Deck 15: Chemical Equilibrium
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/146
Play
Full screen (f)
Deck 15: Chemical Equilibrium
1
Which substances are included in the equilibrium constant expression, Kc?
A) Only pure solids
B) Only pure liquids
C) Only pure solids and liquids
D) Only gases and dissolved substances
E) All participating substances
A) Only pure solids
B) Only pure liquids
C) Only pure solids and liquids
D) Only gases and dissolved substances
E) All participating substances
Only gases and dissolved substances
2
For the following reaction at 25ºC, is 3 × 1024. 2SO2(g) + O2(g)  2SO3(g)
2SO3(g)
What is Kc at this temperature? (R = 0.08206 L • atm/K • mol)
A) 1 × 1023
B) 1 × 1024
C) 3 × 1024
D) 6 × 1024
E) 7 × 1025
 2SO3(g)
2SO3(g)What is Kc at this temperature? (R = 0.08206 L • atm/K • mol)
A) 1 × 1023
B) 1 × 1024
C) 3 × 1024
D) 6 × 1024
E) 7 × 1025
7 × 1025
3
Phosgene, COCl2, a poisonous gas, decomposes as follows. COCl2(g)  CO(g) + Cl2(g).
CO(g) + Cl2(g).
At 900.ºC, Kc = 0.083. What is KP at this temperature? (R = 0.08206 L • atm/K • mol)
A) 0.125
B) 8.0
C) 6.1
D) 0.16
E) 0.083
 CO(g) + Cl2(g).
CO(g) + Cl2(g).At 900.ºC, Kc = 0.083. What is KP at this temperature? (R = 0.08206 L • atm/K • mol)
A) 0.125
B) 8.0
C) 6.1
D) 0.16
E) 0.083
8.0
4
If, in a particular process, reactants are able to form products, and products are also able to form reactants, then this process may be described as
A) a reversible process.
B) an elementary process.
C) at equilibrium.
D) forbidden.
E) a forward process.
A) a reversible process.
B) an elementary process.
C) at equilibrium.
D) forbidden.
E) a forward process.

Unlock Deck
Unlock for access to all 146 flashcards in this deck.
Unlock Deck
k this deck
5
What is the name for Qc?
A) Reversibility expression
B) Reaction expression
C) Equilibrium expression
D) Reaction quotient
E) Mass action
A) Reversibility expression
B) Reaction expression
C) Equilibrium expression
D) Reaction quotient
E) Mass action

Unlock Deck
Unlock for access to all 146 flashcards in this deck.
Unlock Deck
k this deck
6
Hydrogen peroxide may decompose to form water and oxygen gas according to the following reaction. 2H2O2(g)  2H2O(g) + O2(g)
2H2O(g) + O2(g)
In a particular experiment, 1.75 moles of H2O2 were placed in a 2.5-L reaction chamber at 307ºC. After equilibrium was reached, 1.20 moles of H2O2 remained. What is Kc for the reaction?
A) 2.0 × 10-4
B) 2.3 × 10-2
C) 2.4 × 10-3
D) 5.5 × 10-3
E) 3.9 × 10-4
 2H2O(g) + O2(g)
2H2O(g) + O2(g)In a particular experiment, 1.75 moles of H2O2 were placed in a 2.5-L reaction chamber at 307ºC. After equilibrium was reached, 1.20 moles of H2O2 remained. What is Kc for the reaction?
A) 2.0 × 10-4
B) 2.3 × 10-2
C) 2.4 × 10-3
D) 5.5 × 10-3
E) 3.9 × 10-4

Unlock Deck
Unlock for access to all 146 flashcards in this deck.
Unlock Deck
k this deck
7
The observation that at equilibrium, the reaction quotient equals the equilibrium constant, is representative of which law?
A) Law of equal states
B) Reversibility law
C) Law of equivalence
D) Law of reactant-product equivalence
E) Law of mass action
A) Law of equal states
B) Reversibility law
C) Law of equivalence
D) Law of reactant-product equivalence
E) Law of mass action

Unlock Deck
Unlock for access to all 146 flashcards in this deck.
Unlock Deck
k this deck
8
Which is the correct equilibrium constant expression for the following reaction? 2BrCl3(g) ![<strong>Which is the correct equilibrium constant expression for the following reaction? 2BrCl<sub>3</sub>(g) Br<sub>2</sub>(g) + 3Cl<sub>2</sub>(g)</strong> A) K<sub>c</sub> = [Br<sub>2</sub>] [Cl<sub>2</sub>]/[BrCl<sub>3</sub>] B) K<sub>c</sub> = [Br<sub>2</sub>] [Cl<sub>2</sub>]<sup>5</sup>/[BrCl<sub>3</sub>]<sup>2</sup> C) K<sub>c</sub> = [Br<sub>2</sub>] [Cl<sub>2</sub>]<sup>3</sup>/[BrCl<sub>3</sub>]<sup>2</sup> D) K<sub>c</sub> = [BrCl<sub>3</sub>]<sup>2</sup>/([Br<sub>2</sub>] × [Cl<sub>2</sub>]<sup>3</sup>) E) K<sub>c</sub> = 2[BrCl<sub>3</sub>]<sup>2</sup>/([Br<sub>2</sub>] × 3[Cl<sub>2</sub>]<sup>3</sup>)](https://d2lvgg3v3hfg70.cloudfront.net/TB8482/11eb6c5c_3a7d_8ec4_8d9d_037841bf3a67_TB8482_11.jpg) Br2(g) + 3Cl2(g)
Br2(g) + 3Cl2(g)
A) Kc = [Br2] [Cl2]/[BrCl3]
B) Kc = [Br2] [Cl2]5/[BrCl3]2
C) Kc = [Br2] [Cl2]3/[BrCl3]2
D) Kc = [BrCl3]2/([Br2] × [Cl2]3)
E) Kc = 2[BrCl3]2/([Br2] × 3[Cl2]3)
![<strong>Which is the correct equilibrium constant expression for the following reaction? 2BrCl<sub>3</sub>(g) Br<sub>2</sub>(g) + 3Cl<sub>2</sub>(g)</strong> A) K<sub>c</sub> = [Br<sub>2</sub>] [Cl<sub>2</sub>]/[BrCl<sub>3</sub>] B) K<sub>c</sub> = [Br<sub>2</sub>] [Cl<sub>2</sub>]<sup>5</sup>/[BrCl<sub>3</sub>]<sup>2</sup> C) K<sub>c</sub> = [Br<sub>2</sub>] [Cl<sub>2</sub>]<sup>3</sup>/[BrCl<sub>3</sub>]<sup>2</sup> D) K<sub>c</sub> = [BrCl<sub>3</sub>]<sup>2</sup>/([Br<sub>2</sub>] × [Cl<sub>2</sub>]<sup>3</sup>) E) K<sub>c</sub> = 2[BrCl<sub>3</sub>]<sup>2</sup>/([Br<sub>2</sub>] × 3[Cl<sub>2</sub>]<sup>3</sup>)](https://d2lvgg3v3hfg70.cloudfront.net/TB8482/11eb6c5c_3a7d_8ec4_8d9d_037841bf3a67_TB8482_11.jpg) Br2(g) + 3Cl2(g)
Br2(g) + 3Cl2(g)A) Kc = [Br2] [Cl2]/[BrCl3]
B) Kc = [Br2] [Cl2]5/[BrCl3]2
C) Kc = [Br2] [Cl2]3/[BrCl3]2
D) Kc = [BrCl3]2/([Br2] × [Cl2]3)
E) Kc = 2[BrCl3]2/([Br2] × 3[Cl2]3)

Unlock Deck
Unlock for access to all 146 flashcards in this deck.
Unlock Deck
k this deck
9
What is defined as a fraction with equilibrium product concentrations in the numerator and equilibrium reactant concentrations in the denominator and each concentration raised to a power equal to the corresponding stoichiometric coefficient in the balanced chemical equation?
A) Reversibility expression
B) Reaction expression
C) Equilibrium expression
D) Product quotient
E) Mass action
A) Reversibility expression
B) Reaction expression
C) Equilibrium expression
D) Product quotient
E) Mass action

Unlock Deck
Unlock for access to all 146 flashcards in this deck.
Unlock Deck
k this deck
10
The equilibrium constant for the reaction Ni(s) + 4CO(g)  Ni(CO)4(g) is 5.0 × 104 at 25ºC. What is the equilibrium constant for the following reaction?
Ni(CO)4(g) is 5.0 × 104 at 25ºC. What is the equilibrium constant for the following reaction?
Ni(CO)4(g) Ni(s) + 4CO(g)?
Ni(s) + 4CO(g)?
A) 2.0 × 10-5
B) 2.5 × 109
C) 5.0 × 104
D) 5.0 × 10-4
E) 2.0 × 10-3
 Ni(CO)4(g) is 5.0 × 104 at 25ºC. What is the equilibrium constant for the following reaction?
Ni(CO)4(g) is 5.0 × 104 at 25ºC. What is the equilibrium constant for the following reaction?Ni(CO)4(g)
 Ni(s) + 4CO(g)?
Ni(s) + 4CO(g)?A) 2.0 × 10-5
B) 2.5 × 109
C) 5.0 × 104
D) 5.0 × 10-4
E) 2.0 × 10-3

Unlock Deck
Unlock for access to all 146 flashcards in this deck.
Unlock Deck
k this deck
11
A 6.0-L vessel was found to contain 1.0 mol BrCl3, 2.0 mol Br2 and 6.0 mol Cl2 at equilibrium. What is the equilibrium constant, Kc, for this equilibrium mixture for the reaction
2BrCl3(g) Br2(g) + 3Cl2(g)?
Br2(g) + 3Cl2(g)?
A) 0.014
B) 108
C) 18
D) 12
E) 432
2BrCl3(g)
 Br2(g) + 3Cl2(g)?
Br2(g) + 3Cl2(g)?A) 0.014
B) 108
C) 18
D) 12
E) 432

Unlock Deck
Unlock for access to all 146 flashcards in this deck.
Unlock Deck
k this deck
12
Which is the correct equilibrium constant expression for the following reaction? 2C6H6(g) + 15O2(g)  12CO2(g) + 6H2O(g)
12CO2(g) + 6H2O(g)
A)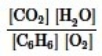
B)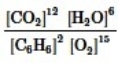
C)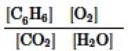
D)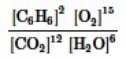
E)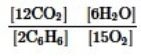
 12CO2(g) + 6H2O(g)
12CO2(g) + 6H2O(g)A)
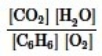
B)
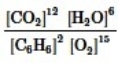
C)
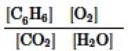
D)
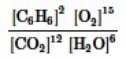
E)
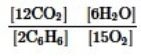

