Deck 9: Chemical Bonds
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/96
Play
Full screen (f)
Deck 9: Chemical Bonds
1
The Lewis electron diagram of an atom is shown below. This atom has a valence shell configuration of ns2np1. 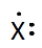
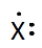
True
2
Electronegativity is a unitless number
True
3
The following is the accurate Lewis electron diagram for a P atom. 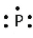
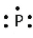
False
4
When electrons are transferred to a central atom to form a compound, a covalent bond is formed.

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
5
Lone electron pairs are electrons that do not make covalent bonds.

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
6
BeCl2 is an example of an electron deficient molecule.

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
7
O is the central atom in an H2O molecule.

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
8
The equal sharing of electrons in a covalent bond is called a nonpolar covalent bond.

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
9
Most odd electron compounds are chemically inactive.

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
10
The measured strength of ionic bonding is called the lattice energy.

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
11
A Cl- ion has fulfilled the octet rule of valence electrons.

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
12
NO2 is an example of an odd electron molecule.

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
13
A Na atom needs to gain two electrons to fulfill the octet rule.

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
14
The H−H bond is an example of a covalent bond.

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
15
A C-H bond is a nonpolar covalent bond.

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
16
A Lewis electron dot diagram represents only the protons of an atom.

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
17
The following is the accurate Lewis electron diagram for a carbon atom. 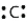
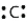

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
18
A bond will definitely be polar covalent if the electronegativity difference between the atoms involved is 1.4.

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
19
An ionic bond is formed between two atoms with the same charge.

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
20
Strength of an ionic bond decreases with an increase in the magnitude of the charge.

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
21
Two hydrogen atoms share electrons to form a stable compound. This type of a bond is called a(n)_____ bond.
A) ionic
B) hydrogen
C) dipole
D) covalent
E) metallic
A) ionic
B) hydrogen
C) dipole
D) covalent
E) metallic

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
22
Which of the following ions contain an octet of valence electrons?
A) N2-
B) O-
C) Na2+
D) F-
E) Ba+
A) N2-
B) O-
C) Na2+
D) F-
E) Ba+

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
23
How many electrons should a Ca atom lose to satisfy the octet rule?
A) 1
B) 2
C) 3
D) 4
E) 8
A) 1
B) 2
C) 3
D) 4
E) 8

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
24
The molecule CH2O takes a trigonal pyramidal shape.

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
25
The measured strength of ionic bonding is called the _____ energy.
A) kinetic
B) valence
C) covalent
D) lattice
E) latent
A) kinetic
B) valence
C) covalent
D) lattice
E) latent

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
26
A molecule with four surrounding atoms takes a bent molecular shape.

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
27
How many electrons should a Zn atom lose to satisfy the octet rule?
A) 3
B) 4
C) 0
D) 1
E) 2
A) 3
B) 4
C) 0
D) 1
E) 2

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
28
Which of the following ions do not contain an octet of valence electrons?
A) Na+
B) Rb2+
C) S2-
D) Cl-
E) Ba2+
A) Na+
B) Rb2+
C) S2-
D) Cl-
E) Ba2+

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
29
Which of the following occurs when Ca reacts with Cl2 to form CaCl2?
A) Ca gains two electrons to become Ca2+
B) Cl2 loses two electrons to become Cl22-
C) Ca loses two electrons to become Ca2+
D) Cl2 gains two electrons to become Cl22+
E) Cl atoms loses 2 electrons each to become Cl2-
A) Ca gains two electrons to become Ca2+
B) Cl2 loses two electrons to become Cl22-
C) Ca loses two electrons to become Ca2+
D) Cl2 gains two electrons to become Cl22+
E) Cl atoms loses 2 electrons each to become Cl2-

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
30
Which of the following is used to represent the Na atom using the Lewis electron dot diagram?
A)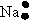
B)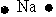
C)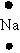
D)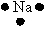
E)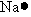
A)
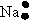
B)
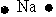
C)
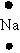
D)
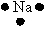
E)
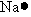

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
31
NaCl remains stable due to the attraction between the Na+ and Cl- ions. This attraction is called a(n)_____.
A) chemical bond
B) ionic bond
C) covalent bond
D) metallic bond
E) dipole attraction
A) chemical bond
B) ionic bond
C) covalent bond
D) metallic bond
E) dipole attraction

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
32
For a chlorine atom to complete an octet, it must _____.
A) lose an electron
B) gain two electrons
C) gain an electron
D) gain five electrons
E) lose two electrons
A) lose an electron
B) gain two electrons
C) gain an electron
D) gain five electrons
E) lose two electrons

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
33
Which of the following is the common convention to represent a helium atom using the Lewis electron dot diagram? 
A)
B)
C)
D)
E)

A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
34
A(n) _____ is a representation of the valence electrons of an atom that uses dots around the symbol of the element.
A) Lewis electron dot diagram
B) molecular electron graph
C) structural electron formula
D) HYPERLINK "http://en.wikipedia.org/wiki/Molecular_geometry" molecular electron geometry
E) orbital electron diagram
A) Lewis electron dot diagram
B) molecular electron graph
C) structural electron formula
D) HYPERLINK "http://en.wikipedia.org/wiki/Molecular_geometry" molecular electron geometry
E) orbital electron diagram

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
35
CH3OH is an example of a molecule that violates the octet rule.

