Deck 16: Organic Chemistry
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/129
Play
Full screen (f)
Deck 16: Organic Chemistry
1
Which of the following is the molecular formula for the molecule represented in the figure? 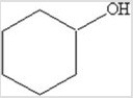
A)C7H15O
B)C7H13O
C)C6H10O
D)C6H12O
E)C6H6O

A)C7H15O
B)C7H13O
C)C6H10O
D)C6H12O
E)C6H6O
C6H12O
2
Which of the following molecules has a carbon-to-carbon double bond?
A)CH3CCH
B)CHCH
C)CH3CH3
D)CH3CH2CH3
E)CH2CHCH3
A)CH3CCH
B)CHCH
C)CH3CH3
D)CH3CH2CH3
E)CH2CHCH3
CH2CHCH3
3
To which class of compounds does the molecule shown in the figure belong? 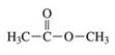
A)ketone
B)aldehyde
C)alcohol
D)ester
E)carboxylic acid
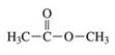
A)ketone
B)aldehyde
C)alcohol
D)ester
E)carboxylic acid
ester
4
List the number and type of bonds in the compound CH2CHCH2CH3.
A)12 single bonds
B)11 single bonds, and 1 double bond
C)10 single bonds, and 1 double bond
D)11 single bonds
E)13 single bonds
A)12 single bonds
B)11 single bonds, and 1 double bond
C)10 single bonds, and 1 double bond
D)11 single bonds
E)13 single bonds

Unlock Deck
Unlock for access to all 129 flashcards in this deck.
Unlock Deck
k this deck
5
Which of the following is the molecular formula for the molecule represented in the figure? 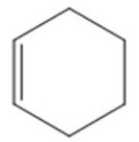
A)C6H6
B)C6H8
C)C6H10
D)C6H12
E)C6H16

A)C6H6
B)C6H8
C)C6H10
D)C6H12
E)C6H16

Unlock Deck
Unlock for access to all 129 flashcards in this deck.
Unlock Deck
k this deck
6
To which class of compounds does the compound shown in the figure belong? 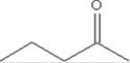
A)ketone
B)aldehyde
C)alcohol
D)amine
E)carboxylic acid

A)ketone
B)aldehyde
C)alcohol
D)amine
E)carboxylic acid

Unlock Deck
Unlock for access to all 129 flashcards in this deck.
Unlock Deck
k this deck
7
To which class of compounds does the molecule shown in the figure belong? 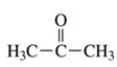
A)ketone
B)aldehyde
C)alcohol
D)amine
E)carboxylic acid
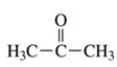
A)ketone
B)aldehyde
C)alcohol
D)amine
E)carboxylic acid

Unlock Deck
Unlock for access to all 129 flashcards in this deck.
Unlock Deck
k this deck
8
To which class of compounds does the molecule CH3CH2OCH3 belong?
A)ketone
B)aldehyde
C)alcohol
D)ether
E)carboxylic acid
A)ketone
B)aldehyde
C)alcohol
D)ether
E)carboxylic acid

Unlock Deck
Unlock for access to all 129 flashcards in this deck.
Unlock Deck
k this deck
9
Which of the following is the structural formula for the molecule represented in the figure? 
A)CH3CH2CH2CH2CH3
B)CH3CH2CH3
C)CH2CH2CH2CH2CH2
D)CH2CHCHCHCH2
E)CHCHCHCHCH

A)CH3CH2CH2CH2CH3
B)CH3CH2CH3
C)CH2CH2CH2CH2CH2
D)CH2CHCHCHCH2
E)CHCHCHCHCH

Unlock Deck
Unlock for access to all 129 flashcards in this deck.
Unlock Deck
k this deck
10
Which of the following molecules has only single bonds?
A)CH3CHCH2
B)CH3CH2CH3
C)CH2CHCH2CH3
D)CHCH
E)CH3CCH
A)CH3CHCH2
B)CH3CH2CH3
C)CH2CHCH2CH3
D)CHCH
E)CH3CCH

Unlock Deck
Unlock for access to all 129 flashcards in this deck.
Unlock Deck
k this deck
11
To which class of compounds does the compound shown belong? 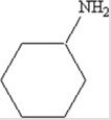
A)ketone
B)aldehyde
C)alcohol
D)amine
E)aromatic compound

A)ketone
B)aldehyde
C)alcohol
D)amine
E)aromatic compound

Unlock Deck
Unlock for access to all 129 flashcards in this deck.
Unlock Deck
k this deck
12
List the number and type of bonds in the molecule CH3CCCH3.
A)7 single bonds, and 2 double bonds
B)6 single bonds, and 3 double bonds
C)9 single bonds
D)8 single bonds, and 1 double bond
E)8 single bonds, and 1 triple bond
A)7 single bonds, and 2 double bonds
B)6 single bonds, and 3 double bonds
C)9 single bonds
D)8 single bonds, and 1 double bond
E)8 single bonds, and 1 triple bond