Unlock Deck
Unlock for access to all 146 flashcards in this deck.
Unlock Deck
k this deck
13
Which is the correct equilibrium constant expression for the following reaction? FeO(s) + H2(g) ![<strong>Which is the correct equilibrium constant expression for the following reaction? FeO(s) + H<sub>2</sub>(g) Fe(s) + H<sub>2</sub>O(g)</strong> A) K<sub>c</sub> = [H<sub>2</sub>O]/[H<sub>2</sub>] B) K<sub>c</sub> = [Fe] [H<sub>2</sub>O]/[Fe<sub>2</sub>O<sub>3</sub>] C) K<sub>c</sub> = [Fe<sub>2</sub>O<sub>3</sub>] [H<sub>2</sub>]/[Fe][H<sub>2</sub>O] D) K<sub>c</sub> = [Fe][H<sub>2</sub>O]/[Fe<sub>2</sub>O<sub>3</sub>] [H<sub>2</sub>] E) K<sub>c</sub> = [H<sub>2</sub>]/[H<sub>2</sub>O]](https://d2lvgg3v3hfg70.cloudfront.net/TB8482/11eb6c5c_3a7d_40a3_8d9d_8dc327802c50_TB8482_11.jpg) Fe(s) + H2O(g)
Fe(s) + H2O(g)
A) Kc = [H2O]/[H2]
B) Kc = [Fe] [H2O]/[Fe2O3]
C) Kc = [Fe2O3] [H2]/[Fe][H2O]
D) Kc = [Fe][H2O]/[Fe2O3] [H2]
E) Kc = [H2]/[H2O]
![<strong>Which is the correct equilibrium constant expression for the following reaction? FeO(s) + H<sub>2</sub>(g) Fe(s) + H<sub>2</sub>O(g)</strong> A) K<sub>c</sub> = [H<sub>2</sub>O]/[H<sub>2</sub>] B) K<sub>c</sub> = [Fe] [H<sub>2</sub>O]/[Fe<sub>2</sub>O<sub>3</sub>] C) K<sub>c</sub> = [Fe<sub>2</sub>O<sub>3</sub>] [H<sub>2</sub>]/[Fe][H<sub>2</sub>O] D) K<sub>c</sub> = [Fe][H<sub>2</sub>O]/[Fe<sub>2</sub>O<sub>3</sub>] [H<sub>2</sub>] E) K<sub>c</sub> = [H<sub>2</sub>]/[H<sub>2</sub>O]](https://d2lvgg3v3hfg70.cloudfront.net/TB8482/11eb6c5c_3a7d_40a3_8d9d_8dc327802c50_TB8482_11.jpg) Fe(s) + H2O(g)
Fe(s) + H2O(g)A) Kc = [H2O]/[H2]
B) Kc = [Fe] [H2O]/[Fe2O3]
C) Kc = [Fe2O3] [H2]/[Fe][H2O]
D) Kc = [Fe][H2O]/[Fe2O3] [H2]
E) Kc = [H2]/[H2O]

Unlock Deck
Unlock for access to all 146 flashcards in this deck.
Unlock Deck
k this deck
14
What is defined as a fraction with product concentrations in the numerator and reactant concentrations in the denominator and with each concentration raised to a power equal to the corresponding stoichiometric coefficient in the balanced chemical equation?
A) Reversibility expression
B) Reaction expression
C) Equilibrium expression
D) Reaction quotient
E) Mass action
A) Reversibility expression
B) Reaction expression
C) Equilibrium expression
D) Reaction quotient
E) Mass action

Unlock Deck
Unlock for access to all 146 flashcards in this deck.
Unlock Deck
k this deck
15
At elevated temperatures, hydrogen iodide may decompose to form hydrogen gas and iodine gas, as follows. 2HI(g) ![<strong>At elevated temperatures, hydrogen iodide may decompose to form hydrogen gas and iodine gas, as follows. 2HI(g) H<sub>2</sub>(g) + I<sub>2</sub>(g) In a particular experiment, the concentrations at equilibrium were measured to be [HI] = 0.85 mol/L, [I<sub>2</sub>] = 0.60 mol/L, and [H<sub>2</sub>] = 0.27 mol/L. What is K<sub>c</sub> for the above reaction?</strong> A) 5.3 B) 0.22 C) 4.5 D) 0.19 E) 1.6 × 10<sup>2</sup>](https://d2lvgg3v3hfg70.cloudfront.net/TB8482/11eb6c5c_3a7f_b1b6_8d9d_5943cd9d9077_TB8482_11.jpg) H2(g) + I2(g)
H2(g) + I2(g)
In a particular experiment, the concentrations at equilibrium were measured to be [HI] = 0.85 mol/L, [I2] = 0.60 mol/L, and [H2] = 0.27 mol/L. What is Kc for the above reaction?
A) 5.3
B) 0.22
C) 4.5
D) 0.19
E) 1.6 × 102
![<strong>At elevated temperatures, hydrogen iodide may decompose to form hydrogen gas and iodine gas, as follows. 2HI(g) H<sub>2</sub>(g) + I<sub>2</sub>(g) In a particular experiment, the concentrations at equilibrium were measured to be [HI] = 0.85 mol/L, [I<sub>2</sub>] = 0.60 mol/L, and [H<sub>2</sub>] = 0.27 mol/L. What is K<sub>c</sub> for the above reaction?</strong> A) 5.3 B) 0.22 C) 4.5 D) 0.19 E) 1.6 × 10<sup>2</sup>](https://d2lvgg3v3hfg70.cloudfront.net/TB8482/11eb6c5c_3a7f_b1b6_8d9d_5943cd9d9077_TB8482_11.jpg) H2(g) + I2(g)
H2(g) + I2(g)In a particular experiment, the concentrations at equilibrium were measured to be [HI] = 0.85 mol/L, [I2] = 0.60 mol/L, and [H2] = 0.27 mol/L. What is Kc for the above reaction?
A) 5.3
B) 0.22
C) 4.5
D) 0.19
E) 1.6 × 102

Unlock Deck
Unlock for access to all 146 flashcards in this deck.
Unlock Deck
k this deck
16
Carbon tetrachloride reacts at high temperatures with oxygen to produce two toxic gases, phosgene and chlorine. CCl4(g) + ½O2(g)  COCl2(g) + Cl2(g), Kc = 4.4 × 109 at 1000 K
COCl2(g) + Cl2(g), Kc = 4.4 × 109 at 1000 K
What is Kc for the following reaction?
2CCl4(g) + O2(g) 2COCl2(g) + 2Cl2(g)
2COCl2(g) + 2Cl2(g)
A) 6.6 × 104
B) 4.4 × 109
C) 8.8 × 109
D) 1.9 × 1019
E) 2.3 × 10-10
 COCl2(g) + Cl2(g), Kc = 4.4 × 109 at 1000 K
COCl2(g) + Cl2(g), Kc = 4.4 × 109 at 1000 KWhat is Kc for the following reaction?
2CCl4(g) + O2(g)
 2COCl2(g) + 2Cl2(g)
2COCl2(g) + 2Cl2(g)A) 6.6 × 104
B) 4.4 × 109
C) 8.8 × 109
D) 1.9 × 1019
E) 2.3 × 10-10

Unlock Deck
Unlock for access to all 146 flashcards in this deck.
Unlock Deck
k this deck
17
During a chemical reaction, what defines when the concentrations of the reactants and products reach a constant level?
A) Elementary process
B) Reversible reaction
C) Rate law
D) Rate constant
E) Equilibrium
A) Elementary process
B) Reversible reaction
C) Rate law
D) Rate constant
E) Equilibrium

Unlock Deck
Unlock for access to all 146 flashcards in this deck.
Unlock Deck
k this deck
18
Which statement is correct?
A) If Q < K, then products must be converted to reactants.
B) If Q > K, then reactants must be converted to products.
C) If Q = K, then the system is at equilibrium.
D) If Q < K, then more reactants are produced.
E) None of the answers is correct.
A) If Q < K, then products must be converted to reactants.
B) If Q > K, then reactants must be converted to products.
C) If Q = K, then the system is at equilibrium.
D) If Q < K, then more reactants are produced.
E) None of the answers is correct.

Unlock Deck
Unlock for access to all 146 flashcards in this deck.
Unlock Deck
k this deck
19
Which is the correct mass-action expression, Qc, for the following chemical reaction? Sn2+(aq) + ½ O2(g) + 3H2O(l)  SnO2(s) + 2H3O+(aq)
SnO2(s) + 2H3O+(aq)
A)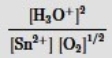
B)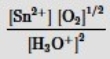
C)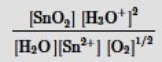
D)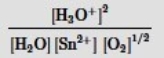
E) None of these expressions is correct.
 SnO2(s) + 2H3O+(aq)
SnO2(s) + 2H3O+(aq)A)
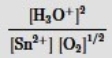
B)
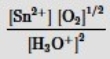
C)
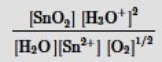
D)
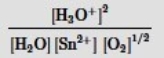
E) None of these expressions is correct.

Unlock Deck
Unlock for access to all 146 flashcards in this deck.
Unlock Deck
k this deck
20
Consider the reversible reaction: 2NO2(g)  N2O4(g) If the concentrations of both NO2 and N2O4 are each 0.016 M, what is the value of Qc?
N2O4(g) If the concentrations of both NO2 and N2O4 are each 0.016 M, what is the value of Qc?
A) 0.016
B) 0.50
C) 1.0
D) 2.0
E) 63
 N2O4(g) If the concentrations of both NO2 and N2O4 are each 0.016 M, what is the value of Qc?
N2O4(g) If the concentrations of both NO2 and N2O4 are each 0.016 M, what is the value of Qc?A) 0.016
B) 0.50
C) 1.0
D) 2.0
E) 63

Unlock Deck
Unlock for access to all 146 flashcards in this deck.
Unlock Deck
k this deck
21
The equilibrium constant, KP, for the reaction H2(g) + I2(g)  2HI(g) is 55.2 at 425°C.
2HI(g) is 55.2 at 425°C.
A rigid cylinder at that temperature contains 0.127 atm of hydrogen, 0.134 atm of iodine, and 1.055 atm of hydrogen iodide. Is the system at equilibrium?
A) Yes.
B) No, the forward reaction must proceed to establish equilibrium.
C) No, the reverse reaction must proceed to establish equilibrium.
D) I need to know the volume of the container before deciding.
E) Need to know the starting pressures of all substances before deciding.
 2HI(g) is 55.2 at 425°C.
2HI(g) is 55.2 at 425°C.A rigid cylinder at that temperature contains 0.127 atm of hydrogen, 0.134 atm of iodine, and 1.055 atm of hydrogen iodide. Is the system at equilibrium?
A) Yes.
B) No, the forward reaction must proceed to establish equilibrium.
C) No, the reverse reaction must proceed to establish equilibrium.
D) I need to know the volume of the container before deciding.
E) Need to know the starting pressures of all substances before deciding.

Unlock Deck
Unlock for access to all 146 flashcards in this deck.
Unlock Deck
k this deck
22
Nitrogen dioxide decomposes according to the reaction 2NO2(g)  2NO(g) + O2(g)
2NO(g) + O2(g)
Where KP = 4.48 × 10-13 at 25°C. What is the value for Kc?(R = 0.08206 L • atm/K • mol)
A) 1.83 × 10-14
B) 4.48 × 10-14
C) 9.19 × 10-13
D) 2.18 × 10-13
E) 1.10 × 10-11
 2NO(g) + O2(g)
2NO(g) + O2(g)Where KP = 4.48 × 10-13 at 25°C. What is the value for Kc?(R = 0.08206 L • atm/K • mol)
A) 1.83 × 10-14
B) 4.48 × 10-14
C) 9.19 × 10-13
D) 2.18 × 10-13
E) 1.10 × 10-11

Unlock Deck
Unlock for access to all 146 flashcards in this deck.
Unlock Deck
k this deck
23
A mixture of 0.500 mole of carbon monoxide and 0.400 mole of bromine was placed into a rigid 1.00-L container and the system was allowed to come to equilibrium. The equilibrium concentration of COBr2 was 0.233 M. What is Kc for this reaction? CO(g) + Br2(g)  COBr2(g)
COBr2(g)
A) 5.23
B) 2.14
C) 1.17
D) 0.467
E) 0.191
 COBr2(g)
COBr2(g)A) 5.23
B) 2.14
C) 1.17
D) 0.467
E) 0.191

Unlock Deck
Unlock for access to all 146 flashcards in this deck.
Unlock Deck
k this deck
24
Suppose 15.00 g of solid ammonium hydrogen sulfide is introduced into a 500.-mL flask at 25°C, the flask is sealed, and the system is allowed to reach equilibrium. What is the partial pressure of ammonia in this flask if KP = 0.108 at 25°C for the following reaction? NH4HS(s)  NH3(g) + H2S(g)
NH3(g) + H2S(g)
A) 0.657 atm
B) 1.25 atm
C) 0.329 atm
D) 14.4 atm
E) 2.50 atm
 NH3(g) + H2S(g)
NH3(g) + H2S(g)A) 0.657 atm
B) 1.25 atm
C) 0.329 atm
D) 14.4 atm
E) 2.50 atm

Unlock Deck
Unlock for access to all 146 flashcards in this deck.
Unlock Deck
k this deck
25
At a certain temperature the reaction CO2(g) + H2(g)  CO(g) + H2O(g)
CO(g) + H2O(g)
Has Kc = 2.50. If 2.00 mol of carbon dioxide and 1.50 mol of hydrogen are placed in a 5.00-L vessel and equilibrium is established, what is the equilibrium concentration of carbon monoxide?
A) 0.209 M
B) 1.33 M
C) 0.267 M
D) 0.667 M
E) 0.600 M
 CO(g) + H2O(g)
CO(g) + H2O(g)Has Kc = 2.50. If 2.00 mol of carbon dioxide and 1.50 mol of hydrogen are placed in a 5.00-L vessel and equilibrium is established, what is the equilibrium concentration of carbon monoxide?
A) 0.209 M
B) 1.33 M
C) 0.267 M
D) 0.667 M
E) 0.600 M

Unlock Deck
Unlock for access to all 146 flashcards in this deck.
Unlock Deck
k this deck
26
Consider the following equilibria: 2SO3(g)  2SO2(g) + O2(g) Kc = 2.3 × 10-7
2SO2(g) + O2(g) Kc = 2.3 × 10-7
2NO3(g) 2NO2(g) + O2(g) Kc = 1.4 × 10-3
2NO2(g) + O2(g) Kc = 1.4 × 10-3
Calculate the equilibrium constant for the reaction
SO2(g) + NO3(g) SO3(g) + NO2(g).
SO3(g) + NO2(g).
A) 78
B) 1.3 × 10-2
C) 1.6 × 10-4
D) 3.2 × 10-10
E) 6.1 × 103
 2SO2(g) + O2(g) Kc = 2.3 × 10-7
2SO2(g) + O2(g) Kc = 2.3 × 10-72NO3(g)
 2NO2(g) + O2(g) Kc = 1.4 × 10-3
2NO2(g) + O2(g) Kc = 1.4 × 10-3Calculate the equilibrium constant for the reaction
SO2(g) + NO3(g)
 SO3(g) + NO2(g).
SO3(g) + NO2(g).A) 78
B) 1.3 × 10-2
C) 1.6 × 10-4
D) 3.2 × 10-10
E) 6.1 × 103

Unlock Deck
Unlock for access to all 146 flashcards in this deck.
Unlock Deck
k this deck
27
Consider the equilibrium reaction: N2O4(g)  2NO2(g) Which of the following correctly describes the relationship between Kc and KP for the reaction?
2NO2(g) Which of the following correctly describes the relationship between Kc and KP for the reaction?
A) KP = Kc
B) KP = RT × Kc
C) KP = (RT × Kc )-1
D) KP = Kc/RT
E) KP = RT/Kc
 2NO2(g) Which of the following correctly describes the relationship between Kc and KP for the reaction?
2NO2(g) Which of the following correctly describes the relationship between Kc and KP for the reaction?A) KP = Kc
B) KP = RT × Kc
C) KP = (RT × Kc )-1
D) KP = Kc/RT
E) KP = RT/Kc

Unlock Deck
Unlock for access to all 146 flashcards in this deck.
Unlock Deck
k this deck
28
Nitric oxide is formed in automobile exhaust when nitrogen and oxygen in air react at high temperatures. N2(g) + O2(g)  2NO(g)
2NO(g)
The equilibrium constant KP for the reaction is 0.0025 at 2127°C. If a container is charged with 8.00 atm of nitrogen and 5.00 atm of oxygen and the mixture is allowed to reach equilibrium, what will be the equilibrium partial pressure of nitrogen?
A) 0.15 atm
B) 0.31 atm
C) 3.08 atm
D) 7.69 atm
E) 7.85 atm
 2NO(g)
2NO(g)The equilibrium constant KP for the reaction is 0.0025 at 2127°C. If a container is charged with 8.00 atm of nitrogen and 5.00 atm of oxygen and the mixture is allowed to reach equilibrium, what will be the equilibrium partial pressure of nitrogen?
A) 0.15 atm
B) 0.31 atm
C) 3.08 atm
D) 7.69 atm
E) 7.85 atm

Unlock Deck
Unlock for access to all 146 flashcards in this deck.
Unlock Deck
k this deck
29
At 850°C, the equilibrium constant, KP, for the reaction C(s) + CO2(g)  2CO(g) has a value of 10.7.
2CO(g) has a value of 10.7.
If the total pressure in the system at equilibrium is 1.000 atm, what is the partial pressure of carbon monoxide at equilibrium?
A) 0.362 atm
B) 0.489 atm
C) 0.667 atm
D) 0.915 atm
E) 0.921 atm
 2CO(g) has a value of 10.7.
2CO(g) has a value of 10.7.If the total pressure in the system at equilibrium is 1.000 atm, what is the partial pressure of carbon monoxide at equilibrium?
A) 0.362 atm
B) 0.489 atm
C) 0.667 atm
D) 0.915 atm
E) 0.921 atm

Unlock Deck
Unlock for access to all 146 flashcards in this deck.
Unlock Deck
k this deck
30
The equilibrium constant, KP, has a value of 6.5 × 10-4 at 308 K for the reaction of nitrogen monoxide with chlorine. 2NO(g) + Cl2(g)  2NOCl(g)
2NOCl(g)
What is the value of Kc? (R = 0.08206 L • atm/K • mol)
A) 2.5 × 10-7
B) 6.5 × 10-4
C) 1.6 × 10-2
D) 1.7
E) None of these choices is correct.
 2NOCl(g)
2NOCl(g)What is the value of Kc? (R = 0.08206 L • atm/K • mol)
A) 2.5 × 10-7
B) 6.5 × 10-4
C) 1.6 × 10-2
D) 1.7
E) None of these choices is correct.

Unlock Deck
Unlock for access to all 146 flashcards in this deck.
Unlock Deck
k this deck
31
The reaction of nitrogen with oxygen to form nitrogen monoxide can be represented by the following equation. N2(g) + O2(g)  2NO(g)
2NO(g)
At 2000.°C, the equilibrium constant, Kc, has a value of 4.10 × 10-4. What is the value of KP? (R = 0.08206 L • atm/K • mol)
A) 2.17 × 10-8
B) 4.10 × 10-4
C) 7.65 × 10-2
D) 2.20 ×10-6
E) 2.44 ×103
 2NO(g)
2NO(g)At 2000.°C, the equilibrium constant, Kc, has a value of 4.10 × 10-4. What is the value of KP? (R = 0.08206 L • atm/K • mol)
A) 2.17 × 10-8
B) 4.10 × 10-4
C) 7.65 × 10-2
D) 2.20 ×10-6
E) 2.44 ×103

Unlock Deck
Unlock for access to all 146 flashcards in this deck.
Unlock Deck
k this deck
32
For the nitrogen fixation reaction, 3H2(g) + N2(g)  2NH3(g), Kc = 6.0 × 10-2 at 500°C. If 0.250 M H2 and 0.050 M NH3 are present at equilibrium, what is the equilibrium concentration of N2?
2NH3(g), Kc = 6.0 × 10-2 at 500°C. If 0.250 M H2 and 0.050 M NH3 are present at equilibrium, what is the equilibrium concentration of N2?
A) 3.3 M
B) 2.7 M
C) 0.20 M
D) 0.083 M
E) 0.058 M
 2NH3(g), Kc = 6.0 × 10-2 at 500°C. If 0.250 M H2 and 0.050 M NH3 are present at equilibrium, what is the equilibrium concentration of N2?
2NH3(g), Kc = 6.0 × 10-2 at 500°C. If 0.250 M H2 and 0.050 M NH3 are present at equilibrium, what is the equilibrium concentration of N2?A) 3.3 M
B) 2.7 M
C) 0.20 M
D) 0.083 M
E) 0.058 M

Unlock Deck
Unlock for access to all 146 flashcards in this deck.
Unlock Deck
k this deck
33
At 500°C the equilibrium constant, KP , is 4.00 × 10-4 for the equilibrium: 2HCN(g)  H2(g) + C2N2(g)
H2(g) + C2N2(g)
What is Kp for the following reaction?
H2(g) + C2 N2(g) 2HCN(g)
2HCN(g)
A) 2.00 × 10-4
B) -4.00 × 10-4
C) 1.25 × 103
D) 2.50 × 103
E) 4.00 × 104
 H2(g) + C2N2(g)
H2(g) + C2N2(g)What is Kp for the following reaction?
H2(g) + C2 N2(g)
 2HCN(g)
2HCN(g)A) 2.00 × 10-4
B) -4.00 × 10-4
C) 1.25 × 103
D) 2.50 × 103
E) 4.00 × 104

Unlock Deck
Unlock for access to all 146 flashcards in this deck.
Unlock Deck
k this deck
34
Which of the following is the expression that relates Kc to KP?
A) Kc = KPR/T
B) KP = Kc(RT)Δn
C) KP = Kc/(RT)Δn
D) KcKP = RT/V
E) KP = KcP/RT
A) Kc = KPR/T
B) KP = Kc(RT)Δn
C) KP = Kc/(RT)Δn
D) KcKP = RT/V
E) KP = KcP/RT

Unlock Deck
Unlock for access to all 146 flashcards in this deck.
Unlock Deck
k this deck
35
Nitric oxide and bromine were allowed to react in a sealed container. When equilibrium was reached, the following partial pressures of three gases were measured: NO: 0.526 atm; Br2 : 1.59 atm; NOBr: 7.68 atm. Calculate KP for the reaction. 2NO(g) + Br2(g)  2NOBr(g)
2NOBr(g)
A) 7.45 × 10-3
B) 0.109
C) 9.18
D) 91.8
E) 134
 2NOBr(g)
2NOBr(g)A) 7.45 × 10-3
B) 0.109
C) 9.18
D) 91.8
E) 134