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
36
Which of the following compounds is characterized by the strongest ionic bond?
A) LiCl
B) NaCl
C) NaBr
D) LiF
E) MgO
A) LiCl
B) NaCl
C) NaBr
D) LiF
E) MgO

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
37
A GeF2 molecule has linear geometry.

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
38
Any molecule with only two atoms has a linear shape.

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
39
What column of the periodic table has Lewis electron dot diagrams that have four electrons in them?
A) the column headed by oxygen
B) the column headed by fluorine
C) the column headed by carbon
D) the column headed by nitrogen
E) the column headed by boron
A) the column headed by oxygen
B) the column headed by fluorine
C) the column headed by carbon
D) the column headed by nitrogen
E) the column headed by boron

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
40
Which of the following bonds is formed when electrons are shared between atoms?
A) ionic bond
B) covalent bond
C) dipole interaction
D) valence bond
E) metallic bond
A) ionic bond
B) covalent bond
C) dipole interaction
D) valence bond
E) metallic bond

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
41
What is the polarity of a Na-Br bond?
A) likely ionic
B) slightly polar covalent
C) definitely polar covalent
D) slightly ionic
E) nonpolar covalent
A) likely ionic
B) slightly polar covalent
C) definitely polar covalent
D) slightly ionic
E) nonpolar covalent

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
42
What is the energy change for this reaction? (Refer to Table 9.2 in the text.) 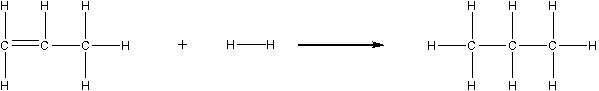
A) -183 kJ/mol
B) -477 kJ/mol
C) 477 kJ/mol
D) -129 kJ/mol
E) -4.00 kJ/mol

A) -183 kJ/mol
B) -477 kJ/mol
C) 477 kJ/mol
D) -129 kJ/mol
E) -4.00 kJ/mol

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
43
What is the polarity of a C-Si bond?
A) likely ionic
B) slightly polar covalent
C) definitely polar covalent
D) slightly ionic
E) nonpolar covalent
A) likely ionic
B) slightly polar covalent
C) definitely polar covalent
D) slightly ionic
E) nonpolar covalent

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
44
Which of the following bonds is most likely ionic?
A) C-C
B) Li-F
C) P-H
D) H-I
E) H-Br
A) C-C
B) Li-F
C) P-H
D) H-I
E) H-Br

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
45
In the molecule CO2, how many electrons are shared by C and the two O atoms?
A) 4
B) 5
C) 8
D) 6
E) 10
A) 4
B) 5
C) 8
D) 6
E) 10

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
46
Which of the following molecules is not linear in shape?
A) PCl3
B) O2
C) CO2
D) NO
E) BeH2
A) PCl3
B) O2
C) CO2
D) NO
E) BeH2

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
47
Which of the following molecules is likely to have a trigonal planar electron group distribution?
A) H2O
B) CO2
C) NO
D) NO2
E) BF3
A) H2O
B) CO2
C) NO
D) NO2
E) BF3

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
48
Which of the following bonds is most likely to be nonpolar covalent bond?
A) P-H
B) C-H
C) O-H (electronegativity chart in text shows a "D" instead of "O" for oxygen
D) Na-Cl
E) Li-Cl
A) P-H
B) C-H
C) O-H (electronegativity chart in text shows a "D" instead of "O" for oxygen
D) Na-Cl
E) Li-Cl

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
49
Covalent compounds of _____ usually form electron deficient molecules.
A) hydrogen
B) boron
C) chlorine
D) lithium
E) sodium
A) hydrogen
B) boron
C) chlorine
D) lithium
E) sodium

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
50
Which of the following is a stable odd-electron molecule?
A) N3
B) HF
C) CO2
D) HCl
E) NO
A) N3
B) HF
C) CO2
D) HCl
E) NO

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
51
Which of the following molecules is formed by triple bonds alone?
A) F2
B) N2
C) O2
D) O3
E) CO2
A) F2
B) N2
C) O2
D) O3
E) CO2

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
52
Which of the following has the strongest covalent bond?
A) HF
B) H2S
C) H2
D) N2
E) CO2
A) HF
B) H2S
C) H2
D) N2
E) CO2

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
53
A covalent bond of any type is called a(n)_____.
A) neutron group
B) proton group
C) core group
D) nucleus group
E) electron group
A) neutron group
B) proton group
C) core group
D) nucleus group
E) electron group

