF; HI
C) HI; HF
D) HI; HI
E) HCl; HBr

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
31
Are all the carbons in this structure in the same plane? 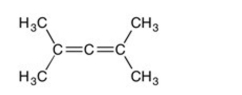
A) Yes
B) No

A) Yes
B) No

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
32
How many sp2 carbons are present in H2C=C=CH2
A) 0
B) 1
C) 1.5
D) 2
E) 3
A) 0
B) 1
C) 1.5
D) 2
E) 3

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
33
CH3CN contains ________ sigma bonds and ________ pi bonds.
A) 5; 2
B) 4; 3
C) 4; 2
D) 2; 2
E) 4; 0
A) 5; 2
B) 4; 3
C) 4; 2
D) 2; 2
E) 4; 0

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
34
What orbitals overlap to form the H-C bond in CH3+?
A) sp3-sp3
B) sp2-sp3
C) s-p
D) s-sp2
E) s-sp3
A) sp3-sp3
B) sp2-sp3
C) s-p
D) s-sp2
E) s-sp3

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
35
The nitrogen atom in (CH3CH2)3N ________ is hybridized and the C-N-C bond angle is ________.
A) sp2 ;>109.5°
B) sp2 ;<109.5°
C) sp3 ;>109.5°
D) sp3 ;<109.5°
E) sp;109.5°
A) sp2 ;>109.5°
B) sp2 ;<109.5°
C) sp3 ;>109.5°
D) sp3 ;<109.5°
E) sp;109.5°

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
36
How many carbon-carbon sigma bonds are in the following compound?
A) 0
B) 1
C) 2
D) 3
E) 4
A) 0
B) 1
C) 2
D) 3
E) 4

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
37
Which of the following angles is closest to the C-O-C bond angle in CH3-O-CH3?
A) 180°
B) 120°
C) 109.5°
D) 90°
E) 160°
A) 180°
B) 120°
C) 109.5°
D) 90°
E) 160°

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
38
What orbitals overlap to form the C-H bond in ethene (H2C=CH2)?
A) s-sp
B) s-sp2
C) s-sp3
D) s-p
E) p-p
A) s-sp
B) s-sp2
C) s-sp3
D) s-p
E) p-p

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
39
How many sp-hybridized atoms are in the following compound? 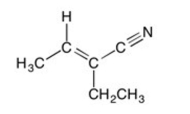
A) One
B) Two
C) Three
D) Four

A) One
B) Two
C) Three
D) Four

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
40
The lone-pair electrons of the methyl anion occupy a(n) ________ orbital.
A) s
B) p
C) sp
D) sp2
E) sp3
A) s
B) p
C) sp
D) sp2
E) sp3

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
41
Which of the following compounds does not have a dipole moment of zero?
A) CH3NH2
B) CO2
C) CH3OCH3
D)
E) CHCl3
A) CH3NH2
B) CO2
C) CH3OCH3
D)
E) CHCl3

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
42
Using the symbols δ+ and δ- , show the direction of the polarity in the O-H bond. 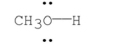
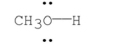

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
43
Which bond in the following compound is the shortest? 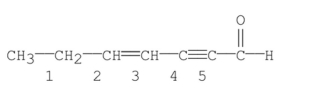
A) bond 1
B) bond 2
C) bond 3
D) bond 4
E) bond 5

A) bond 1
B) bond 2
C) bond 3
D) bond 4
E) bond 5

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
44
What two factors determine the size of a bond's dipole moment?

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
45
What is the H-C-H bond angle in H2CO
A) 60°
B) 90°
C) 109.5°
D) 120°
E) 180°
A) 60°
B) 90°
C) 109.5°
D) 120°
E) 180°

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
46
The carbon-carbon double bond in ethene is ________ and ________ than the carbon-carbon triple bond in ethyne.
A) stronger; shorter
B) stronger; longer
C) weaker; shorter
D) weaker; longer
E) stronger; more polar
A) stronger; shorter
B) stronger; longer
C) weaker; shorter
D) weaker; longer
E) stronger; more polar

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
47
Convert the following Kekulé structure into a condensed structure. 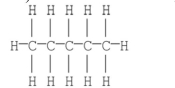


Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
48
Which of the following compounds does not have a dipole moment of zero?
A) CO2
B) CH4
C) CCl4
D) H2O
E) SO3
A) CO2
B) CH4
C) CCl4
D) H2O
E) SO3

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
49
What is the bond angle in the following compound?
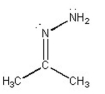
A) ~60°
B) ~90°
C) ~110°
D) ~120°
E) ~ 180°

A) ~60°
B) ~90°
C) ~110°
D) ~120°
E) ~ 180°

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
50
What is the hybridization of the oxygen in CH3OCH3?
A) sp
B) sp2
C) sp3
D) sp4
E) sp5
A) sp
B) sp2
C) sp3
D) sp4
E) sp5

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
51
The hydrogen-halogen bond becomes ________ and ________ as the size of the halogen increases.
A) longer; weaker
B) longer; stronger
C) shorter; weaker
D) shorter; stronger
A) longer; weaker
B) longer; stronger
C) shorter; weaker
D) shorter; stronger

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
52
What atomic property determines the polarity of a chemical bond?

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
53
In which orbital are the lone-pair electrons of CH3O-.
A) s
B) p
C) sp
D) sp2
E) sp3
A) s
B) p
C) sp
D) sp2
E) sp3

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
54
What is the hybridization of the carbon H2CO
A) sp
B) sp2
C) sp3
D) sp4
E) s3p
A) sp
B) sp2
C) sp3
D) sp4
E) s3p

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
55
Atoms with the same number of protons but different numbers of neutrons are called
________.
________.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
56
What is the electronic configuration of Ca2+ ?

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
57
Which of the following compounds has the smallest dipole moment?
A) Br2
B) NH3
C) HCl
D) HBr
E) HI
A) Br2
B) NH3
C) HCl
D) HBr
E) HI

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
58
Ar 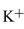 , and Cl- each have 18 electrons. What orbital does the highest-energy electron
, and Cl- each have 18 electrons. What orbital does the highest-energy electron
occupy?
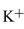 , and Cl- each have 18 electrons. What orbital does the highest-energy electron
, and Cl- each have 18 electrons. What orbital does the highest-energy electronoccupy?

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
59
Which of the following compounds has a dipole moment of zero?
A)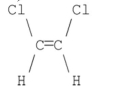
B)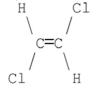
C)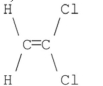
D)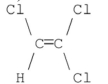
E)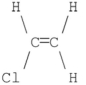
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
60
Which of the following compounds has the weakest bond?
A) H2
B) HF
C) HCl
D) HBr
E) HI
A) H2
B) HF
C) HCl
D) HBr
E) HI

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
61
Expand the condensed structure shown below to show the covalent bonds and the lone-pair electrons. 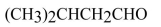
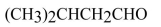

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
62
What is the hybridization of the nitrogen in
(CH3)3N
(CH3)3N

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
63
Draw the structure of a compound that contains only carbon and hydrogen atoms and that has two sp2 carbons and one sp carbon.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
64
What is the hybridizations of the carbons, going from left to right, in 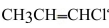
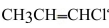

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
65
Draw the Kekul  structure o CH2Cl2 and show the direction of its dipole moment.
structure o CH2Cl2 and show the direction of its dipole moment.
 structure o CH2Cl2 and show the direction of its dipole moment.
structure o CH2Cl2 and show the direction of its dipole moment.
Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
66
What is the hybridization and bond angles of each carbon in CH3CN

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
67
Draw a 2p orbital.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
68
How many nonbonding electron pairs, bonding electron pairs, pi bonds, and sigma bonds are present CO2?

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
69
Draw the Lewis structure fo CH3N2+

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
70
What is the hybridization and bond angles of the carbon 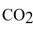 ?
?
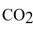 ?
?
Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
71
Draw condensed structures for the four compounds with molecular formula C3H9N.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
72
Why is the 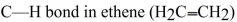 horter and stronger than the
horter and stronger than the 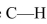 bond in
bond in
ethane (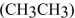
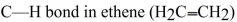 horter and stronger than the
horter and stronger than the 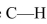 bond in
bond inethane (
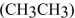

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
73
Draw the Lewis structure of CH2CO.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
74
What orbitals are used to form the covalent bonds in 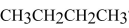 ?
?
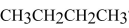 ?
?
Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck



