Deck 13: Chemical Equilibrium
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/99
Play
Full screen (f)
Deck 13: Chemical Equilibrium
1
The Ka for HCN is 6.20 × 10−10. The concentration of CN- in the ionization of 0.0400 M HCN in H2O is 4.89 × 10−6 M
False
2
Given: 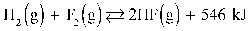 To maximize the reactants in the reaction above, the temperature of the reaction should be decreased.
To maximize the reactants in the reaction above, the temperature of the reaction should be decreased.
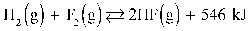 To maximize the reactants in the reaction above, the temperature of the reaction should be decreased.
To maximize the reactants in the reaction above, the temperature of the reaction should be decreased.False
3
Given: 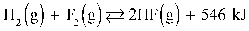 Increasing the temperature in the reaction above shifts the equilibrium toward the reactants.
Increasing the temperature in the reaction above shifts the equilibrium toward the reactants.
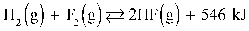 Increasing the temperature in the reaction above shifts the equilibrium toward the reactants.
Increasing the temperature in the reaction above shifts the equilibrium toward the reactants.True
4
Given: 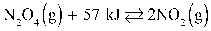 Decreasing the pressure in the reaction above shifts the equilibrium toward the reactants.
Decreasing the pressure in the reaction above shifts the equilibrium toward the reactants.
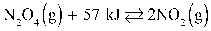 Decreasing the pressure in the reaction above shifts the equilibrium toward the reactants.
Decreasing the pressure in the reaction above shifts the equilibrium toward the reactants.
Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
5
The equilibrium equation that exists between calcium sulfate and water as reactants and calcium hydroxide and sulfuric acid as products can be represented as 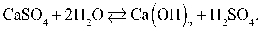
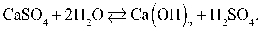

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
6
Equilibrium reactions do not stop; both the forward and reverse reaction continue to occur.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
7
The equilibrium concentration of H2S(g) in the reaction below is 0.17 M. 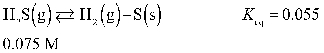

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
8
Given: 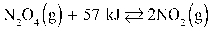 To maximize the amount of N2O4 in the reaction above, the number of moles of NO2 should be increased.
To maximize the amount of N2O4 in the reaction above, the number of moles of NO2 should be increased.
 To maximize the amount of N2O4 in the reaction above, the number of moles of NO2 should be increased.
To maximize the amount of N2O4 in the reaction above, the number of moles of NO2 should be increased.
Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
9
A catalyst does not affect the extent or position of a reaction at equilibrium.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
10
In a chemical equilibrium reaction all the three phases of matter are included. This equation is in a state of homogeneous equilibrium.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
11
Given: ![Given: If [C<sub>2</sub>H<sub>6</sub>], [O<sub>2</sub>], [CO<sub>2</sub>], and K<sub>eq</sub> are 0.451 M, 0.589 M, 0.638 M, and 25.0, respectively, the equilibrium [H<sub>2</sub>O] is 0.326 M.](https://d2lvgg3v3hfg70.cloudfront.net/TB8373/11eb7747_828a_3129_be6a_978f6b0a4505_TB8373_11.jpg) If [C2H6], [O2], [CO2], and Keq are 0.451 M, 0.589 M, 0.638 M, and 25.0, respectively, the equilibrium [H2O] is 0.326 M.
If [C2H6], [O2], [CO2], and Keq are 0.451 M, 0.589 M, 0.638 M, and 25.0, respectively, the equilibrium [H2O] is 0.326 M.
![Given: If [C<sub>2</sub>H<sub>6</sub>], [O<sub>2</sub>], [CO<sub>2</sub>], and K<sub>eq</sub> are 0.451 M, 0.589 M, 0.638 M, and 25.0, respectively, the equilibrium [H<sub>2</sub>O] is 0.326 M.](https://d2lvgg3v3hfg70.cloudfront.net/TB8373/11eb7747_828a_3129_be6a_978f6b0a4505_TB8373_11.jpg) If [C2H6], [O2], [CO2], and Keq are 0.451 M, 0.589 M, 0.638 M, and 25.0, respectively, the equilibrium [H2O] is 0.326 M.
If [C2H6], [O2], [CO2], and Keq are 0.451 M, 0.589 M, 0.638 M, and 25.0, respectively, the equilibrium [H2O] is 0.326 M.
Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
12
Chemical equilibrium is static rather than dynamic.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
13
Given: 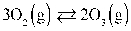 If the Keq at 57.0°C for the reaction above is 13.6, then KP is 0.0474.
If the Keq at 57.0°C for the reaction above is 13.6, then KP is 0.0474.
Assume R = 0.08205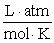
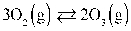 If the Keq at 57.0°C for the reaction above is 13.6, then KP is 0.0474.
If the Keq at 57.0°C for the reaction above is 13.6, then KP is 0.0474.Assume R = 0.08205
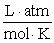