Unlock Deck
Unlock for access to all 146 flashcards in this deck.
Unlock Deck
k this deck
36
Ammonium iodide dissociates reversibly to ammonia and hydrogen iodide. NH4I(s)  NH3(g) + HI(g)
NH3(g) + HI(g)
At 400°C, KP = 0.215. Calculate the partial pressure of ammonia at equilibrium when a sufficient quantity of ammonium iodide is heated to 400°C.
A) 0.103 atm
B) 0.215 atm
C) 0.232 atm
D) 0.464 atm
E) 2.00 atm
 NH3(g) + HI(g)
NH3(g) + HI(g)At 400°C, KP = 0.215. Calculate the partial pressure of ammonia at equilibrium when a sufficient quantity of ammonium iodide is heated to 400°C.
A) 0.103 atm
B) 0.215 atm
C) 0.232 atm
D) 0.464 atm
E) 2.00 atm

Unlock Deck
Unlock for access to all 146 flashcards in this deck.
Unlock Deck
k this deck
37
At 340. K, KP = 69 for the reaction H2(g) + I2(g)  2HI(g). Suppose 50.0 g of HI is injected into an evacuated 5.00-L rigid cylinder at 340. K. What is the total pressure inside the cylinder when the system comes to equilibrium?
2HI(g). Suppose 50.0 g of HI is injected into an evacuated 5.00-L rigid cylinder at 340. K. What is the total pressure inside the cylinder when the system comes to equilibrium?
A) 2.60 atm
B) 1.76 atm
C) 0.424 atm
D) 2.18 atm
E) 0.171 atm
 2HI(g). Suppose 50.0 g of HI is injected into an evacuated 5.00-L rigid cylinder at 340. K. What is the total pressure inside the cylinder when the system comes to equilibrium?
2HI(g). Suppose 50.0 g of HI is injected into an evacuated 5.00-L rigid cylinder at 340. K. What is the total pressure inside the cylinder when the system comes to equilibrium?A) 2.60 atm
B) 1.76 atm
C) 0.424 atm
D) 2.18 atm
E) 0.171 atm

Unlock Deck
Unlock for access to all 146 flashcards in this deck.
Unlock Deck
k this deck
38
At 25°C, the equilibrium constant, Kc, for the reaction 2A(g)  B(g) + C(g) is 0.0350.
B(g) + C(g) is 0.0350.
A mixture of 8.00 moles of B and 12.00 moles of C in a 20.0-L container is allowed to come to equilibrium. What is the equilibrium concentration of A?
A) 0.339 M
B) 0.378 M
C) 0.400 M
D) 0.677 M
E) 0.755 M
 B(g) + C(g) is 0.0350.
B(g) + C(g) is 0.0350.A mixture of 8.00 moles of B and 12.00 moles of C in a 20.0-L container is allowed to come to equilibrium. What is the equilibrium concentration of A?
A) 0.339 M
B) 0.378 M
C) 0.400 M
D) 0.677 M
E) 0.755 M

Unlock Deck
Unlock for access to all 146 flashcards in this deck.
Unlock Deck
k this deck
39
The equilibrium constant, Kc, for the reaction PCl3(g) + Cl2(g)  PCl5(g) is 49 at 230°C.
PCl5(g) is 49 at 230°C.
If 0.70 mol of PCl3 is added to 0.70 mol of Cl2 in a 1.00-L reaction vessel at 230°C, what is the concentration of PCl3 when equilibrium has been established?
A) 0.049 M
B) 0.11 M
C) 0.35 M
D) 0.59 M
E) 0.83 M
 PCl5(g) is 49 at 230°C.
PCl5(g) is 49 at 230°C.If 0.70 mol of PCl3 is added to 0.70 mol of Cl2 in a 1.00-L reaction vessel at 230°C, what is the concentration of PCl3 when equilibrium has been established?
A) 0.049 M
B) 0.11 M
C) 0.35 M
D) 0.59 M
E) 0.83 M

Unlock Deck
Unlock for access to all 146 flashcards in this deck.
Unlock Deck
k this deck
40
Compounds A, B, and C react according to the following equation. 3A(g) + 2B(g) ![<strong>Compounds A, B, and C react according to the following equation. 3A(g) + 2B(g) 2C(g) At 100°C a mixture of these gases at equilibrium showed that [A] = 0.855 M, [B] = 1.23 M, and [C] = 1.75 M. What is the value of K<sub>c</sub> for this reaction?</strong> A) 0.309 B) 0.601 C) 1.66 D) 2.25 E) 3.24](https://d2lvgg3v3hfg70.cloudfront.net/TB8482/11eb6c5c_3a80_750a_8d9d_3fcafa62e0f3_TB8482_11.jpg) 2C(g)
2C(g)
At 100°C a mixture of these gases at equilibrium showed that [A] = 0.855 M, [B] = 1.23 M, and [C] = 1.75 M. What is the value of Kc for this reaction?
A) 0.309
B) 0.601
C) 1.66
D) 2.25
E) 3.24
![<strong>Compounds A, B, and C react according to the following equation. 3A(g) + 2B(g) 2C(g) At 100°C a mixture of these gases at equilibrium showed that [A] = 0.855 M, [B] = 1.23 M, and [C] = 1.75 M. What is the value of K<sub>c</sub> for this reaction?</strong> A) 0.309 B) 0.601 C) 1.66 D) 2.25 E) 3.24](https://d2lvgg3v3hfg70.cloudfront.net/TB8482/11eb6c5c_3a80_750a_8d9d_3fcafa62e0f3_TB8482_11.jpg) 2C(g)
2C(g)At 100°C a mixture of these gases at equilibrium showed that [A] = 0.855 M, [B] = 1.23 M, and [C] = 1.75 M. What is the value of Kc for this reaction?
A) 0.309
B) 0.601
C) 1.66
D) 2.25
E) 3.24

Unlock Deck
Unlock for access to all 146 flashcards in this deck.
Unlock Deck
k this deck
41
Consider the reaction: 2A(g) + B(g) → 2C(g) If ΔG° = 50.0 kJ/mol at T = 25°C and PA = PB = 1 atm and PC = 2 atm, what is the value of ΔG? (R = 8.314 J/K • mol)
A) 50.0 kJ/mol
B) 49.7 kJ/mol
C) 46.5 kJ/mol
D) 53.4 kJ/mol
E) -49.7 kJ/mol
A) 50.0 kJ/mol
B) 49.7 kJ/mol
C) 46.5 kJ/mol
D) 53.4 kJ/mol
E) -49.7 kJ/mol

Unlock Deck
Unlock for access to all 146 flashcards in this deck.
Unlock Deck
k this deck
42
For the reaction HCONH2(g)  NH3(g) + CO(g), Kc = 4.84 at 400 K. If ΔH° for this reaction is 29 kJ/mol, find Kc at 500 K.
NH3(g) + CO(g), Kc = 4.84 at 400 K. If ΔH° for this reaction is 29 kJ/mol, find Kc at 500 K.
A) 5.8
B) 0.17
C) 27.5
D) 0.88
E) 10.3
 NH3(g) + CO(g), Kc = 4.84 at 400 K. If ΔH° for this reaction is 29 kJ/mol, find Kc at 500 K.
NH3(g) + CO(g), Kc = 4.84 at 400 K. If ΔH° for this reaction is 29 kJ/mol, find Kc at 500 K.A) 5.8
B) 0.17
C) 27.5
D) 0.88
E) 10.3

Unlock Deck
Unlock for access to all 146 flashcards in this deck.
Unlock Deck
k this deck
43
The standard free energy of formation of gaseous hydrogen iodide is 1.30 kJ/mol at 25°C. What is KP for the reaction H2(g) + I2(g)  2HI(g) at this temperature?
2HI(g) at this temperature?
A) 0.35
B) 0.59
C) 1.0
D) 1.7
E) 2.9
 2HI(g) at this temperature?
2HI(g) at this temperature?A) 0.35
B) 0.59
C) 1.0
D) 1.7
E) 2.9

Unlock Deck
Unlock for access to all 146 flashcards in this deck.
Unlock Deck
k this deck
44
For the reaction H2(g) + Br2(g) ![<strong>For the reaction H<sub>2</sub>(g) + Br<sub>2</sub>(g) 2HBr(g), K<sub>c</sub> = 81.4 at 385ºC. If [H<sub>2</sub>] = [Br<sub>2</sub>] = [HBr] = 2.4 × 10<sup>-4</sup><sup>M</sup> at 385ºC, which one of the following is correct?</strong> A) [H<sub>2</sub>] and [HBr] decrease as the system moves toward equilibrium. B) The system is at equilibrium. C) [H<sub>2</sub>] and [Br<sub>2</sub>] increase as the system approaches equilibrium. D) [HBr] increases as the system approaches equilibrium. E) [HBr] and [Br<sub>2</sub>] increases as the system approaches equilibrium.](https://d2lvgg3v3hfg70.cloudfront.net/TB8482/11eb6c5c_3a84_1da1_8d9d_f78a4f21a64e_TB8482_11.jpg) 2HBr(g), Kc = 81.4 at 385ºC. If [H2] = [Br2] = [HBr] = 2.4 × 10-4M at 385ºC, which one of the following is correct?
2HBr(g), Kc = 81.4 at 385ºC. If [H2] = [Br2] = [HBr] = 2.4 × 10-4M at 385ºC, which one of the following is correct?
A) [H2] and [HBr] decrease as the system moves toward equilibrium.
B) The system is at equilibrium.
C) [H2] and [Br2] increase as the system approaches equilibrium.
D) [HBr] increases as the system approaches equilibrium.
E) [HBr] and [Br2] increases as the system approaches equilibrium.
![<strong>For the reaction H<sub>2</sub>(g) + Br<sub>2</sub>(g) 2HBr(g), K<sub>c</sub> = 81.4 at 385ºC. If [H<sub>2</sub>] = [Br<sub>2</sub>] = [HBr] = 2.4 × 10<sup>-4</sup><sup>M</sup> at 385ºC, which one of the following is correct?</strong> A) [H<sub>2</sub>] and [HBr] decrease as the system moves toward equilibrium. B) The system is at equilibrium. C) [H<sub>2</sub>] and [Br<sub>2</sub>] increase as the system approaches equilibrium. D) [HBr] increases as the system approaches equilibrium. E) [HBr] and [Br<sub>2</sub>] increases as the system approaches equilibrium.](https://d2lvgg3v3hfg70.cloudfront.net/TB8482/11eb6c5c_3a84_1da1_8d9d_f78a4f21a64e_TB8482_11.jpg) 2HBr(g), Kc = 81.4 at 385ºC. If [H2] = [Br2] = [HBr] = 2.4 × 10-4M at 385ºC, which one of the following is correct?
2HBr(g), Kc = 81.4 at 385ºC. If [H2] = [Br2] = [HBr] = 2.4 × 10-4M at 385ºC, which one of the following is correct?A) [H2] and [HBr] decrease as the system moves toward equilibrium.
B) The system is at equilibrium.
C) [H2] and [Br2] increase as the system approaches equilibrium.
D) [HBr] increases as the system approaches equilibrium.
E) [HBr] and [Br2] increases as the system approaches equilibrium.