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
54
Identify the correct Lewis electron diagram for CO2.
A)
B)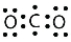
C)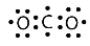
D)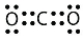
E)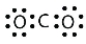
A)

B)
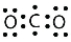
C)
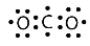
D)
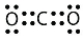
E)
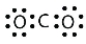

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
55
From the following identify the molecule which has a bent or angular shape.
A) CO2
B) CaCl2
C) GeF2
D) CaF2
E) SiO2
A) CO2
B) CaCl2
C) GeF2
D) CaF2
E) SiO2

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
56
If the electronegativity of a bond formed between two atoms is 0.15, it is likely to be a(n)_____ bond.
A) likely ionic
B) slightly polar covalent
C) definitely polar covalent
D) slightly ionic
E) nonpolar covalent
A) likely ionic
B) slightly polar covalent
C) definitely polar covalent
D) slightly ionic
E) nonpolar covalent

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
57
Diatomic molecules are linear because _____.
A) they form odd-electron molecules around the central atom
B) electron-deficient molecules cannot be formed of only two electron groups
C) only polar covalent bonds are formed in such molecules
D) electron groups repel to get as far away from each other
E) bonds in such compounds have a polarity of less than 0.4
A) they form odd-electron molecules around the central atom
B) electron-deficient molecules cannot be formed of only two electron groups
C) only polar covalent bonds are formed in such molecules
D) electron groups repel to get as far away from each other
E) bonds in such compounds have a polarity of less than 0.4

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
58
Which of the following compounds is characterized by electron deficient molecules?
A) NaCl
B) NO2
C) NO
D) CO2
E) BeF2
A) NaCl
B) NO2
C) NO
D) CO2
E) BeF2

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
59
What is the energy change when one carbon-carbon double bond is broken to form a carbon-carbon single bond and two H-H single bonds? (Refer to Table 9.2 in the text.)
A) -565 kJ/mol
B) -129 kJ/mol
C) 609 kJ/mol
D) -1,176 kJ/mol
E) 129 kJ/mol
A) -565 kJ/mol
B) -129 kJ/mol
C) 609 kJ/mol
D) -1,176 kJ/mol
E) 129 kJ/mol

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
60
_____ is a scale for judging how much atoms of any element attract electrons.
A) Electron density
B) Reactivity
C) Electron affinity
D) Magnetivity
E) Electronegetavity
A) Electron density
B) Reactivity
C) Electron affinity
D) Magnetivity
E) Electronegetavity

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
61
A molecule has three electron groups on the central atom and has two surrounding atoms. What is the likely shape of the atom?
A) Trigonal pyramidal
B) Bent
C) Trigonal planar
D) Linear
E) Tetrahedral
A) Trigonal pyramidal
B) Bent
C) Trigonal planar
D) Linear
E) Tetrahedral

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
62
What is a single bond? Provide an example.

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
63
What are the factors that determine the strength of ionic bonds?

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
64
Explain why it is difficult to violate the octet rule.

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
65
What are Lewis electron dot diagrams?

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
66
Explain the concept of valence shell electron pair repulsion?

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
67
What are polar covalent bonds and nonpolar covalent bonds?

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
68
Identify the molecule with a tetrahedral molecular structure.
A) NOF
B) CH2O
C) NH3
D) CH4
E) PCl3
A) NOF
B) CH2O
C) NH3
D) CH4
E) PCl3

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
69
How many electron groups can likely be found on the central atom of a molecule with a trigonal planar shape?
A) 1
B) 2
C) 3
D) 4
E) 5
A) 1
B) 2
C) 3
D) 4
E) 5

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
70
What are odd-electron molecules?

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
71
Explain the steps for determining the Lewis electron dot diagram of a simple molecule.

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
72
Which of the following molecules will likely have a bent molecular shape?
A) NOF
B) CH2O
C) CH2Cl2
D) CH4
E) PCl3
A) NOF
B) CH2O
C) CH2Cl2
D) CH4
E) PCl3

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
73
What are electron-deficient molecules and expanded valence shell molecules?

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
74
Draw the Lewis electron diagram of a boron atom. Explain the diagram.

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
75
What is the octet rule? Do atoms always follow the octet rule when forming compounds?

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
76
Draw the electron diagram for an atom with the valence shell electron configuration of ns2np3.

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
77
Which of the following molecules will have a trigonal pyramidal molecular structure?
A) PCl3
B) NO2
C) CH4
D) CaCl2
E) NaCl
A) PCl3
B) NO2
C) CH4
D) CaCl2
E) NaCl

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
78
Explain the concept of electronegativity.

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
79
What is the most likely shape for a molecule with a central atom and only one atom surrounding it?
A) Angular
B) Linear
C) Trigonal planar
D) Trigonal pyramidal
E) Tetrahedral
A) Angular
B) Linear
C) Trigonal planar
D) Trigonal pyramidal
E) Tetrahedral

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
80
What is a covalent bond? What is an ionic bond? Give an example that demonstrates each type of bond.

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck