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
14
Given: ![Given: If [C<sub>2</sub>H<sub>6</sub>], [O<sub>2</sub>], [CO<sub>2</sub>], and K<sub>eq</sub> are 0.451 M, 0.589 M, 0.638 M, and 25.0, respectively, the equilibrium [H<sub>2</sub>O] is 0.632 M.](https://d2lvgg3v3hfg70.cloudfront.net/TB8373/11eb7747_828a_0a18_be6a_193e3606f94d_TB8373_11.jpg) If [C2H6], [O2], [CO2], and Keq are 0.451 M, 0.589 M, 0.638 M, and 25.0, respectively, the equilibrium [H2O] is 0.632 M.
If [C2H6], [O2], [CO2], and Keq are 0.451 M, 0.589 M, 0.638 M, and 25.0, respectively, the equilibrium [H2O] is 0.632 M.
![Given: If [C<sub>2</sub>H<sub>6</sub>], [O<sub>2</sub>], [CO<sub>2</sub>], and K<sub>eq</sub> are 0.451 M, 0.589 M, 0.638 M, and 25.0, respectively, the equilibrium [H<sub>2</sub>O] is 0.632 M.](https://d2lvgg3v3hfg70.cloudfront.net/TB8373/11eb7747_828a_0a18_be6a_193e3606f94d_TB8373_11.jpg) If [C2H6], [O2], [CO2], and Keq are 0.451 M, 0.589 M, 0.638 M, and 25.0, respectively, the equilibrium [H2O] is 0.632 M.
If [C2H6], [O2], [CO2], and Keq are 0.451 M, 0.589 M, 0.638 M, and 25.0, respectively, the equilibrium [H2O] is 0.632 M.
Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
15
The Ka for HClO2 is 1.10 × 10−2. The pH of 0.165 M HClO2 in H2O is 1.37.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
16
An equilibrium equation should be balanced.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
17
The equilibrium concentration of H2O(g) for the chemical reaction below is 0.900 M. 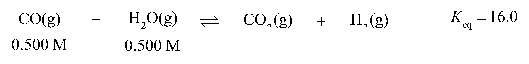
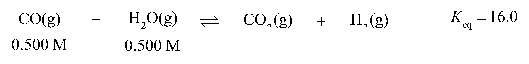

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
18
Given: ![Given: The equilibrium partial pressures of [H<sub>2</sub>O<sub>2</sub>] and [O<sub>2</sub>] are 1.45 atm and 1.89 atm, respectively. If K<sub>P</sub> = 6.00 the equilibrium partial pressure of H<sub>2</sub>O is 0.134 atm.](https://d2lvgg3v3hfg70.cloudfront.net/TB8373/11eb7747_828a_312a_be6a_1bea6212f8ea_TB8373_11.jpg) The equilibrium partial pressures of [H2O2] and [O2] are 1.45 atm and 1.89 atm, respectively. If KP = 6.00 the equilibrium partial pressure of H2O is 0.134 atm.
The equilibrium partial pressures of [H2O2] and [O2] are 1.45 atm and 1.89 atm, respectively. If KP = 6.00 the equilibrium partial pressure of H2O is 0.134 atm.
![Given: The equilibrium partial pressures of [H<sub>2</sub>O<sub>2</sub>] and [O<sub>2</sub>] are 1.45 atm and 1.89 atm, respectively. If K<sub>P</sub> = 6.00 the equilibrium partial pressure of H<sub>2</sub>O is 0.134 atm.](https://d2lvgg3v3hfg70.cloudfront.net/TB8373/11eb7747_828a_312a_be6a_1bea6212f8ea_TB8373_11.jpg) The equilibrium partial pressures of [H2O2] and [O2] are 1.45 atm and 1.89 atm, respectively. If KP = 6.00 the equilibrium partial pressure of H2O is 0.134 atm.
The equilibrium partial pressures of [H2O2] and [O2] are 1.45 atm and 1.89 atm, respectively. If KP = 6.00 the equilibrium partial pressure of H2O is 0.134 atm.
Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
19
For a given reaction 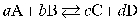 , the equilibrium constant can be defined as
, the equilibrium constant can be defined as 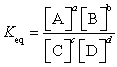 .
.
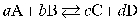 , the equilibrium constant can be defined as
, the equilibrium constant can be defined as 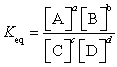 .
.
Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
20
Given: ![Given: If [HCl], [Cl<sub>2</sub>], and K<sub>eq</sub> are 0.586 M, 0.486 M, and 2.37, respectively, the equilibrium [H<sub>2</sub>] is 3.35 M.](https://d2lvgg3v3hfg70.cloudfront.net/TB8373/11eb7747_828a_0a17_be6a_4baa87b2006b_TB8373_11.jpg) If [HCl], [Cl2], and Keq are 0.586 M, 0.486 M, and 2.37, respectively, the equilibrium [H2] is 3.35 M.
If [HCl], [Cl2], and Keq are 0.586 M, 0.486 M, and 2.37, respectively, the equilibrium [H2] is 3.35 M.
![Given: If [HCl], [Cl<sub>2</sub>], and K<sub>eq</sub> are 0.586 M, 0.486 M, and 2.37, respectively, the equilibrium [H<sub>2</sub>] is 3.35 M.](https://d2lvgg3v3hfg70.cloudfront.net/TB8373/11eb7747_828a_0a17_be6a_4baa87b2006b_TB8373_11.jpg) If [HCl], [Cl2], and Keq are 0.586 M, 0.486 M, and 2.37, respectively, the equilibrium [H2] is 3.35 M.
If [HCl], [Cl2], and Keq are 0.586 M, 0.486 M, and 2.37, respectively, the equilibrium [H2] is 3.35 M.
Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
21
What is the expression for  for this reaction?
for this reaction?
A)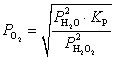
B)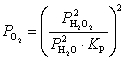
C)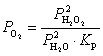
D)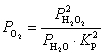
E)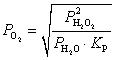
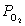 for this reaction?
for this reaction?A)
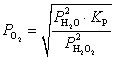
B)
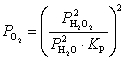
C)
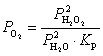
D)
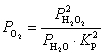
E)
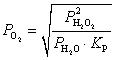