Unlock Deck
Unlock for access to all 146 flashcards in this deck.
Unlock Deck
k this deck
45
The equilibrium constant KP at 427°C for the reaction N2(g) + 3H2(g)  2NH3(g) is 9.4 × 10-5. What is ΔG° for the reaction under these conditions? (R = 8.314 J/K • mol)
2NH3(g) is 9.4 × 10-5. What is ΔG° for the reaction under these conditions? (R = 8.314 J/K • mol)
A) -33 kJ/mol
B) -54 kJ/mol
C) 54 kJ/mol
D) 33 kJ/mol
E) 1.3 J/mol
 2NH3(g) is 9.4 × 10-5. What is ΔG° for the reaction under these conditions? (R = 8.314 J/K • mol)
2NH3(g) is 9.4 × 10-5. What is ΔG° for the reaction under these conditions? (R = 8.314 J/K • mol)A) -33 kJ/mol
B) -54 kJ/mol
C) 54 kJ/mol
D) 33 kJ/mol
E) 1.3 J/mol

Unlock Deck
Unlock for access to all 146 flashcards in this deck.
Unlock Deck
k this deck
46
A sample of solid naphthalene is introduced into an evacuated flask. Using the data below, what is the equilibrium vapor pressure of naphthalene (C10H8) in the flask at 35°C? 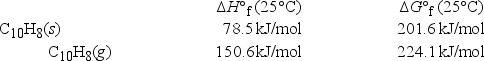
A) 890. mmHg
B) 0.22 mmHg
C) 696 mmHg
D) 0.086 mmHg
E) 833 mmHg
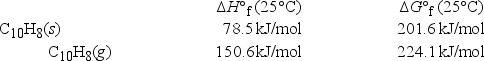
A) 890. mmHg
B) 0.22 mmHg
C) 696 mmHg
D) 0.086 mmHg
E) 833 mmHg

Unlock Deck
Unlock for access to all 146 flashcards in this deck.
Unlock Deck
k this deck
47
In the gas phase, methyl isonitrile (CH3NC) isomerizes to acetonitrile (CH3CN), H3C-N≡C(g)  H3C-C≡N(g)
H3C-C≡N(g)
With ΔH° = -89.5 kJ/mol and ΔG° = - 73.8 kJ/mol at 25°C. Find the equilibrium constant for this reaction at 100°C.
A) 1.68 × 10-10
B) 5.96 × 109
C) 2.16 × 1010
D) 4.63 × 10-11
E) 8.64 × 1012
 H3C-C≡N(g)
H3C-C≡N(g)With ΔH° = -89.5 kJ/mol and ΔG° = - 73.8 kJ/mol at 25°C. Find the equilibrium constant for this reaction at 100°C.
A) 1.68 × 10-10
B) 5.96 × 109
C) 2.16 × 1010
D) 4.63 × 10-11
E) 8.64 × 1012

Unlock Deck
Unlock for access to all 146 flashcards in this deck.
Unlock Deck
k this deck
48
What is KP at 298 K for the following reaction? (R = 8.314 J/K • mol) SO2(g) + NO2(g) → SO3(g) + NO(g) 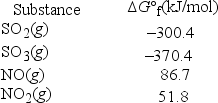
A) 6.99 × 10-7
B) 5.71 × 10-8
C) 14.2
D) 475
E) 1.42 × 106
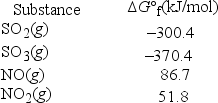
A) 6.99 × 10-7
B) 5.71 × 10-8
C) 14.2
D) 475
E) 1.42 × 106

Unlock Deck
Unlock for access to all 146 flashcards in this deck.
Unlock Deck
k this deck
49
At 1500°C the equilibrium constant for the reaction CO(g) + 2H2(g)  CH3OH(g) has the value KP = 1.4 × 10-7. What is ΔG° for this reaction at 1500°C? (R = 8.314 J/K • mol)
CH3OH(g) has the value KP = 1.4 × 10-7. What is ΔG° for this reaction at 1500°C? (R = 8.314 J/K • mol)
A) 105 kJ/mol
B) 1.07 kJ/mol
C) -233 kJ/mol
D) -105 kJ/mol
E) 233 kJ/mol
 CH3OH(g) has the value KP = 1.4 × 10-7. What is ΔG° for this reaction at 1500°C? (R = 8.314 J/K • mol)
CH3OH(g) has the value KP = 1.4 × 10-7. What is ΔG° for this reaction at 1500°C? (R = 8.314 J/K • mol)A) 105 kJ/mol
B) 1.07 kJ/mol
C) -233 kJ/mol
D) -105 kJ/mol
E) 233 kJ/mol

Unlock Deck
Unlock for access to all 146 flashcards in this deck.
Unlock Deck
k this deck
50
Which is correct?
A) If ΔG < 0, then Q > K.
B) If ΔG < 0, then Q < K.
C) If ΔG < 0, then Q = K.
D) If ΔGo < 0, then Q = K.
E) If ΔGo < 0, then Q > K.
A) If ΔG < 0, then Q > K.
B) If ΔG < 0, then Q < K.
C) If ΔG < 0, then Q = K.
D) If ΔGo < 0, then Q = K.
E) If ΔGo < 0, then Q > K.

Unlock Deck
Unlock for access to all 146 flashcards in this deck.
Unlock Deck
k this deck
51
Which represents the correct relationship between the Gibbs free energy and the equilibrium constant?
A) ΔG = -RT lnK
B) ΔG = RT lnK
C) ΔGo = -RT lnK
D) ΔGo = -RT lnQ
E) ΔGo = RT lnQ
A) ΔG = -RT lnK
B) ΔG = RT lnK
C) ΔGo = -RT lnK
D) ΔGo = -RT lnQ
E) ΔGo = RT lnQ

Unlock Deck
Unlock for access to all 146 flashcards in this deck.
Unlock Deck
k this deck
52
Find the temperature at which KP = 42.0 for the reaction H2(g) + I2(g) ![<strong>Find the temperature at which K<sub>P</sub> = 42.0 for the reaction H<sub>2</sub>(g) + I<sub>2</sub>(g) 2HI(g). [Given: at 25°C, for H<sub>2</sub>(g), ΔH°<sub>f</sub> = 0, S° = 131.0 J/mol • K; for I<sub>2</sub>(g), ΔH°<sub>f</sub> = 62.26 kJ/mol, S° = 260.6 J/mol • K; for HI(g), ΔH°<sub>f</sub> = 25.9 kJ/mol, S° = 206.3 J/mol • K; assume that ΔH° and ΔS° are independent of temperature.]</strong> A) 1040 K B) 168 K C) 539 K D) 1400 K E) 34,200 K](https://d2lvgg3v3hfg70.cloudfront.net/TB8482/11eb6c5c_3a87_790d_8d9d_818e78f2d612_TB8482_11.jpg) 2HI(g). [Given: at 25°C, for H2(g), ΔH°f = 0, S° = 131.0 J/mol • K; for I2(g), ΔH°f = 62.26 kJ/mol, S° = 260.6 J/mol • K; for HI(g), ΔH°f = 25.9 kJ/mol, S° = 206.3 J/mol • K; assume that ΔH° and ΔS° are independent of temperature.]
2HI(g). [Given: at 25°C, for H2(g), ΔH°f = 0, S° = 131.0 J/mol • K; for I2(g), ΔH°f = 62.26 kJ/mol, S° = 260.6 J/mol • K; for HI(g), ΔH°f = 25.9 kJ/mol, S° = 206.3 J/mol • K; assume that ΔH° and ΔS° are independent of temperature.]
A) 1040 K
B) 168 K
C) 539 K
D) 1400 K
E) 34,200 K
![<strong>Find the temperature at which K<sub>P</sub> = 42.0 for the reaction H<sub>2</sub>(g) + I<sub>2</sub>(g) 2HI(g). [Given: at 25°C, for H<sub>2</sub>(g), ΔH°<sub>f</sub> = 0, S° = 131.0 J/mol • K; for I<sub>2</sub>(g), ΔH°<sub>f</sub> = 62.26 kJ/mol, S° = 260.6 J/mol • K; for HI(g), ΔH°<sub>f</sub> = 25.9 kJ/mol, S° = 206.3 J/mol • K; assume that ΔH° and ΔS° are independent of temperature.]</strong> A) 1040 K B) 168 K C) 539 K D) 1400 K E) 34,200 K](https://d2lvgg3v3hfg70.cloudfront.net/TB8482/11eb6c5c_3a87_790d_8d9d_818e78f2d612_TB8482_11.jpg) 2HI(g). [Given: at 25°C, for H2(g), ΔH°f = 0, S° = 131.0 J/mol • K; for I2(g), ΔH°f = 62.26 kJ/mol, S° = 260.6 J/mol • K; for HI(g), ΔH°f = 25.9 kJ/mol, S° = 206.3 J/mol • K; assume that ΔH° and ΔS° are independent of temperature.]
2HI(g). [Given: at 25°C, for H2(g), ΔH°f = 0, S° = 131.0 J/mol • K; for I2(g), ΔH°f = 62.26 kJ/mol, S° = 260.6 J/mol • K; for HI(g), ΔH°f = 25.9 kJ/mol, S° = 206.3 J/mol • K; assume that ΔH° and ΔS° are independent of temperature.]A) 1040 K
B) 168 K
C) 539 K
D) 1400 K
E) 34,200 K

Unlock Deck
Unlock for access to all 146 flashcards in this deck.
Unlock Deck
k this deck
53
At what temperature is KP = 4.00 for the reaction N2O4(g)  2NO2(g)?
2NO2(g)? 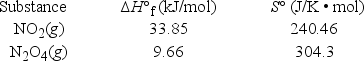 (R = 8.314 J/K • mol)
(R = 8.314 J/K • mol)
A) 197°C
B) 56°C
C) 36°C
D) 79°C
E) 476°C
 2NO2(g)?
2NO2(g)? 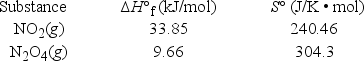 (R = 8.314 J/K • mol)
(R = 8.314 J/K • mol)A) 197°C
B) 56°C
C) 36°C
D) 79°C
E) 476°C

Unlock Deck
Unlock for access to all 146 flashcards in this deck.
Unlock Deck
k this deck
54
Nitrosyl chloride (NOCl) decomposes at elevated temperatures according to the equation 2NOCl(g)  2NO(g) + Cl2(g). What is KP for this reaction at 227°C?
2NO(g) + Cl2(g). What is KP for this reaction at 227°C?
For this reaction ΔH° = 81.2 kJ/mol and ΔS° = 128 J/K • mol. (R = 8.314 J/K • mol)
A) 1.60 × 10-2
B) 2.10 × 10-7
C) 62.8
D) 4.90 × 106
E) 3.20 × 109
 2NO(g) + Cl2(g). What is KP for this reaction at 227°C?
2NO(g) + Cl2(g). What is KP for this reaction at 227°C?For this reaction ΔH° = 81.2 kJ/mol and ΔS° = 128 J/K • mol. (R = 8.314 J/K • mol)
A) 1.60 × 10-2
B) 2.10 × 10-7
C) 62.8
D) 4.90 × 106
E) 3.20 × 109

Unlock Deck
Unlock for access to all 146 flashcards in this deck.
Unlock Deck
k this deck
55
Hydrogen peroxide (H2O2) decomposes according to the following equation. H2O2(l) → H2O(l) + ½O2(g).
What is KP for this reaction at 25°C? (ΔH° = -98.2 kJ/mol, ΔS° = 70.1 J/K • mol, R = 8.314 J/K • mol)
A) 1.3 × 10-21
B) 20.9
C) 3.46 × 1017
D) 7.4 × 1020
E) 8.6 × 104
What is KP for this reaction at 25°C? (ΔH° = -98.2 kJ/mol, ΔS° = 70.1 J/K • mol, R = 8.314 J/K • mol)
A) 1.3 × 10-21
B) 20.9
C) 3.46 × 1017
D) 7.4 × 1020
E) 8.6 × 104