Unlock Deck
Unlock for access to all 129 flashcards in this deck.
Unlock Deck
k this deck
13
To which class of compounds does the molecule CH3CH=CH2 belong?
A)alkane
B)aldehyde
C)alcohol
D)alkene
E)carboxylic acid
A)alkane
B)aldehyde
C)alcohol
D)alkene
E)carboxylic acid

Unlock Deck
Unlock for access to all 129 flashcards in this deck.
Unlock Deck
k this deck
14
Which of the following molecules has a carbon-to-carbon triple bond?
A)CH3CH3
B)CH2CHCH3
C)CH3CCH
D)CH3CH2CH3
E)CH2CHCH2CH3
A)CH3CH3
B)CH2CHCH3
C)CH3CCH
D)CH3CH2CH3
E)CH2CHCH2CH3

Unlock Deck
Unlock for access to all 129 flashcards in this deck.
Unlock Deck
k this deck
15
To which class of compounds does the molecule CH3CH2NH2 belong?
A)ketone
B)aldehyde
C)alcohol
D)amine
E)carboxylic acid
A)ketone
B)aldehyde
C)alcohol
D)amine
E)carboxylic acid

Unlock Deck
Unlock for access to all 129 flashcards in this deck.
Unlock Deck
k this deck
16
To which class of compounds does the molecule CH3CH2CO2H belong?
A)ketone
B)aldehyde
C)alcohol
D)carboxylic acid
A)ketone
B)aldehyde
C)alcohol
D)carboxylic acid

Unlock Deck
Unlock for access to all 129 flashcards in this deck.
Unlock Deck
k this deck
17
To which class of compounds does the compound shown in the figure belong? 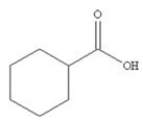
A)ketone
B)aldehyde
C)alcohol
D)amine
E)carboxylic acid

A)ketone
B)aldehyde
C)alcohol
D)amine
E)carboxylic acid

Unlock Deck
Unlock for access to all 129 flashcards in this deck.
Unlock Deck
k this deck
18
Which of the following molecules has only single bonds?
A)CHCHCH3
B)CH2CHCH3
C)CH3CH2CCH
D)CH3CH3
E)CH2CH2
A)CHCHCH3
B)CH2CHCH3
C)CH3CH2CCH
D)CH3CH3
E)CH2CH2

Unlock Deck
Unlock for access to all 129 flashcards in this deck.
Unlock Deck
k this deck
19
To which class of compounds does the molecule shown in the figure belong? 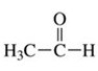
A)ketone
B)aldehyde
C)alcohol
D)amine
E)carboxylic acid
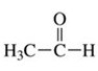
A)ketone
B)aldehyde
C)alcohol
D)amine
E)carboxylic acid

Unlock Deck
Unlock for access to all 129 flashcards in this deck.
Unlock Deck
k this deck
20
To which class of compounds does the molecule CH3CH2OH belong?
A)ketone
B)aldehyde
C)alcohol
D)amine
E)carboxylic acid
A)ketone
B)aldehyde
C)alcohol
D)amine
E)carboxylic acid

Unlock Deck
Unlock for access to all 129 flashcards in this deck.
Unlock Deck
k this deck
21
What is the name of the straight-chain alkane CH3CH2CH2CH3?
A)ethane
B)propane
C)pentane
D)hexane
E)butane
A)ethane
B)propane
C)pentane
D)hexane
E)butane

Unlock Deck
Unlock for access to all 129 flashcards in this deck.
Unlock Deck
k this deck
22
What is the IUPAC name of the compound shown in the figure? 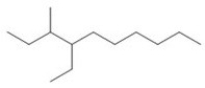
A)3-methyl-4-ethyldecane
B)4-ethyl-3-methyldecane
C)ethyl-methyl-decane
D)methyl-ethyl-decane
E)3-methyl-4-propyldecane

A)3-methyl-4-ethyldecane
B)4-ethyl-3-methyldecane
C)ethyl-methyl-decane
D)methyl-ethyl-decane
E)3-methyl-4-propyldecane

Unlock Deck
Unlock for access to all 129 flashcards in this deck.
Unlock Deck
k this deck
23
What is the name of the straight-chain alkane CH3CH2CH3?
A)ethane
B)propane
C)pentane
D)methane
E)butane
A)ethane
B)propane
C)pentane
D)methane
E)butane

Unlock Deck
Unlock for access to all 129 flashcards in this deck.
Unlock Deck
k this deck
24
Which of the following formulas represents a straight-chain molecule that is saturated?
A)C3H8
B)C5H10
C)CH2CHCH3
D)CH2CHCH2CH3
E)C4H8
A)C3H8
B)C5H10
C)CH2CHCH3
D)CH2CHCH2CH3
E)C4H8

Unlock Deck
Unlock for access to all 129 flashcards in this deck.
Unlock Deck
k this deck
25
The condensed structural formula for the molecule shown in the figure is ________. 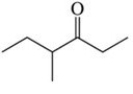
A)C8H14O
B)CH3CH2CH(CH3)COCH2CH3
C)CH3CH2CH(CH3)2COCH2CH3
D)CH3CH2CH(CH3)CH2OCH2CH3
E)CH3CH2CH(CH3)CHOCH2CH3