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
22
If the equilibrium partial pressures of [PCl5], [PCl3], and [Cl2] are 0.240 atm, 0.291 atm, and 0.655 atm, respectively, determine KP.
A) 3.14
B) 1.43
C) 1.26
D) 4.31
E) 4.13
Refer to the reaction below for 38-39.
Given:![<strong>If the equilibrium partial pressures of [PCl<sub>5</sub>], [PCl<sub>3</sub>], and [Cl<sub>2</sub>] are 0.240 atm, 0.291 atm, and 0.655 atm, respectively, determine K<sub>P</sub>.</strong> A) 3.14 B) 1.43 C) 1.26 D) 4.31 E) 4.13 Refer to the reaction below for 38-39. Given:](https://d2lvgg3v3hfg70.cloudfront.net/TB8373/11eb7747_828b_b7fc_be6a_65e37de827b6_TB8373_11.jpg)
A) 3.14
B) 1.43
C) 1.26
D) 4.31
E) 4.13
Refer to the reaction below for 38-39.
Given:
![<strong>If the equilibrium partial pressures of [PCl<sub>5</sub>], [PCl<sub>3</sub>], and [Cl<sub>2</sub>] are 0.240 atm, 0.291 atm, and 0.655 atm, respectively, determine K<sub>P</sub>.</strong> A) 3.14 B) 1.43 C) 1.26 D) 4.31 E) 4.13 Refer to the reaction below for 38-39. Given:](https://d2lvgg3v3hfg70.cloudfront.net/TB8373/11eb7747_828b_b7fc_be6a_65e37de827b6_TB8373_11.jpg)

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
23
If [C2H2], [O2], [H2O], and [CO2] are 0.295 M, 0.400 M, 0.500 M, and 0.686 M, respectively, determine Keq.
A) 84.4
B) 48.4
C) 62.3
D) 42.2
E) 22.4
Refer to the reaction below for 34-35.
Given:![<strong>If [C<sub>2</sub>H<sub>2</sub>], [O<sub>2</sub>], [H<sub>2</sub>O], and [CO<sub>2</sub>] are 0.295 M, 0.400 M, 0.500 M, and 0.686 M, respectively, determine K<sub>eq</sub>.</strong> A) 84.4 B) 48.4 C) 62.3 D) 42.2 E) 22.4 Refer to the reaction below for 34-35. Given:](https://d2lvgg3v3hfg70.cloudfront.net/TB8373/11eb7747_828b_69d0_be6a_8f1e6e73322a_TB8373_11.jpg)
A) 84.4
B) 48.4
C) 62.3
D) 42.2
E) 22.4
Refer to the reaction below for 34-35.
Given:
![<strong>If [C<sub>2</sub>H<sub>2</sub>], [O<sub>2</sub>], [H<sub>2</sub>O], and [CO<sub>2</sub>] are 0.295 M, 0.400 M, 0.500 M, and 0.686 M, respectively, determine K<sub>eq</sub>.</strong> A) 84.4 B) 48.4 C) 62.3 D) 42.2 E) 22.4 Refer to the reaction below for 34-35. Given:](https://d2lvgg3v3hfg70.cloudfront.net/TB8373/11eb7747_828b_69d0_be6a_8f1e6e73322a_TB8373_11.jpg)

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
24
What is the Keq expression for the reaction?
A)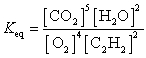
B)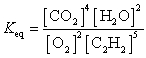
C)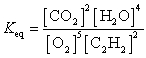
D)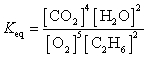
E)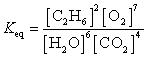
A)
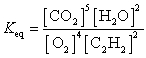
B)
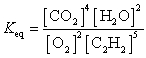
C)
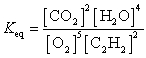
D)
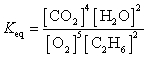
E)
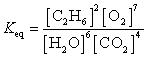

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
25
What is the Keq expression for the reaction?
A)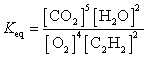
B)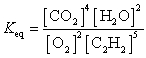
C)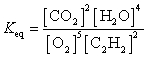
D)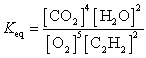
E)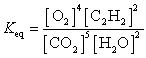
A)
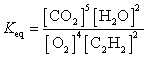
B)
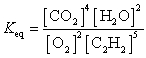
C)
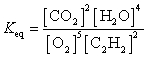
D)
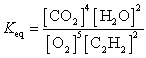
E)
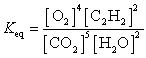

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
26
What is the Keq expression for the reaction?
A)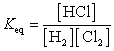
B)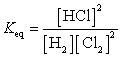
C)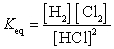
D)
E)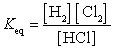
A)
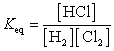
B)
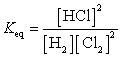
C)
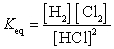
D)

E)
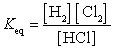

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
27
What is the KP at 37.0°C for this reaction if the Keq is 1.20 × 10−2? 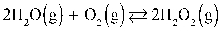 Assume R = 0.08205
Assume R = 0.08205 
A) 4.27 * 10- 4
B) 2.47 *10- 4
C) 2.74 * 10- 4
D) 7.42 * 10- 4
E) 4.72 *10- 4
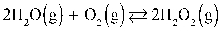 Assume R = 0.08205
Assume R = 0.08205 
A) 4.27 * 10- 4
B) 2.47 *10- 4
C) 2.74 * 10- 4
D) 7.42 * 10- 4
E) 4.72 *10- 4