Unlock Deck
Unlock for access to all 146 flashcards in this deck.
Unlock Deck
k this deck
56
For the reaction PCl3(g) + Cl2(g) ![<strong>For the reaction PCl<sub>3</sub>(g) + Cl<sub>2</sub>(g) PCl<sub>5</sub>(g) at a particular temperature, K<sub>c</sub> = 32.4. Suppose a system at that temperature is prepared with [PCl<sub>5</sub>] = 0.50 M, [Cl<sub>2</sub>] = 0.4 M, and [PCl<sub>3</sub>] = 0.10 M. Which of the following is correct?</strong> A) The system will proceed in the direction of forming more PCl<sub>5</sub> and Cl<sub>2</sub> until equilibrium is reached. B) The system is at equilibrium. C) The system will proceed in the direction of forming more PCl<sub>5</sub> until equilibrium is reached. D) The system will proceed in the direction of forming more PCl<sub>3</sub> and Cl<sub>2</sub> until equilibrium is reached. E) The system will proceed in the direction of forming more PCl<sub>3</sub> and PCl<sub>5</sub> until equilibrium is reached.](https://d2lvgg3v3hfg70.cloudfront.net/TB8482/11eb6c5c_3a84_44b2_8d9d_07f81f9a81cd_TB8482_11.jpg) PCl5(g) at a particular temperature, Kc = 32.4. Suppose a system at that temperature is prepared with [PCl5] = 0.50 M, [Cl2] = 0.4 M, and [PCl3] = 0.10 M. Which of the following is correct?
PCl5(g) at a particular temperature, Kc = 32.4. Suppose a system at that temperature is prepared with [PCl5] = 0.50 M, [Cl2] = 0.4 M, and [PCl3] = 0.10 M. Which of the following is correct?
A) The system will proceed in the direction of forming more PCl5 and Cl2 until equilibrium is reached.
B) The system is at equilibrium.
C) The system will proceed in the direction of forming more PCl5 until equilibrium is reached.
D) The system will proceed in the direction of forming more PCl3 and Cl2 until equilibrium is reached.
E) The system will proceed in the direction of forming more PCl3 and PCl5 until equilibrium is reached.
![<strong>For the reaction PCl<sub>3</sub>(g) + Cl<sub>2</sub>(g) PCl<sub>5</sub>(g) at a particular temperature, K<sub>c</sub> = 32.4. Suppose a system at that temperature is prepared with [PCl<sub>5</sub>] = 0.50 M, [Cl<sub>2</sub>] = 0.4 M, and [PCl<sub>3</sub>] = 0.10 M. Which of the following is correct?</strong> A) The system will proceed in the direction of forming more PCl<sub>5</sub> and Cl<sub>2</sub> until equilibrium is reached. B) The system is at equilibrium. C) The system will proceed in the direction of forming more PCl<sub>5</sub> until equilibrium is reached. D) The system will proceed in the direction of forming more PCl<sub>3</sub> and Cl<sub>2</sub> until equilibrium is reached. E) The system will proceed in the direction of forming more PCl<sub>3</sub> and PCl<sub>5</sub> until equilibrium is reached.](https://d2lvgg3v3hfg70.cloudfront.net/TB8482/11eb6c5c_3a84_44b2_8d9d_07f81f9a81cd_TB8482_11.jpg) PCl5(g) at a particular temperature, Kc = 32.4. Suppose a system at that temperature is prepared with [PCl5] = 0.50 M, [Cl2] = 0.4 M, and [PCl3] = 0.10 M. Which of the following is correct?
PCl5(g) at a particular temperature, Kc = 32.4. Suppose a system at that temperature is prepared with [PCl5] = 0.50 M, [Cl2] = 0.4 M, and [PCl3] = 0.10 M. Which of the following is correct?A) The system will proceed in the direction of forming more PCl5 and Cl2 until equilibrium is reached.
B) The system is at equilibrium.
C) The system will proceed in the direction of forming more PCl5 until equilibrium is reached.
D) The system will proceed in the direction of forming more PCl3 and Cl2 until equilibrium is reached.
E) The system will proceed in the direction of forming more PCl3 and PCl5 until equilibrium is reached.

Unlock Deck
Unlock for access to all 146 flashcards in this deck.
Unlock Deck
k this deck
57
The equilibrium constant for the reaction AgBr(s) ![<strong>The equilibrium constant for the reaction AgBr(s) Ag<sup>+</sup>(aq) + Br<sup>- </sup>(aq) is the solubility product constant, K<sub>sp</sub> = 7.7 × 10<sup>-13</sup> at 25°C. Calculate ΔG for the reaction when [Ag<sup>+</sup>] = 1.0 × 10<sup>-2 </sup><sup>M</sup> and [Br<sup>-</sup>] = 1.0 × 10<sup>-3 </sup><sup>M</sup>. Is the reaction spontaneous or nonspontaneous at these concentrations? (R = 8.314 J/K • mol)</strong> A) ΔG = 69.1 kJ/mol, nonspontaneous B) ΔG = -69.1 kJ/mol, spontaneous C) ΔG = 97.5 kJ/mol, spontaneous D) ΔG = 40.6 kJ/mol, nonspontaneous E) ΔG = -97.5 kJ/mol, nonspontaneous](https://d2lvgg3v3hfg70.cloudfront.net/TB8482/11eb6c5c_3a85_f267_8d9d_cb2996c90085_TB8482_11.jpg) Ag+(aq) + Br- (aq) is the solubility product constant, Ksp = 7.7 × 10-13 at 25°C. Calculate ΔG for the reaction when [Ag+] = 1.0 × 10-2 M and [Br-] = 1.0 × 10-3 M. Is the reaction spontaneous or nonspontaneous at these concentrations? (R = 8.314 J/K • mol)
Ag+(aq) + Br- (aq) is the solubility product constant, Ksp = 7.7 × 10-13 at 25°C. Calculate ΔG for the reaction when [Ag+] = 1.0 × 10-2 M and [Br-] = 1.0 × 10-3 M. Is the reaction spontaneous or nonspontaneous at these concentrations? (R = 8.314 J/K • mol)
A) ΔG = 69.1 kJ/mol, nonspontaneous
B) ΔG = -69.1 kJ/mol, spontaneous
C) ΔG = 97.5 kJ/mol, spontaneous
D) ΔG = 40.6 kJ/mol, nonspontaneous
E) ΔG = -97.5 kJ/mol, nonspontaneous
![<strong>The equilibrium constant for the reaction AgBr(s) Ag<sup>+</sup>(aq) + Br<sup>- </sup>(aq) is the solubility product constant, K<sub>sp</sub> = 7.7 × 10<sup>-13</sup> at 25°C. Calculate ΔG for the reaction when [Ag<sup>+</sup>] = 1.0 × 10<sup>-2 </sup><sup>M</sup> and [Br<sup>-</sup>] = 1.0 × 10<sup>-3 </sup><sup>M</sup>. Is the reaction spontaneous or nonspontaneous at these concentrations? (R = 8.314 J/K • mol)</strong> A) ΔG = 69.1 kJ/mol, nonspontaneous B) ΔG = -69.1 kJ/mol, spontaneous C) ΔG = 97.5 kJ/mol, spontaneous D) ΔG = 40.6 kJ/mol, nonspontaneous E) ΔG = -97.5 kJ/mol, nonspontaneous](https://d2lvgg3v3hfg70.cloudfront.net/TB8482/11eb6c5c_3a85_f267_8d9d_cb2996c90085_TB8482_11.jpg) Ag+(aq) + Br- (aq) is the solubility product constant, Ksp = 7.7 × 10-13 at 25°C. Calculate ΔG for the reaction when [Ag+] = 1.0 × 10-2 M and [Br-] = 1.0 × 10-3 M. Is the reaction spontaneous or nonspontaneous at these concentrations? (R = 8.314 J/K • mol)
Ag+(aq) + Br- (aq) is the solubility product constant, Ksp = 7.7 × 10-13 at 25°C. Calculate ΔG for the reaction when [Ag+] = 1.0 × 10-2 M and [Br-] = 1.0 × 10-3 M. Is the reaction spontaneous or nonspontaneous at these concentrations? (R = 8.314 J/K • mol)A) ΔG = 69.1 kJ/mol, nonspontaneous
B) ΔG = -69.1 kJ/mol, spontaneous
C) ΔG = 97.5 kJ/mol, spontaneous
D) ΔG = 40.6 kJ/mol, nonspontaneous
E) ΔG = -97.5 kJ/mol, nonspontaneous

Unlock Deck
Unlock for access to all 146 flashcards in this deck.
Unlock Deck
k this deck
58
The solubility product constant, Ksp, at 25°C for AgI(s) in water has the value 8.3 × 10-17. Calculate ΔG at 25°C for the process AgI(s) ![<strong>The solubility product constant, K<sub>sp</sub>, at 25°C for AgI(s) in water has the value 8.3 × 10<sup>-17</sup>. Calculate ΔG at 25°C for the process AgI(s) Ag<sup>+</sup>(aq) + I<sup>-</sup>(aq) where [Ag<sup>+</sup>] = 9.1 × 10<sup>-9 </sup><sup>M</sup> and [I<sup>-</sup>] = 9.1 × 10<sup>-9 </sup><sup>M</sup>. (R = 8.314 J/K • mol)</strong> A) +4.4 kJ/mol B) +91.7 kJ/mol C) 0.0 kJ/mol D) -91.7 kJ/mol E) -4.4 kJ/mol](https://d2lvgg3v3hfg70.cloudfront.net/TB8482/11eb6c5c_3a86_4088_8d9d_8745113416de_TB8482_11.jpg) Ag+(aq) + I-(aq) where [Ag+] = 9.1 × 10-9 M and [I-] = 9.1 × 10-9 M. (R = 8.314 J/K • mol)
Ag+(aq) + I-(aq) where [Ag+] = 9.1 × 10-9 M and [I-] = 9.1 × 10-9 M. (R = 8.314 J/K • mol)
A) +4.4 kJ/mol
B) +91.7 kJ/mol
C) 0.0 kJ/mol
D) -91.7 kJ/mol
E) -4.4 kJ/mol
![<strong>The solubility product constant, K<sub>sp</sub>, at 25°C for AgI(s) in water has the value 8.3 × 10<sup>-17</sup>. Calculate ΔG at 25°C for the process AgI(s) Ag<sup>+</sup>(aq) + I<sup>-</sup>(aq) where [Ag<sup>+</sup>] = 9.1 × 10<sup>-9 </sup><sup>M</sup> and [I<sup>-</sup>] = 9.1 × 10<sup>-9 </sup><sup>M</sup>. (R = 8.314 J/K • mol)</strong> A) +4.4 kJ/mol B) +91.7 kJ/mol C) 0.0 kJ/mol D) -91.7 kJ/mol E) -4.4 kJ/mol](https://d2lvgg3v3hfg70.cloudfront.net/TB8482/11eb6c5c_3a86_4088_8d9d_8745113416de_TB8482_11.jpg) Ag+(aq) + I-(aq) where [Ag+] = 9.1 × 10-9 M and [I-] = 9.1 × 10-9 M. (R = 8.314 J/K • mol)
Ag+(aq) + I-(aq) where [Ag+] = 9.1 × 10-9 M and [I-] = 9.1 × 10-9 M. (R = 8.314 J/K • mol)A) +4.4 kJ/mol
B) +91.7 kJ/mol
C) 0.0 kJ/mol
D) -91.7 kJ/mol
E) -4.4 kJ/mol

Unlock Deck
Unlock for access to all 146 flashcards in this deck.
Unlock Deck
k this deck
59
In the gas phase, formic acid forms a dimer, 2HCOOH(g)  (HCOOH)2(g). For this reaction, ΔH° = -60.1 kJ/mol and ΔG° = -13.9 kJ/mol at 25°C. Find the equilibrium constant (KP) for this reaction at 75 °C.
(HCOOH)2(g). For this reaction, ΔH° = -60.1 kJ/mol and ΔG° = -13.9 kJ/mol at 25°C. Find the equilibrium constant (KP) for this reaction at 75 °C.
A) 8960
B) 273
C) 0.120
D) 8.33
E) 1.12 × 10-4
 (HCOOH)2(g). For this reaction, ΔH° = -60.1 kJ/mol and ΔG° = -13.9 kJ/mol at 25°C. Find the equilibrium constant (KP) for this reaction at 75 °C.
(HCOOH)2(g). For this reaction, ΔH° = -60.1 kJ/mol and ΔG° = -13.9 kJ/mol at 25°C. Find the equilibrium constant (KP) for this reaction at 75 °C.A) 8960
B) 273
C) 0.120
D) 8.33
E) 1.12 × 10-4

Unlock Deck
Unlock for access to all 146 flashcards in this deck.
Unlock Deck
k this deck
60
The reaction system POBr3(g)  POBr(g) + Br2(g) is at equilibrium. Which of the following statements describes the behavior of the system if the partial pressure of bromine is reduced by 75% as equilibrium is established?
POBr(g) + Br2(g) is at equilibrium. Which of the following statements describes the behavior of the system if the partial pressure of bromine is reduced by 75% as equilibrium is established?
A) POBr will be consumed as equilibrium is established.
B) The partial pressure of POBr will decrease while the partial pressure of Br2 increases as equilibrium is established.
C) POBr3 will be consumed as equilibrium is established.
D) The volume will have to decrease before equilibrium can be reestablished.
E) Bromine will be generated as equilibrium is established.
 POBr(g) + Br2(g) is at equilibrium. Which of the following statements describes the behavior of the system if the partial pressure of bromine is reduced by 75% as equilibrium is established?
POBr(g) + Br2(g) is at equilibrium. Which of the following statements describes the behavior of the system if the partial pressure of bromine is reduced by 75% as equilibrium is established?A) POBr will be consumed as equilibrium is established.
B) The partial pressure of POBr will decrease while the partial pressure of Br2 increases as equilibrium is established.
C) POBr3 will be consumed as equilibrium is established.
D) The volume will have to decrease before equilibrium can be reestablished.
E) Bromine will be generated as equilibrium is established.

Unlock Deck
Unlock for access to all 146 flashcards in this deck.
Unlock Deck
k this deck
61
The reaction system POBr3(g)  POBr(g) + Br2(g) is at equilibrium. Which of the following statements describes the behavior of the system if POBr is added to the container?
POBr(g) + Br2(g) is at equilibrium. Which of the following statements describes the behavior of the system if POBr is added to the container?
A) POBr will be consumed in order to establish a new equilibrium.
B) The partial pressures of POBr3 and POBr will remain steady while the partial pressure of bromine increases.
C) The partial pressure of bromine will increase while the partial pressure of POBr decreases.
D) The partial pressure of bromine remains steady while the partial pressures of POBr3 and POBr increase.
E) The forward reaction will proceed to establish equilibrium.
 POBr(g) + Br2(g) is at equilibrium. Which of the following statements describes the behavior of the system if POBr is added to the container?
POBr(g) + Br2(g) is at equilibrium. Which of the following statements describes the behavior of the system if POBr is added to the container?A) POBr will be consumed in order to establish a new equilibrium.
B) The partial pressures of POBr3 and POBr will remain steady while the partial pressure of bromine increases.
C) The partial pressure of bromine will increase while the partial pressure of POBr decreases.
D) The partial pressure of bromine remains steady while the partial pressures of POBr3 and POBr increase.
E) The forward reaction will proceed to establish equilibrium.

Unlock Deck
Unlock for access to all 146 flashcards in this deck.
Unlock Deck
k this deck
62
Based on the following data, what is Ksp at 298 K of barium carbonate, BaCO3? (R = 8.314 J/K • mol) 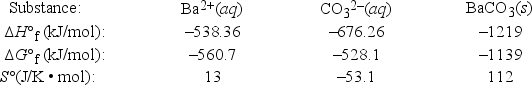
A) 5.86
B) 6.30 × 108
C) 1.59 × 10-9
D) 5.47 × 10-21
E) 2.18 × 10-27
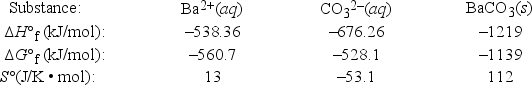
A) 5.86
B) 6.30 × 108
C) 1.59 × 10-9
D) 5.47 × 10-21
E) 2.18 × 10-27

Unlock Deck
Unlock for access to all 146 flashcards in this deck.
Unlock Deck
k this deck
63
Which equation is correct?
A) ΔG = ΔG° - RT logKeq
B) ΔG° = - RT lnK
C) ΔG = RT lnQ
D) ΔG = -RT logQ
E) ΔG° = -RT logKeq
A) ΔG = ΔG° - RT logKeq
B) ΔG° = - RT lnK
C) ΔG = RT lnQ
D) ΔG = -RT logQ
E) ΔG° = -RT logKeq

Unlock Deck
Unlock for access to all 146 flashcards in this deck.
Unlock Deck
k this deck
64
Calculate KP for the reaction 2NOCl(g)  2NO(g) + Cl2(g) at 400.°C if Kc at 400.°C for this reaction is 2.1 × 10-2. (R = 0.08206 L • atm/K • mol)
2NO(g) + Cl2(g) at 400.°C if Kc at 400.°C for this reaction is 2.1 × 10-2. (R = 0.08206 L • atm/K • mol)
A) 6.4 × 10-3
B) 1.7 × 10-3
C) 0.69
D) 1.2
E) 3.8 × 10-4
 2NO(g) + Cl2(g) at 400.°C if Kc at 400.°C for this reaction is 2.1 × 10-2. (R = 0.08206 L • atm/K • mol)
2NO(g) + Cl2(g) at 400.°C if Kc at 400.°C for this reaction is 2.1 × 10-2. (R = 0.08206 L • atm/K • mol)A) 6.4 × 10-3
B) 1.7 × 10-3
C) 0.69
D) 1.2
E) 3.8 × 10-4

Unlock Deck
Unlock for access to all 146 flashcards in this deck.
Unlock Deck
k this deck
65
Which statement is correct?
A) When Q < K then ΔG = 1.
B) When Q < K then ΔG = -ΔS.
C) When Q = K then ΔG = 0.
D) When Q > K then ΔG = 1.
E) When Q > K then ΔG = - RT.
A) When Q < K then ΔG = 1.
B) When Q < K then ΔG = -ΔS.
C) When Q = K then ΔG = 0.
D) When Q > K then ΔG = 1.
E) When Q > K then ΔG = - RT.

Unlock Deck
Unlock for access to all 146 flashcards in this deck.
Unlock Deck
k this deck
66
At 450°C, tert-butyl alcohol decomposes into water and isobutene. (CH3)3COH(g)  (CH3)2CCH2(g) + H2O(g)
(CH3)2CCH2(g) + H2O(g)
A reaction vessel contains these compounds at equilibrium. What will happen if the volume of the container is reduced by 50% at constant temperature?
A) The forward reaction will proceed in order to reestablish equilibrium.
B) The reverse reaction will proceed in order to reestablish equilibrium.
C) No change occurs.
D) The equilibrium constant will increase.
E) The equilibrium constant will decrease.
 (CH3)2CCH2(g) + H2O(g)
(CH3)2CCH2(g) + H2O(g)A reaction vessel contains these compounds at equilibrium. What will happen if the volume of the container is reduced by 50% at constant temperature?
A) The forward reaction will proceed in order to reestablish equilibrium.
B) The reverse reaction will proceed in order to reestablish equilibrium.
C) No change occurs.
D) The equilibrium constant will increase.
E) The equilibrium constant will decrease.

Unlock Deck
Unlock for access to all 146 flashcards in this deck.
Unlock Deck
k this deck
67
Iron(III) oxide can be reduced by carbon monoxide. Fe2O3(s) + 3CO(g)  2Fe(s) + 3CO2(g)
2Fe(s) + 3CO2(g)
Use the following thermodynamic data at 298 K to determine the equilibrium constant at this temperature. (R = 8.314 J/K • mol)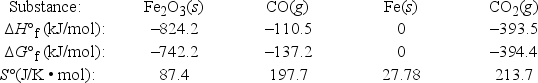
A) 7.0 × 10-6
B) 1.3 × 10-3
C) 2.2 × 104
D) 1.4 × 105
E) > 2.0 × 105
 2Fe(s) + 3CO2(g)
2Fe(s) + 3CO2(g)Use the following thermodynamic data at 298 K to determine the equilibrium constant at this temperature. (R = 8.314 J/K • mol)
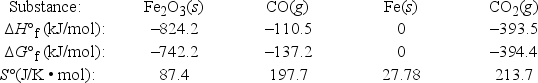
A) 7.0 × 10-6
B) 1.3 × 10-3
C) 2.2 × 104
D) 1.4 × 105
E) > 2.0 × 105

Unlock Deck
Unlock for access to all 146 flashcards in this deck.
Unlock Deck
k this deck
68
Hydrogen sulfide can be formed in the following reaction: H2(g) + 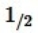 S2(g)
S2(g)  H2S(g); ΔH°rxn = -92 kJ/mol
H2S(g); ΔH°rxn = -92 kJ/mol
The equilibrium constant,KP,is 106 at 1023 K. Estimate the value of KP at 1218 K. (R = 8.314 J/K • mol)
A) 5.05
B) 18.8
C) 34.7
D) 88.9
E) 598
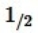 S2(g)
S2(g)  H2S(g); ΔH°rxn = -92 kJ/mol
H2S(g); ΔH°rxn = -92 kJ/molThe equilibrium constant,KP,is 106 at 1023 K. Estimate the value of KP at 1218 K. (R = 8.314 J/K • mol)
A) 5.05
B) 18.8
C) 34.7
D) 88.9
E) 598

Unlock Deck
Unlock for access to all 146 flashcards in this deck.
Unlock Deck
k this deck
69
The following reactions occur at 500 K. Arrange them in order of increasing tendency to proceed to completion (least completion → greatest completion). 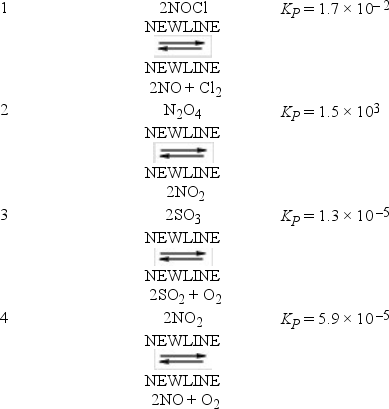
A) 2 < 1 < 3 < 4
B) 3 < 1 < 4 < 2
C) 3 < 4 < 1 < 2
D) 4 < 3 < 2 < 1
E) 4 < 3 < 1 < 2