A)C8H14O
B)CH3CH2CH(CH3)COCH2CH3
C)CH3CH2CH(CH3)2COCH2CH3
D)CH3CH2CH(CH3)CH2OCH2CH3
E)CH3CH2CH(CH3)CHOCH2CH3

Unlock Deck
Unlock for access to all 129 flashcards in this deck.
Unlock Deck
k this deck
26
What is the IUPAC name of the compound shown in the figure? 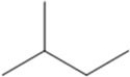
A)pentane
B)butane
C)methylbutane
D)3-methylbutane
E)methylpentane

A)pentane
B)butane
C)methylbutane
D)3-methylbutane
E)methylpentane

Unlock Deck
Unlock for access to all 129 flashcards in this deck.
Unlock Deck
k this deck
27
Which of the following formulas represents a molecule that is unsaturated?
A)C3H8
B)C5H12
C)CH2CHCH3
D)CH3CH3
E)C7H16
A)C3H8
B)C5H12
C)CH2CHCH3
D)CH3CH3
E)C7H16

Unlock Deck
Unlock for access to all 129 flashcards in this deck.
Unlock Deck
k this deck
28
Arrange the following compounds in order of increasing strength of intermolecular forces: CH3CH3, CH3CH2CH3, CH4
A)CH3CH3 < CH3CH2CH3 < CH4
B)CH3CH3 < CH4 < CH3CH2CH3
C)CH4 < CH3CH2CH3 < CH3CH3
D)CH4 < CH3CH3 < CH3CH2CH3
E)CH3CH2CH3 < CH3CH3 < CH4
A)CH3CH3 < CH3CH2CH3 < CH4
B)CH3CH3 < CH4 < CH3CH2CH3
C)CH4 < CH3CH2CH3 < CH3CH3
D)CH4 < CH3CH3 < CH3CH2CH3
E)CH3CH2CH3 < CH3CH3 < CH4

Unlock Deck
Unlock for access to all 129 flashcards in this deck.
Unlock Deck
k this deck
29
Arrange the following compounds in order of increasing strength of intermolecular forces: CH3CH3, CH3CH2CH2CH3, CH3CH2CH3
A)CH3CH3 < CH3CH2CH2CH3 < CH3CH2CH3
B)CH3CH2CH2CH33CH2CH3 3CH3
C)CH3CH2CH33CH3 < CH3CH2CH2CH3
D)CH3CH3 < CH3CH2CH3 < CH3CH2CH2CH3
E)CH3CH2CH2CH3 < CH3CH3 < CH3CH2CH3
A)CH3CH3 < CH3CH2CH2CH3 < CH3CH2CH3
B)CH3CH2CH2CH3
C)CH3CH2CH3
D)CH3CH3 < CH3CH2CH3 < CH3CH2CH2CH3
E)CH3CH2CH2CH3 < CH3CH3 < CH3CH2CH3

Unlock Deck
Unlock for access to all 129 flashcards in this deck.
Unlock Deck
k this deck
30
The condensed structural formula for the molecule shown in the figure is ________. 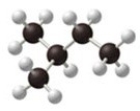
A)(CH3)2CH(CH2)2CH3
B)(CH3)2CHCH2CH3
C)CH3CH2CH3(CH2)2
D)CH3CH2CH3(CH3)2
E)CH3CHCH3(CH3)2

A)(CH3)2CH(CH2)2CH3
B)(CH3)2CHCH2CH3
C)CH3CH2CH3(CH2)2
D)CH3CH2CH3(CH3)2
E)CH3CHCH3(CH3)2

Unlock Deck
Unlock for access to all 129 flashcards in this deck.
Unlock Deck
k this deck
31
Arrange the alkanes in the figure in order of increasing boiling point. 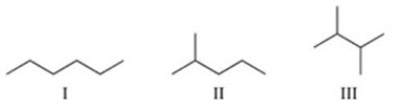
A)I < II < III
B)I < III < II
C)II < I < III
D)II < III < I
E)III < II < I

A)I < II < III
B)I < III < II
C)II < I < III
D)II < III < I
E)III < II < I

Unlock Deck
Unlock for access to all 129 flashcards in this deck.
Unlock Deck
k this deck
32
What is the name of the straight-chain alkane CH3CH2CH2CH2CH3?
A)ethane
B)propane
C)pentane
D)hexane
E)butane
A)ethane
B)propane
C)pentane
D)hexane
E)butane

Unlock Deck
Unlock for access to all 129 flashcards in this deck.
Unlock Deck
k this deck
33
Which of the following formulas represent an alkene?
A)C3H8
B)C5H12
C)CH2CHCH3
D)CH3CH3
E)C7H16
A)C3H8
B)C5H12
C)CH2CHCH3
D)CH3CH3
E)C7H16