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
28
What is the Keq expression for the reaction?
A)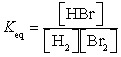
B)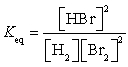
C)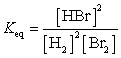
D)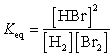
E)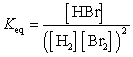
A)
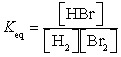
B)
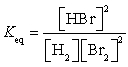
C)
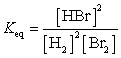
D)
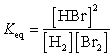
E)
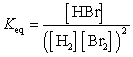

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
29
If [H2], [Br2], and [HBr] are 0.0350 M, 0.0600 M, and 0.0850 M, respectively, determine Keq.
A) 6.00
B) 4.50
C) 3.44
D) 1.80
E) 404
Refer to the reaction below for 30-31.
Given:![<strong>If [H<sub>2</sub>], [Br<sub>2</sub>], and [HBr] are 0.0350 M, 0.0600 M, and 0.0850 M, respectively, determine K<sub>eq</sub>.</strong> A) 6.00 B) 4.50 C) 3.44 D) 1.80 E) 404 Refer to the reaction below for 30-31. Given:](https://d2lvgg3v3hfg70.cloudfront.net/TB8373/11eb7747_828b_1ba4_be6a_cd88a90e396d_TB8373_11.jpg)
A) 6.00
B) 4.50
C) 3.44
D) 1.80
E) 404
Refer to the reaction below for 30-31.
Given:
![<strong>If [H<sub>2</sub>], [Br<sub>2</sub>], and [HBr] are 0.0350 M, 0.0600 M, and 0.0850 M, respectively, determine K<sub>eq</sub>.</strong> A) 6.00 B) 4.50 C) 3.44 D) 1.80 E) 404 Refer to the reaction below for 30-31. Given:](https://d2lvgg3v3hfg70.cloudfront.net/TB8373/11eb7747_828b_1ba4_be6a_cd88a90e396d_TB8373_11.jpg)

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
30
The equilibrium partial pressures of [H2O2] and [O2] are 0.239 atm and 0.281 atm, respectively. If KP = 5.00, determine the equilibrium partial pressure of [H2O].
A) 2.20 atm
B) 0.220 atm
C) 0.0022 atm
D) 0.022 atm
E) 0.202 atm
A) 2.20 atm
B) 0.220 atm
C) 0.0022 atm
D) 0.022 atm
E) 0.202 atm

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
31
If [C2H6], [O2], [CO2], and Keq are 0.391 M, 0.486 M, 0.400 M, and 25.0, respectively, determine the equilibrium [H2O].
A) 0.993 M
B) 0.339 M
C) 0.393 M
D) 0.933 M
E) 0.399 M
Refer to the reaction below for 36-37.
Given:![<strong>If [C<sub>2</sub>H<sub>6</sub>], [O<sub>2</sub>], [CO<sub>2</sub>], and K<sub>eq</sub> are 0.391 M, 0.486 M, 0.400 M, and 25.0, respectively, determine the equilibrium [H<sub>2</sub>O].</strong> A) 0.993 M B) 0.339 M C) 0.393 M D) 0.933 M E) 0.399 M Refer to the reaction below for 36-37. Given:](https://d2lvgg3v3hfg70.cloudfront.net/TB8373/11eb7747_828b_90e6_be6a_a55990b91664_TB8373_11.jpg)
A) 0.993 M
B) 0.339 M
C) 0.393 M
D) 0.933 M
E) 0.399 M
Refer to the reaction below for 36-37.
Given:
![<strong>If [C<sub>2</sub>H<sub>6</sub>], [O<sub>2</sub>], [CO<sub>2</sub>], and K<sub>eq</sub> are 0.391 M, 0.486 M, 0.400 M, and 25.0, respectively, determine the equilibrium [H<sub>2</sub>O].</strong> A) 0.993 M B) 0.339 M C) 0.393 M D) 0.933 M E) 0.399 M Refer to the reaction below for 36-37. Given:](https://d2lvgg3v3hfg70.cloudfront.net/TB8373/11eb7747_828b_90e6_be6a_a55990b91664_TB8373_11.jpg)

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
32
If the Kb for CN− is 1.6 × 10−5, Ka for HCN is 6.3 × 10−10.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
33
If [OH−] is 2.02 × 10−7 M, [H+] in a solution is 4.59 × 10−8.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
34
If [H2], [Cl2], and Keq are 0.315 M, 0.218 M, and 3.85, respectively, determine the equilibrium [HCl].
A) 0.240 M
B) 0.514 M
C) 0.0354 M
D) 0.134 M
E) 0.0530 M
Refer to the reaction below for 32-33.
Given:![<strong>If [H<sub>2</sub>], [Cl<sub>2</sub>], and K<sub>eq</sub> are 0.315 M, 0.218 M, and 3.85, respectively, determine the equilibrium [HCl].</strong> A) 0.240 M B) 0.514 M C) 0.0354 M D) 0.134 M E) 0.0530 M Refer to the reaction below for 32-33. Given:](https://d2lvgg3v3hfg70.cloudfront.net/TB8373/11eb7747_828b_42ba_be6a_854c649d4e76_TB8373_11.jpg)
A) 0.240 M
B) 0.514 M
C) 0.0354 M
D) 0.134 M
E) 0.0530 M
Refer to the reaction below for 32-33.
Given:
![<strong>If [H<sub>2</sub>], [Cl<sub>2</sub>], and K<sub>eq</sub> are 0.315 M, 0.218 M, and 3.85, respectively, determine the equilibrium [HCl].</strong> A) 0.240 M B) 0.514 M C) 0.0354 M D) 0.134 M E) 0.0530 M Refer to the reaction below for 32-33. Given:](https://d2lvgg3v3hfg70.cloudfront.net/TB8373/11eb7747_828b_42ba_be6a_854c649d4e76_TB8373_11.jpg)