A) 2 < 1 < 3 < 4
B) 3 < 1 < 4 < 2
C) 3 < 4 < 1 < 2
D) 4 < 3 < 2 < 1
E) 4 < 3 < 1 < 2

Unlock Deck
Unlock for access to all 146 flashcards in this deck.
Unlock Deck
k this deck
70
What is the free energy change, ΔG°, for the equilibrium between hydrogen iodide, hydrogen, and iodine at 453°C? (R = 8.314 J/K• mol) 2HI(g)  H2(g) + I2(g)Kc = 0.020 at T = 453°C
H2(g) + I2(g)Kc = 0.020 at T = 453°C
A) 6.4 kJ/mol
B) 8.8 kJ/mol
C) 15 kJ/mol
D) 19 kJ/mol
E) 24 kJ/mol
 H2(g) + I2(g)Kc = 0.020 at T = 453°C
H2(g) + I2(g)Kc = 0.020 at T = 453°CA) 6.4 kJ/mol
B) 8.8 kJ/mol
C) 15 kJ/mol
D) 19 kJ/mol
E) 24 kJ/mol

Unlock Deck
Unlock for access to all 146 flashcards in this deck.
Unlock Deck
k this deck
71
When the following reaction is at equilibrium, which relationship is always true? 2NOCl(g) ![<strong>When the following reaction is at equilibrium, which relationship is always true? 2NOCl(g) 2NO(g) + Cl<sub>2</sub>(g)</strong> A) [NO] [Cl<sub>2</sub>] = [NOCl] B) [NO]<sup>2</sup> [Cl<sub>2</sub>] = [NOCl]<sup>2</sup> C) [NOCl] = [NO] D) 2[NO] = [Cl<sub>2</sub>] E) [NO]<sup>2</sup> [Cl<sub>2</sub>] = K<sub>c</sub>[NOCl]<sup>2</sup>](https://d2lvgg3v3hfg70.cloudfront.net/TB8482/11eb6c5c_3a8c_0d00_8d9d_352aadde2440_TB8482_11.jpg) 2NO(g) + Cl2(g)
2NO(g) + Cl2(g)
A) [NO] [Cl2] = [NOCl]
B) [NO]2 [Cl2] = [NOCl]2
C) [NOCl] = [NO]
D) 2[NO] = [Cl2]
E) [NO]2 [Cl2] = Kc[NOCl]2
![<strong>When the following reaction is at equilibrium, which relationship is always true? 2NOCl(g) 2NO(g) + Cl<sub>2</sub>(g)</strong> A) [NO] [Cl<sub>2</sub>] = [NOCl] B) [NO]<sup>2</sup> [Cl<sub>2</sub>] = [NOCl]<sup>2</sup> C) [NOCl] = [NO] D) 2[NO] = [Cl<sub>2</sub>] E) [NO]<sup>2</sup> [Cl<sub>2</sub>] = K<sub>c</sub>[NOCl]<sup>2</sup>](https://d2lvgg3v3hfg70.cloudfront.net/TB8482/11eb6c5c_3a8c_0d00_8d9d_352aadde2440_TB8482_11.jpg) 2NO(g) + Cl2(g)
2NO(g) + Cl2(g)A) [NO] [Cl2] = [NOCl]
B) [NO]2 [Cl2] = [NOCl]2
C) [NOCl] = [NO]
D) 2[NO] = [Cl2]
E) [NO]2 [Cl2] = Kc[NOCl]2

Unlock Deck
Unlock for access to all 146 flashcards in this deck.
Unlock Deck
k this deck
72
If one starts with pure NO2(g) at a pressure of 0.500 atm, the total pressure inside the reaction vessel when 2NO2(g)  2NO(g) + O2(g) reaches equilibrium is 0.674 atm. What is the equilibrium partial pressure of NO2?
2NO(g) + O2(g) reaches equilibrium is 0.674 atm. What is the equilibrium partial pressure of NO2?
A) 0.152 atm
B) 0.174 atm
C) 0.200 atm
D) 0.326 atm
E) 0.500 atm
 2NO(g) + O2(g) reaches equilibrium is 0.674 atm. What is the equilibrium partial pressure of NO2?
2NO(g) + O2(g) reaches equilibrium is 0.674 atm. What is the equilibrium partial pressure of NO2?A) 0.152 atm
B) 0.174 atm
C) 0.200 atm
D) 0.326 atm
E) 0.500 atm

Unlock Deck
Unlock for access to all 146 flashcards in this deck.
Unlock Deck
k this deck
73
Which statement is correct?
A) If K < 1, lnK is negative, and ΔG° is negative then, the reaction is product favored.
B) If K > 1, lnK is negative, and ΔG° is positive, then the reaction is product favored.
C) If K > 1, lnK is positive, and ΔG° is negative, then the reaction is product favored.
D) If K > 1, lnK is negative, and ΔG° is negative, then the reaction is reactant favored.
E) If K < 1, lnK is positive, and ΔG° is positive, then the reaction is reactant favored.
A) If K < 1, lnK is negative, and ΔG° is negative then, the reaction is product favored.
B) If K > 1, lnK is negative, and ΔG° is positive, then the reaction is product favored.
C) If K > 1, lnK is positive, and ΔG° is negative, then the reaction is product favored.
D) If K > 1, lnK is negative, and ΔG° is negative, then the reaction is reactant favored.
E) If K < 1, lnK is positive, and ΔG° is positive, then the reaction is reactant favored.

Unlock Deck
Unlock for access to all 146 flashcards in this deck.
Unlock Deck
k this deck
74
Stearic acid, nature's most common fatty acid, dimerizes when dissolved in hexane: 2C17H35COOH  (C17H35COOH)2; ΔH°rxn = -172 kJ/mol
(C17H35COOH)2; ΔH°rxn = -172 kJ/mol
The equilibrium constant for this reaction at 28°C is 2.9 × 103. Estimate the equilibrium constant at 38°C. (R = 8.314 J/K• mol)
A) 4.7 × 105
B) 2.6 × 104
C) 1.9 × 103
D) 3.2 × 102
E) 18
 (C17H35COOH)2; ΔH°rxn = -172 kJ/mol
(C17H35COOH)2; ΔH°rxn = -172 kJ/molThe equilibrium constant for this reaction at 28°C is 2.9 × 103. Estimate the equilibrium constant at 38°C. (R = 8.314 J/K• mol)
A) 4.7 × 105
B) 2.6 × 104
C) 1.9 × 103
D) 3.2 × 102
E) 18

Unlock Deck
Unlock for access to all 146 flashcards in this deck.
Unlock Deck
k this deck
75
Which equation is correct?
A) ΔG = ΔG° - RT logKeq
B) ΔG = ΔG° + RT lnQ
C) ΔG = RT lnQ
D) ΔG = -RT logQ
E) ΔG = -RT logKeq
A) ΔG = ΔG° - RT logKeq
B) ΔG = ΔG° + RT lnQ
C) ΔG = RT lnQ
D) ΔG = -RT logQ
E) ΔG = -RT logKeq

Unlock Deck
Unlock for access to all 146 flashcards in this deck.
Unlock Deck
k this deck
76
For the reaction 2X(g) + Y(g)  2Z(g), Kc = 1.00 ×103 at 500 K. If at equilibrium the concentration of X is 0.20 M and the concentration of Y is 0.50 M, what is the equilibrium concentration of Z?
2Z(g), Kc = 1.00 ×103 at 500 K. If at equilibrium the concentration of X is 0.20 M and the concentration of Y is 0.50 M, what is the equilibrium concentration of Z?
A) 2.2 M
B) 3.2 M
C) 3.5 M
D) 4.5 M
E) 7.1 M
 2Z(g), Kc = 1.00 ×103 at 500 K. If at equilibrium the concentration of X is 0.20 M and the concentration of Y is 0.50 M, what is the equilibrium concentration of Z?
2Z(g), Kc = 1.00 ×103 at 500 K. If at equilibrium the concentration of X is 0.20 M and the concentration of Y is 0.50 M, what is the equilibrium concentration of Z?A) 2.2 M
B) 3.2 M
C) 3.5 M
D) 4.5 M
E) 7.1 M

Unlock Deck
Unlock for access to all 146 flashcards in this deck.
Unlock Deck
k this deck
77
The formation constant for the reaction Ag+(aq) + 2NH3(aq)  Ag(NH3)2+(aq)
Ag(NH3)2+(aq)
Is Kf = 1.7 × 107 at 25°C. What is ΔG° at this temperature? (R = 8.314 J/K• mol)
A) -1.5 kJ/mol
B) -3.5 kJ/mol
C) -18 kJ/mol
D) -23 kJ/mol
E) -41 kJ/mol
 Ag(NH3)2+(aq)
Ag(NH3)2+(aq)Is Kf = 1.7 × 107 at 25°C. What is ΔG° at this temperature? (R = 8.314 J/K• mol)
A) -1.5 kJ/mol
B) -3.5 kJ/mol
C) -18 kJ/mol
D) -23 kJ/mol
E) -41 kJ/mol

Unlock Deck
Unlock for access to all 146 flashcards in this deck.
Unlock Deck
k this deck
78
The following reaction is at equilibrium in a sealed container. N2(g) + 3H2(g)  2NH3(g); ΔH°rxn < 0
2NH3(g); ΔH°rxn < 0
Which, if any, of the following actions will increase the value of the equilibrium constant, Kc?
A) Adding more NH3
B) Adding more N2
C) Increasing the pressure
D) Lowering the temperature
E) Adding a catalyst
 2NH3(g); ΔH°rxn < 0
2NH3(g); ΔH°rxn < 0Which, if any, of the following actions will increase the value of the equilibrium constant, Kc?
A) Adding more NH3
B) Adding more N2
C) Increasing the pressure
D) Lowering the temperature
E) Adding a catalyst

Unlock Deck
Unlock for access to all 146 flashcards in this deck.
Unlock Deck
k this deck
79
Calculate the equilibrium constant at 25°C for the reaction of methane with water to form carbon dioxide and hydrogen. The data below are values at 25°C.(R = 8.314 J/K • mol) CH4(g) + 2H2O(g)  CO2(g) + 4H2(g)
CO2(g) + 4H2(g) 
A) 8.2 × 1019
B) 0.96
C) 0.58
D) 1.2 × 10-20
E) 1.4 × 10-46
 CO2(g) + 4H2(g)
CO2(g) + 4H2(g) 
A) 8.2 × 1019
B) 0.96
C) 0.58
D) 1.2 × 10-20
E) 1.4 × 10-46

Unlock Deck
Unlock for access to all 146 flashcards in this deck.
Unlock Deck
k this deck
80
A container was charged with hydrogen, nitrogen, and ammonia gases at 120°C and the system was allowed to reach equilibrium. What will happen if the volume of the container is increased at constant temperature? 3H2(g) + N2(g)  2NH3(g)
2NH3(g)
A) There will be no effect.
B) More ammonia will be produced at the expense of hydrogen and nitrogen.
C) Hydrogen and nitrogen will be produced at the expense of ammonia.
D) The equilibrium constant will increase.
E) The equilibrium constant will decrease.
 2NH3(g)
2NH3(g)A) There will be no effect.
B) More ammonia will be produced at the expense of hydrogen and nitrogen.
C) Hydrogen and nitrogen will be produced at the expense of ammonia.
D) The equilibrium constant will increase.
E) The equilibrium constant will decrease.

Unlock Deck
Unlock for access to all 146 flashcards in this deck.
Unlock Deck
k this deck