Unlock Deck
Unlock for access to all 129 flashcards in this deck.
Unlock Deck
k this deck
34
What is the name of the straight-chain alkane CH3CH2CH2CH2CH2CH3?
A)heptane
B)propane
C)pentane
D)hexane
E)butane
A)heptane
B)propane
C)pentane
D)hexane
E)butane

Unlock Deck
Unlock for access to all 129 flashcards in this deck.
Unlock Deck
k this deck
35
The condensed structural formula for the molecule shown in the figure is ________. 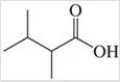
A)C6H16O2
B)CH3CH2CH(CH3)3CO2H
C)(CH3)2CH(CH2)2CO2H
D)(CH3)2CHCH(CH3)CO2H
E)CH3CH2CH(CH3)2CO2H

A)C6H16O2
B)CH3CH2CH(CH3)3CO2H
C)(CH3)2CH(CH2)2CO2H
D)(CH3)2CHCH(CH3)CO2H
E)CH3CH2CH(CH3)2CO2H

Unlock Deck
Unlock for access to all 129 flashcards in this deck.
Unlock Deck
k this deck
36
The condensed structural formula for the molecule shown in the figure is ________. 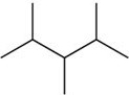
A)C8H14
B)CH3CH2C(CH3)3CH2CH3
C)(CH3)2CHCH(CH3)CH(CH3)2
D)CH3CHCH3CH2(CH3)2
E)CH3CH2CH(CH3)2CH

A)C8H14
B)CH3CH2C(CH3)3CH2CH3
C)(CH3)2CHCH(CH3)CH(CH3)2
D)CH3CHCH3CH2(CH3)2
E)CH3CH2CH(CH3)2CH

Unlock Deck
Unlock for access to all 129 flashcards in this deck.
Unlock Deck
k this deck
37
What is the IUPAC name of the compound shown in the figure? 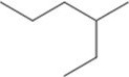
A)2-ethylpentane
B)ethylpentane
C)heptane
D)3-methylhexane
E)4-methylhexane

A)2-ethylpentane
B)ethylpentane
C)heptane
D)3-methylhexane
E)4-methylhexane

Unlock Deck
Unlock for access to all 129 flashcards in this deck.
Unlock Deck
k this deck
38
The molecular formula for the molecule shown in the figure is ________. 
A)C4H4
B)C4H6
C)C4H8
D)C4H10
E)C4H12

A)C4H4
B)C4H6
C)C4H8
D)C4H10
E)C4H12

Unlock Deck
Unlock for access to all 129 flashcards in this deck.
Unlock Deck
k this deck
39
The condensed structural formula for the molecule shown in the figure is ________. 
A)(CH3)2CH(CH2)2CH3
B)(CH3)2CHCH2(CH3)2
C)(CH3)3CCH2CH(CH3)2
D)CH3CH2CH3(CH3)2
E)CH3CHCH3(CH3)2

A)(CH3)2CH(CH2)2CH3
B)(CH3)2CHCH2(CH3)2
C)(CH3)3CCH2CH(CH3)2
D)CH3CH2CH3(CH3)2
E)CH3CHCH3(CH3)2

Unlock Deck
Unlock for access to all 129 flashcards in this deck.
Unlock Deck
k this deck
40
Which of the following formulas could represent a straight-chain molecule that is an alkyne?
A)C3H8
B)C5H12
C)C3H6
D)C4H8
E)C7H12
A)C3H8
B)C5H12
C)C3H6
D)C4H8
E)C7H12

Unlock Deck
Unlock for access to all 129 flashcards in this deck.
Unlock Deck
k this deck
41
What is the IUPAC name of the compound shown in the figure? 
A)butyne
B)1-butene
C)2-butene
D)1-pentene
E)butene

A)butyne
B)1-butene
C)2-butene
D)1-pentene
E)butene

Unlock Deck
Unlock for access to all 129 flashcards in this deck.
Unlock Deck
k this deck
42
There are ________ possible isomers with the formula C5H12.
A)1
B)2
C)3
D)4
E)5
A)1
B)2
C)3
D)4
E)5

Unlock Deck
Unlock for access to all 129 flashcards in this deck.
Unlock Deck
k this deck
43
Predict the product(s)of the reaction between CH3CH(CH3)CH2CH3 and O2 in the presence of a spark.
A)CH3CO(CH3)CH2CH3 + OH
B)CH3CO(CH3)OCH2CH3 + H2
C)no reaction
D)CO2 + H2O
E)CH3CH(CH3)O + CH3CH2O
A)CH3CO(CH3)CH2CH3 + OH
B)CH3CO(CH3)OCH2CH3 + H2
C)no reaction
D)CO2 + H2O
E)CH3CH(CH3)O + CH3CH2O

Unlock Deck
Unlock for access to all 129 flashcards in this deck.
Unlock Deck
k this deck
44
There are ________ possible isomers with the formula C6H14.
A)1
B)2
C)3
D)4
E)5
A)1
B)2
C)3
D)4
E)5

Unlock Deck
Unlock for access to all 129 flashcards in this deck.
Unlock Deck
k this deck
45
Select the line drawing for 2,3-dimethylpentane. 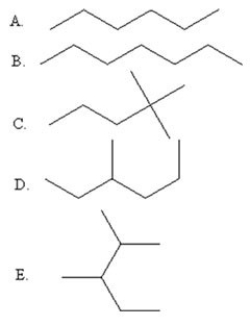
A)A
B)B
C)C
D)D
E)E

A)A
B)B
C)C
D)D
E)E

Unlock Deck
Unlock for access to all 129 flashcards in this deck.
Unlock Deck
k this deck
46
What is the condensed structural formula for 3,3-diethyl-2-methylpentane?
A)CH3CH(CH3)2CH(CH2CH3)CH2CH3
B)CH3CH(CH3)C(CH2CH3)2CH2CH3
C)CH3CH2(CH3)CH(CH2CH3)2CH2CH3
D)CH3CH2(CH3)CH2(CH2CH3)CH2CH3
E)CH3C(CH3)2CH(CH2CH3)CH2CH3
A)CH3CH(CH3)2CH(CH2CH3)CH2CH3
B)CH3CH(CH3)C(CH2CH3)2CH2CH3
C)CH3CH2(CH3)CH(CH2CH3)2CH2CH3
D)CH3CH2(CH3)CH2(CH2CH3)CH2CH3
E)CH3C(CH3)2CH(CH2CH3)CH2CH3

Unlock Deck
Unlock for access to all 129 flashcards in this deck.
Unlock Deck
k this deck
47
What is the name of the compound CH3C(CH3)2CH(CH2CH3)CH2CH3?
A)nonane
B)2-methyl-3-ethylpentane
C)2,2-diethyl-3-ethylpentane
D)2,2-dimethyl-3-ethylpentane
E)3-ethyl-2,2-dimethylpentane
A)nonane
B)2-methyl-3-ethylpentane
C)2,2-diethyl-3-ethylpentane
D)2,2-dimethyl-3-ethylpentane
E)3-ethyl-2,2-dimethylpentane

Unlock Deck
Unlock for access to all 129 flashcards in this deck.
Unlock Deck
k this deck
48
Select the line drawing for 3,3-diethylhexane. 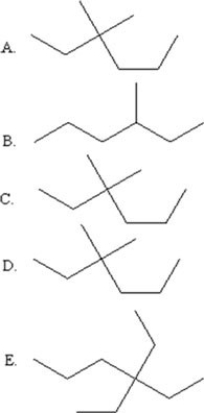
A)A
B)B
C)C
D)D
E)E

A)A
B)B
C)C
D)D
E)E

Unlock Deck
Unlock for access to all 129 flashcards in this deck.
Unlock Deck
k this deck
49
What is the name of the compound CH3CH2CH(CH3)CH3?
A)pentane
B)2-methylbutane
C)3-methylbutane
D)1,1-dimethylpropane
E)3,3-dimethylpropane
A)pentane
B)2-methylbutane
C)3-methylbutane
D)1,1-dimethylpropane
E)3,3-dimethylpropane

Unlock Deck
Unlock for access to all 129 flashcards in this deck.
Unlock Deck
k this deck
50
What is the name of the compound CH3CH2CH2CH(CH3)CH3?
A)hexane
B)5-methylpentane
C)2-methylpentane
D)2-butylethane
E)heptane
A)hexane
B)5-methylpentane
C)2-methylpentane
D)2-butylethane
E)heptane

Unlock Deck
Unlock for access to all 129 flashcards in this deck.
Unlock Deck
k this deck
51
There are many isomers of octane in gasoline. What is the name of the compound with the formula CH3C(CH3)2CH2CH(CH3)CH3?
A)octane
B)2,4-dimethylpentane
C)2,2,4-methylpentane
D)2,2,4-trimethylpentane
E)2,4,4-methylpentane
A)octane
B)2,4-dimethylpentane
C)2,2,4-methylpentane
D)2,2,4-trimethylpentane
E)2,4,4-methylpentane

Unlock Deck
Unlock for access to all 129 flashcards in this deck.
Unlock Deck
k this deck
52
What can be said about the two structural formulas shown in the figure? 
A)They represent isomers.
B)They have different molecular formulas.
C)They represent compounds with the same IUPAC name.
D)They represent the same compound.
E)None of the above.

A)They represent isomers.
B)They have different molecular formulas.
C)They represent compounds with the same IUPAC name.
D)They represent the same compound.
E)None of the above.

Unlock Deck
Unlock for access to all 129 flashcards in this deck.
Unlock Deck
k this deck
53
Predict the product(s)of the reaction CH3CH3 + Cl2 
A)CHClCH3Cl
B)CH2ClCH2Cl + H2
C)no reaction
D)CH3CH2Cl + HCl
E)2 CH3Cl

A)CHClCH3Cl
B)CH2ClCH2Cl + H2
C)no reaction
D)CH3CH2Cl + HCl
E)2 CH3Cl

Unlock Deck
Unlock for access to all 129 flashcards in this deck.
Unlock Deck
k this deck
54
What is the condensed structural formula for 3-ethyl-4-methylheptane?
A)CH3CH2CH2CH2CH(CH3)CH(CH2CH3)CH2CH3
B)CH3CH2CH2CH2C(CH3)2CH(CH2CH3)CH2CH3
C)CH3CH2CH2CH(CH3)CH(CH2CH3)CH2CH3
D)CH3CH2CH2CH(CH2CH3)CH(CH2CH3)CH2CH3
E)CH3CH2CH2CH(CH2CH3)CH2(CH3)CH2CH3
A)CH3CH2CH2CH2CH(CH3)CH(CH2CH3)CH2CH3
B)CH3CH2CH2CH2C(CH3)2CH(CH2CH3)CH2CH3
C)CH3CH2CH2CH(CH3)CH(CH2CH3)CH2CH3
D)CH3CH2CH2CH(CH2CH3)CH(CH2CH3)CH2CH3
E)CH3CH2CH2CH(CH2CH3)CH2(CH3)CH2CH3

Unlock Deck
Unlock for access to all 129 flashcards in this deck.
Unlock Deck
k this deck
55
What is the condensed structural formula for 2-methylpropane?
A)CH3CH(CH3)2
B)CH3CH2(CH3)2
C)CH3C(CH3)3
D)(CH3)2CH3
E)(CH3)2CH
A)CH3CH(CH3)2
B)CH3CH2(CH3)2
C)CH3C(CH3)3
D)(CH3)2CH3
E)(CH3)2CH

Unlock Deck
Unlock for access to all 129 flashcards in this deck.
Unlock Deck
k this deck
56
There are ________ possible isomers with the formula C4H10.
A)1
B)2
C)3
D)4
E)5
A)1
B)2
C)3
D)4
E)5

Unlock Deck
Unlock for access to all 129 flashcards in this deck.
Unlock Deck
k this deck
57
Select the line drawing for 2-methylhexane. 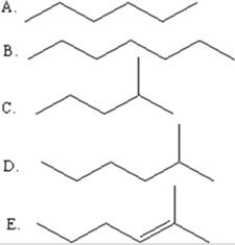
A)A
B)B
C)C
D)D
E)E

A)A
B)B
C)C
D)D
E)E

Unlock Deck
Unlock for access to all 129 flashcards in this deck.
Unlock Deck
k this deck
58
What is the name of the compound CH3CH(CH3)2?
A)2,2-methylethane
B)2,2-dimethylethane
C)1-methylpropane
D)2-methylpropane
E)3-methylpropane
A)2,2-methylethane
B)2,2-dimethylethane
C)1-methylpropane
D)2-methylpropane
E)3-methylpropane

Unlock Deck
Unlock for access to all 129 flashcards in this deck.
Unlock Deck
k this deck
59
Predict the product(s)of the reaction CH3CH3 + Br2
A)CH3BrCH3Br
B)CH2BrCH2Br + H2
C)CH3CH2Br + HBr
D)no reaction
E)2 CH3Br

A)CH3BrCH3Br
B)CH2BrCH2Br + H2
C)CH3CH2Br + HBr
D)no reaction
E)2 CH3Br

Unlock Deck
Unlock for access to all 129 flashcards in this deck.
Unlock Deck
k this deck
60
What is the condensed structural formula for 3,3-diethyl-2-methylhexane?
A)CH3CH2CH(CH2CH3)CH2(CH3)CH2CH3
B)CH3CH2C(CH2CH3)2CH2(CH3)CH2CH3
C)CH3CH2C(CH2CH3)2C(CH3)2CH2CH3
D)CH3CH(CH3)C(CH2CH3)2CH2CH2CH3
E)CH3C(CH3)2C(CH2CH3)2CH2CH2CH3
A)CH3CH2CH(CH2CH3)CH2(CH3)CH2CH3
B)CH3CH2C(CH2CH3)2CH2(CH3)CH2CH3
C)CH3CH2C(CH2CH3)2C(CH3)2CH2CH3
D)CH3CH(CH3)C(CH2CH3)2CH2CH2CH3
E)CH3C(CH3)2C(CH2CH3)2CH2CH2CH3

Unlock Deck
Unlock for access to all 129 flashcards in this deck.
Unlock Deck
k this deck
61
Which of the following compounds can exist as cis/trans isomers?
A)CH3CH2CH=CH2
B)CH3CH=CH2
C)CH2=CH2
D)CH3CH=CHCH3
E)CH2=CHCH2CH2CH3
A)CH3CH2CH=CH2
B)CH3CH=CH2
C)CH2=CH2
D)CH3CH=CHCH3
E)CH2=CHCH2CH2CH3

Unlock Deck
Unlock for access to all 129 flashcards in this deck.
Unlock Deck
k this deck
62
Which of the following represents a cis isomer of a compound that can exhibit cis-trans isomerism? 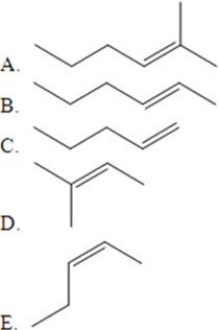
A)A
B)B
C)C
D)D
E)E