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
35
Write the equilibrium equation that exists between calcium sulfate as a reactant and calcium oxide and sulfur trioxide as products.
A)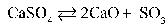
B)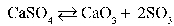
C)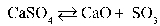
D)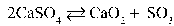
E)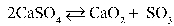
Refer to the reaction below for 28-29.
Given: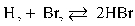
A)
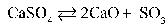
B)
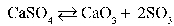
C)
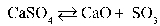
D)
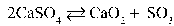
E)
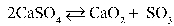
Refer to the reaction below for 28-29.
Given:
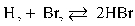

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
36
Write the equilibrium equation that exists between hydrogen and chlorine as reactants and hydrogen chloride as a product.
A)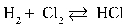
B)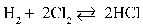
C)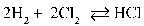
D)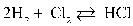
E)
A)
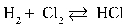
B)
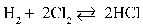
C)
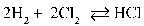
D)
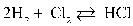
E)


Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
37
What is the KP expression for the reaction?
A)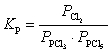
B)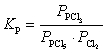
C)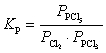
D)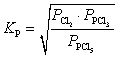
E)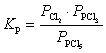
A)
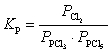
B)
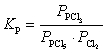
C)
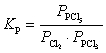
D)
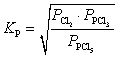
E)
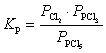

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
38
If the Ksp of SrSO4(s) is 3.8 × 10−4, the concentration of [SO42-] in a saturated solution of SrSO4(s) is 9.1 × 10−12 M.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
39
What is the KP at 47.0°C for this reaction if the Keq is 2.36? 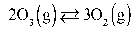 Assume R = 0.08205
Assume R = 0.08205 
A) 31.2
B) 9.88
C) 0.0988
D) 62.0
E) 0.0889
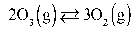 Assume R = 0.08205
Assume R = 0.08205 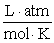
A) 31.2
B) 9.88
C) 0.0988
D) 62.0
E) 0.0889

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
40
The expression for the slight solubility of Al2(SO4)3(s) is 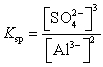 .
.
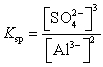 .
.
Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
41
What is the Keq at 7.00°C for this reaction if the KP is 0.00123? 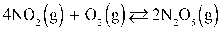 Assume R = 0.08205
Assume R = 0.08205 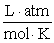
A) 19.4
B) 14.9
C) 49.1
D) 1.94
E) 1.49
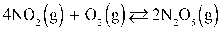 Assume R = 0.08205
Assume R = 0.08205 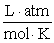
A) 19.4
B) 14.9
C) 49.1
D) 1.94
E) 1.49

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
42
Which of the following will cause the equilibrium to shift toward the products side?
A) Removal of CO
B) Increasing the pressure
C) Addition of CO2
D) Removal of the catalyst
E) Increasing the temperature
A) Removal of CO
B) Increasing the pressure
C) Addition of CO2
D) Removal of the catalyst
E) Increasing the temperature

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
43
Which of the following will cause the equilibrium to shift toward the products?
A) Addition of N2
B) Addition of H2
C) Removal of NH3
D) Increasing the pressure
E) Increasing the temperature
A) Addition of N2
B) Addition of H2
C) Removal of NH3
D) Increasing the pressure
E) Increasing the temperature

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
44
Which of the following is the right ICE chart for the reaction below? 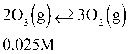
A)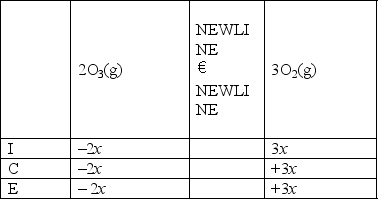
B)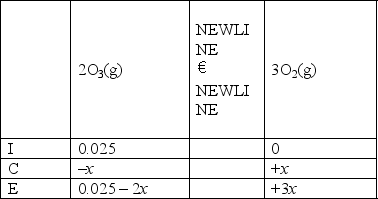
C)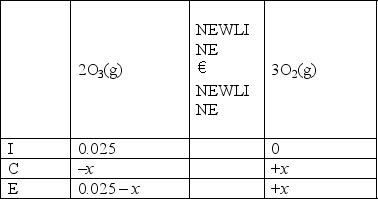
D)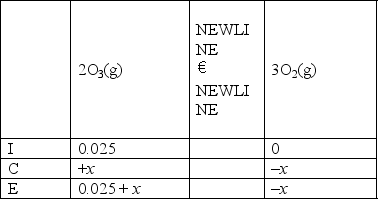
E)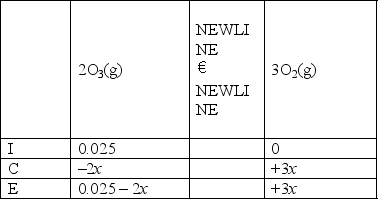
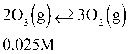
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
45
If concentration is defined as x, formulate Keq for the reaction given below. 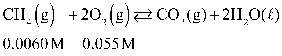
A)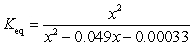
B)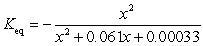
C)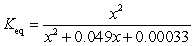
D)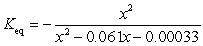
E)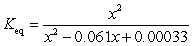
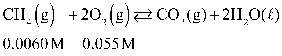
A)
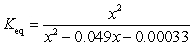
B)
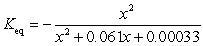
C)
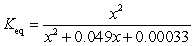
D)
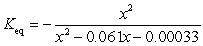
E)
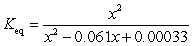

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
46
What is the correct expression for the concentration of [C2H2I4] in this reaction? ![<strong>What is the correct expression for the concentration of [C<sub>2</sub>H<sub>2</sub>I<sub>4</sub>] in this reaction? </strong> A) B) C) D) E)](https://d2lvgg3v3hfg70.cloudfront.net/TB8373/11eb7747_828c_544f_be6a_2b33a6196ac6_TB8373_11.jpg)
A)![<strong>What is the correct expression for the concentration of [C<sub>2</sub>H<sub>2</sub>I<sub>4</sub>] in this reaction? </strong> A) B) C) D) E)](https://d2lvgg3v3hfg70.cloudfront.net/TB8373/11eb7747_828c_5450_be6a_a9cabc4d76ee_TB8373_11.jpg)
B)![<strong>What is the correct expression for the concentration of [C<sub>2</sub>H<sub>2</sub>I<sub>4</sub>] in this reaction? </strong> A) B) C) D) E)](https://d2lvgg3v3hfg70.cloudfront.net/TB8373/11eb7747_828c_7b61_be6a_392eebbbc336_TB8373_11.jpg)
C)![<strong>What is the correct expression for the concentration of [C<sub>2</sub>H<sub>2</sub>I<sub>4</sub>] in this reaction? </strong> A) B) C) D) E)](https://d2lvgg3v3hfg70.cloudfront.net/TB8373/11eb7747_828c_7b62_be6a_951f4a9a5d43_TB8373_11.jpg)
D)![<strong>What is the correct expression for the concentration of [C<sub>2</sub>H<sub>2</sub>I<sub>4</sub>] in this reaction? </strong> A) B) C) D) E)](https://d2lvgg3v3hfg70.cloudfront.net/TB8373/11eb7747_828c_7b63_be6a_0bf8d3b44c71_TB8373_11.jpg)
E)![<strong>What is the correct expression for the concentration of [C<sub>2</sub>H<sub>2</sub>I<sub>4</sub>] in this reaction? </strong> A) B) C) D) E)](https://d2lvgg3v3hfg70.cloudfront.net/TB8373/11eb7747_828c_7b64_be6a_2dfddc324ff4_TB8373_11.jpg)
![<strong>What is the correct expression for the concentration of [C<sub>2</sub>H<sub>2</sub>I<sub>4</sub>] in this reaction? </strong> A) B) C) D) E)](https://d2lvgg3v3hfg70.cloudfront.net/TB8373/11eb7747_828c_544f_be6a_2b33a6196ac6_TB8373_11.jpg)
A)
![<strong>What is the correct expression for the concentration of [C<sub>2</sub>H<sub>2</sub>I<sub>4</sub>] in this reaction? </strong> A) B) C) D) E)](https://d2lvgg3v3hfg70.cloudfront.net/TB8373/11eb7747_828c_5450_be6a_a9cabc4d76ee_TB8373_11.jpg)
B)
![<strong>What is the correct expression for the concentration of [C<sub>2</sub>H<sub>2</sub>I<sub>4</sub>] in this reaction? </strong> A) B) C) D) E)](https://d2lvgg3v3hfg70.cloudfront.net/TB8373/11eb7747_828c_7b61_be6a_392eebbbc336_TB8373_11.jpg)
C)
![<strong>What is the correct expression for the concentration of [C<sub>2</sub>H<sub>2</sub>I<sub>4</sub>] in this reaction? </strong> A) B) C) D) E)](https://d2lvgg3v3hfg70.cloudfront.net/TB8373/11eb7747_828c_7b62_be6a_951f4a9a5d43_TB8373_11.jpg)
D)
![<strong>What is the correct expression for the concentration of [C<sub>2</sub>H<sub>2</sub>I<sub>4</sub>] in this reaction? </strong> A) B) C) D) E)](https://d2lvgg3v3hfg70.cloudfront.net/TB8373/11eb7747_828c_7b63_be6a_0bf8d3b44c71_TB8373_11.jpg)
E)
![<strong>What is the correct expression for the concentration of [C<sub>2</sub>H<sub>2</sub>I<sub>4</sub>] in this reaction? </strong> A) B) C) D) E)](https://d2lvgg3v3hfg70.cloudfront.net/TB8373/11eb7747_828c_7b64_be6a_2dfddc324ff4_TB8373_11.jpg)

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
47
Determine the equilibrium concentration of HCN(g) for this reaction. 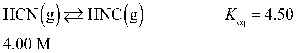
A) 3.27 M
B) 2.37 M
C) 0.372 M
D) 0.370 M
E) 0.730 M
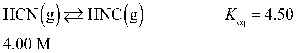
A) 3.27 M
B) 2.37 M
C) 0.372 M
D) 0.370 M
E) 0.730 M