A)A
B)B
C)C
D)D
E)E

Unlock Deck
Unlock for access to all 129 flashcards in this deck.
Unlock Deck
k this deck
63
What is the IUPAC name of the compound shown in the figure? 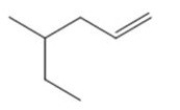
A)methylhexene
B)4-methylhexene
C)2-methylhexene
D)4-methyl-1-hexene
E)4-ethyl-5-pentene

A)methylhexene
B)4-methylhexene
C)2-methylhexene
D)4-methyl-1-hexene
E)4-ethyl-5-pentene

Unlock Deck
Unlock for access to all 129 flashcards in this deck.
Unlock Deck
k this deck
64
Which of the following formulas represents an alcohol?
A)CH3COCH3
B)CH3OCH3
C)CH3OCH2CH3
D)CH3CH2CH2OH
E)CH3CH2CH2CHO
A)CH3COCH3
B)CH3OCH3
C)CH3OCH2CH3
D)CH3CH2CH2OH
E)CH3CH2CH2CHO

Unlock Deck
Unlock for access to all 129 flashcards in this deck.
Unlock Deck
k this deck
65
Predict the product(s)of the reaction of ethene and hydrogen, catalyzed by palladium metal: CH2=CH2 + H2  ?
?
A)no reaction
B)CH2=CH3
C)CH3=CH3
D)CH3CH3
E)2 CH4
 ?
?A)no reaction
B)CH2=CH3
C)CH3=CH3
D)CH3CH3
E)2 CH4

Unlock Deck
Unlock for access to all 129 flashcards in this deck.
Unlock Deck
k this deck
66
Give the IUPAC name for the compound shown in the figure. 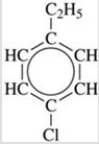
A)1-chloro-3-ethylbenzene
B)1-chloro-4-ethylbenzene
C)1-ethyl-2-chlorobenzene
D)1-ethyl-4-chlorobenzene
E)chloroethylcyclohexane

A)1-chloro-3-ethylbenzene
B)1-chloro-4-ethylbenzene
C)1-ethyl-2-chlorobenzene
D)1-ethyl-4-chlorobenzene
E)chloroethylcyclohexane

Unlock Deck
Unlock for access to all 129 flashcards in this deck.
Unlock Deck
k this deck
67
Which of the following represents a trans isomer of a compound that can exhibit cis-trans isomerism? 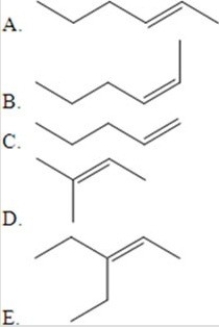
A)A
B)B
C)C
D)D
E)E

A)A
B)B
C)C
D)D
E)E

Unlock Deck
Unlock for access to all 129 flashcards in this deck.
Unlock Deck
k this deck
68
Which of the following compounds can exist as cis/trans isomers?
A)CH3CH=CHC(CH3)3
B)CH3CH=CH2
C)CH2=CH2
D)CH3CH(CH3)2
E)CH2=CHCH2CH3
A)CH3CH=CHC(CH3)3
B)CH3CH=CH2
C)CH2=CH2
D)CH3CH(CH3)2
E)CH2=CHCH2CH3

Unlock Deck
Unlock for access to all 129 flashcards in this deck.
Unlock Deck
k this deck
69
Which of the following formulas represents an alcohol?
A)CH3CH2CHO
B)CH3CH2OCH2CH3
C)CH3CH(OH)CH2CH3
D)CH3COCH2CH3
E)CH3CO2H
A)CH3CH2CHO
B)CH3CH2OCH2CH3
C)CH3CH(OH)CH2CH3
D)CH3COCH2CH3
E)CH3CO2H

Unlock Deck
Unlock for access to all 129 flashcards in this deck.
Unlock Deck
k this deck
70
Which types of compounds undergo addition polymerization reactions?
A)alkanes
B)alkenes
C)alcohols
D)aromatic compounds
E)amines
A)alkanes
B)alkenes
C)alcohols
D)aromatic compounds
E)amines

Unlock Deck
Unlock for access to all 129 flashcards in this deck.
Unlock Deck
k this deck
71
Give the IUPAC name for the compound shown in the figure. 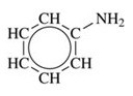
A)2-aminobenzene
B)2-aminocyclohexene
C)aniline
D)ammonia benzene
E)benzene ammonia

A)2-aminobenzene
B)2-aminocyclohexene
C)aniline
D)ammonia benzene
E)benzene ammonia

Unlock Deck
Unlock for access to all 129 flashcards in this deck.
Unlock Deck
k this deck
72
What is the name of the compound shown in the figure? 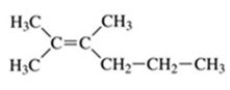
A)trans-2,3-diethyl-2-butene
B)2,3-dimethyl-2-hexene
C)cis-2,3-diethyl-2-butene
D)trans-2,3-dimethyl-2-hexene
E)trans-4,5-dimethyl-4-hexene

A)trans-2,3-diethyl-2-butene
B)2,3-dimethyl-2-hexene
C)cis-2,3-diethyl-2-butene
D)trans-2,3-dimethyl-2-hexene
E)trans-4,5-dimethyl-4-hexene

Unlock Deck
Unlock for access to all 129 flashcards in this deck.
Unlock Deck
k this deck
73
What is the name of the compound shown in the figure? 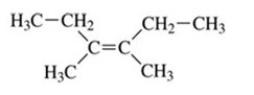
A)trans-2,3-diethyl-2-butene
B)trans-3,4-dimethyl-3-hexene
C)cis-2,3-diethyl-2-butene
D)cis-3,4-dimethyl-3-hexene
E)3-octene

A)trans-2,3-diethyl-2-butene
B)trans-3,4-dimethyl-3-hexene
C)cis-2,3-diethyl-2-butene
D)cis-3,4-dimethyl-3-hexene
E)3-octene

Unlock Deck
Unlock for access to all 129 flashcards in this deck.
Unlock Deck
k this deck
74
Give the IUPAC name for the compound shown in the figure. 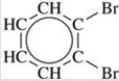
A)2,3-bromobenzene
B)2,3-dibromobenzene
C)1,2-bromobenzene
D)1,2-dibromobenzene
E)benzenedibromide

A)2,3-bromobenzene
B)2,3-dibromobenzene
C)1,2-bromobenzene
D)1,2-dibromobenzene
E)benzenedibromide

Unlock Deck
Unlock for access to all 129 flashcards in this deck.
Unlock Deck
k this deck
75
Give the IUPAC name for the compound shown in the figure. 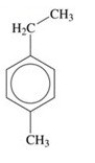
A)4-ethyl-1-methylbenzene
B)3-ethyltoluene
C)4-ethyltoluene
D)1-methyl-phenylethane
E)4-ethyl-1-methylcyclohexane

A)4-ethyl-1-methylbenzene
B)3-ethyltoluene
C)4-ethyltoluene
D)1-methyl-phenylethane
E)4-ethyl-1-methylcyclohexane

Unlock Deck
Unlock for access to all 129 flashcards in this deck.
Unlock Deck
k this deck
76
Predict the product(s)of the reaction of ethene and bromine: CH2=CH2 + Br2 → ?
A)no reaction
B)CH3CH2Br + HBr
C)CH2BrCH2Br + H2
D)CH2BrCH2Br
E)CH2=CHBr + HBr
A)no reaction
B)CH3CH2Br + HBr
C)CH2BrCH2Br + H2
D)CH2BrCH2Br
E)CH2=CHBr + HBr

Unlock Deck
Unlock for access to all 129 flashcards in this deck.
Unlock Deck
k this deck
77
What is the name of the compound shown in the figure? 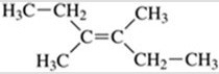
A)trans-2,3-diethyl-2-butene
B)trans-3,4-dimethyl-3-hexene
C)cis-2,3-diethyl-2-butene
D)cis-3,4-dimethyl-3-hexene
E)3-octene

A)trans-2,3-diethyl-2-butene
B)trans-3,4-dimethyl-3-hexene
C)cis-2,3-diethyl-2-butene
D)cis-3,4-dimethyl-3-hexene
E)3-octene

Unlock Deck
Unlock for access to all 129 flashcards in this deck.
Unlock Deck
k this deck
78
What is the name of the compound CH3CH=CHC(CH3)3?
A)2,2-dimethyl-4-pentene
B)trimethylbutene
C)2-heptene
D)4,4-dimethyl-2-pentene
E)1,1,1-trimethyl-2-butene
A)2,2-dimethyl-4-pentene
B)trimethylbutene
C)2-heptene
D)4,4-dimethyl-2-pentene
E)1,1,1-trimethyl-2-butene

Unlock Deck
Unlock for access to all 129 flashcards in this deck.
Unlock Deck
k this deck
79
Identify the major product of the following catalyzed reaction. 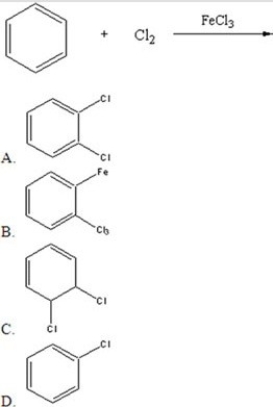
A)A
B)B
C)C
D)D
E)No products.

A)A
B)B
C)C
D)D
E)No products.

Unlock Deck
Unlock for access to all 129 flashcards in this deck.
Unlock Deck
k this deck
80
Predict the product(s)of the reaction between ethene and hydrogen bromide: CH2=CH2 + HBr → ?
A)no reaction
B)CH2=CH2Br
C)CH3=CH2Br
D)CH3CH2Br
E)CH4 + CH3Br
A)no reaction
B)CH2=CH2Br
C)CH3=CH2Br
D)CH3CH2Br
E)CH4 + CH3Br

Unlock Deck
Unlock for access to all 129 flashcards in this deck.
Unlock Deck
k this deck