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
48
To maximize the amount of CO the:
A) pressure of the reaction should be reduced.
B) temperature of the reaction should be reduced.
C) number of moles of CO2 in the reaction should be reduced.
D) amount of catalyst added should be increased.
E) number of moles of CO in the reaction should be increased.
A) pressure of the reaction should be reduced.
B) temperature of the reaction should be reduced.
C) number of moles of CO2 in the reaction should be reduced.
D) amount of catalyst added should be increased.
E) number of moles of CO in the reaction should be increased.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
49
If concentration is defined as x, formulate Keq for the reaction given below. 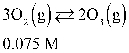
A)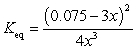
B)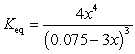
C)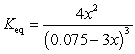
D)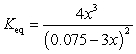
E)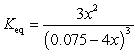
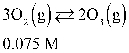
A)
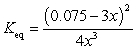
B)
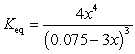
C)
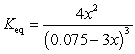
D)
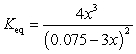
E)
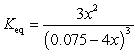

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
50
Determine the equilibrium concentration of H2(g) for this reaction. 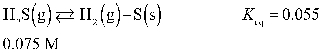
A) 0.039 M
B) 0.0039 M
C) 0.39 M
D) 0.093 M
E) 0.0093 M
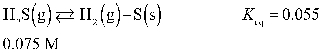
A) 0.039 M
B) 0.0039 M
C) 0.39 M
D) 0.093 M
E) 0.0093 M

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
51
Which of the following will cause the equilibrium to shift toward the reactants side?
A) Addition of CO
B) Removal of CO2
C) Removal of the catalyst
D) Increasing the temperature
E) Increasing the pressure
A) Addition of CO
B) Removal of CO2
C) Removal of the catalyst
D) Increasing the temperature
E) Increasing the pressure

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
52
To maximize the amount of N2 the:
A) pressure of the reaction should be reduced.
B) temperature of the reaction should be decreased.
C) amount of catalyst added should be increased.
D) number of moles of N2 in the reaction should be increased.
E) number of moles of NH3 in the reaction should be reduced.
Refer to the reaction below for 50-53.
Given: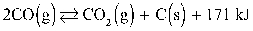
A) pressure of the reaction should be reduced.
B) temperature of the reaction should be decreased.
C) amount of catalyst added should be increased.
D) number of moles of N2 in the reaction should be increased.
E) number of moles of NH3 in the reaction should be reduced.
Refer to the reaction below for 50-53.
Given:
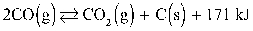

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
53
To maximize the amount of NH3 the:
A) pressure of the reaction should be reduced.
B) temperature of the reaction should be reduced.
C) number of moles of H2 in the reaction should be reduced.
D) number of moles of N2 in the reaction should be reduced.
E) number of moles of NH3 in the reaction should be increased.
A) pressure of the reaction should be reduced.
B) temperature of the reaction should be reduced.
C) number of moles of H2 in the reaction should be reduced.
D) number of moles of N2 in the reaction should be reduced.
E) number of moles of NH3 in the reaction should be increased.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
54
Determine the equilibrium concentration of CO(g) for this reaction. 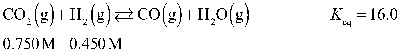
A) 0.836 M
B) 0.683 M
C) 0.386 M
D) 0.863 M
E) 0.368 M
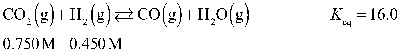
A) 0.836 M
B) 0.683 M
C) 0.386 M
D) 0.863 M
E) 0.368 M

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
55
To maximize the amount of CO2 the:
A) pressure of the reaction should be reduced.
B) temperature of the reaction should be increased.
C) number of moles of CO2 in the reaction should be increased.
D) amount of catalyst added should be reduced.
E) number of moles of CO in the reaction should be increased.
A) pressure of the reaction should be reduced.
B) temperature of the reaction should be increased.
C) number of moles of CO2 in the reaction should be increased.
D) amount of catalyst added should be reduced.
E) number of moles of CO in the reaction should be increased.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
56
Determine the equilibrium concentration of N2O3(g) for this reaction. 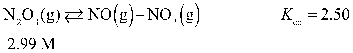
A) 1.76 M
B) 1.23 M
C) 5.61 M
D) 1.65 M
E) 6.51 M
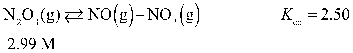
A) 1.76 M
B) 1.23 M
C) 5.61 M
D) 1.65 M
E) 6.51 M

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
57
What is the correct Keq expression for this reaction? 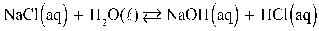
A)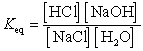
B)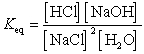
C)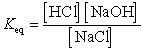
D)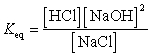
E)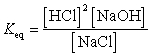
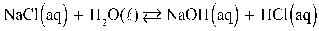
A)
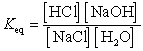
B)
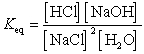
C)
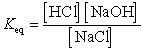
D)
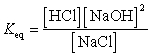
E)
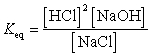

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
58
Determine the equilibrium concentration of Cl2(g) for this reaction. 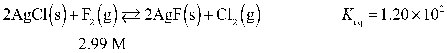
A) 0.729 M
B) 9.27 M
C) 2.97 M
D) 9.72 M
E) 0.297 M
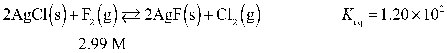
A) 0.729 M
B) 9.27 M
C) 2.97 M
D) 9.72 M
E) 0.297 M

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
59
What is the correct KP expression for this reaction? 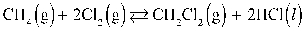
A)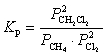
B)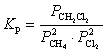
C)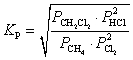
D)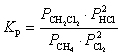
E)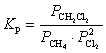
Refer to the reaction below for 46-49.
Given: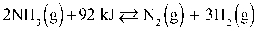
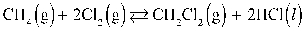
A)
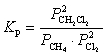
B)
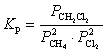
C)
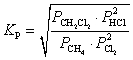
D)
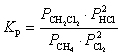
E)
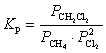
Refer to the reaction below for 46-49.
Given:
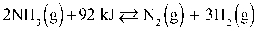

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
60
Which of the following will cause the equilibrium to shift toward the reactants?
A) Removal of N2
B) Removal of H2
C) Removal of NH3
D) Reducing the pressure
E) Increasing the temperature
A) Removal of N2
B) Removal of H2
C) Removal of NH3
D) Reducing the pressure
E) Increasing the temperature

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
61
What is the concentration of [Ag+] in a saturated solution of AgI? The Ksp of AgI is 9.50 × 10−17.
A) 9.75 × 10−9 M
B) 2.92 × 10−10 M
C) 6.20 × 10−10 M
D) 2.60 × 10−9 M
E) 2.29 × 10−9 M
A) 9.75 × 10−9 M
B) 2.92 × 10−10 M
C) 6.20 × 10−10 M
D) 2.60 × 10−9 M
E) 2.29 × 10−9 M

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
62
Which of the following is the equilibrium equation for HF acting as a weak acid?
A)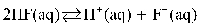
B)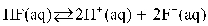
C)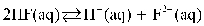
D)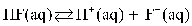
E)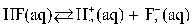
A)
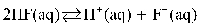
B)
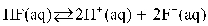
C)
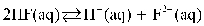
D)
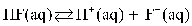
E)
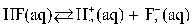

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
63
What is the pOH of a 0.500 M solution of HClO2? The Ka of HClO2 is 1.10 × 10−2.
A) 1.13
B) 12.9
C) 3.11
D) 11.3
E) 31.1
A) 1.13
B) 12.9
C) 3.11
D) 11.3
E) 31.1

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
64
Describe chemical equilibrium as a dynamic process.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
65
What is the effect of temperature changes on an equilibrium?

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
66
Explain the law of mass action and equilibrium constant.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
67
How does the addition or removal of a product or a reactant affect the equilibrium of a reaction?

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
68
What is the concentration of [Ag+] in a saturated solution of Ag2SO4? The Ksp of Ag2SO4 is 1.50 × 10−5.
A) 0.0155 M
B) 0.0310 M
C) 0.0465 M
D) 0.0620 M
E) 0.0775 M
A) 0.0155 M
B) 0.0310 M
C) 0.0465 M
D) 0.0620 M
E) 0.0775 M

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
69
What is the concentration of [PO43-] in a saturated solution of Ca3(PO4)2? The Ksp of Ca3(PO4)2 is 2.1 × 10−33.
A) 3.28 × 10−6 M
B) 1.64 × 10−6 M
C) 2.82 × 10−7 M
D) 2.28 × 10−7 M
E) 8.22 × 10−7 M
A) 3.28 × 10−6 M
B) 1.64 × 10−6 M
C) 2.82 × 10−7 M
D) 2.28 × 10−7 M
E) 8.22 × 10−7 M

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
70
How do we define the equilibrium constant in terms of partial pressures?

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
71
What is the Ksp expression for Mg3(PO4)2?
A)
B)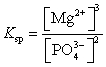
C)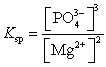
D)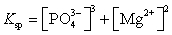
E)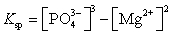
A)

B)
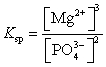
C)
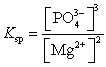
D)
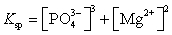
E)
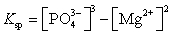

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
72
How does an equilibrium react to a change in pressure?

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
73
Define Le Chatelier's principle. If a reaction is exothermic, if the temperature is increased, what which way will the reaction shift?

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
74
Explain the relationship between Keq and KP.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
75
What is the value of Kb for CN-, which can accept a proton and act as a base? The Ka for HCN is 6.20 × 10−10.
A) 6.20 × 10−10
B) 2.60 × 10−5
C) 1.16 × 10−5
D) 6.11 × 10−5
E) 1.61 × 10−5
A) 6.20 × 10−10
B) 2.60 × 10−5
C) 1.16 × 10−5
D) 6.11 × 10−5
E) 1.61 × 10−5

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
76
Explain heterogeneous equilibrium with an example. What is the rule for heterogeneous equilibria?

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
77
Define chemical equilibrium with an example.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
78
What is the pH of a 0.600 M solution of H2PO4−? The Ka of H2PO4− is 6.2 × 10−8.
A) 3.71
B) 10.3
C) 3.10
D) 30.1
E) 17.7
A) 3.71
B) 10.3
C) 3.10
D) 30.1
E) 17.7

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
79
Which of the following is the Ka expression for HSO4- acting as a weak acid?
A)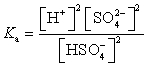
B)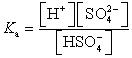
C)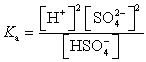
D)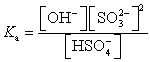
E)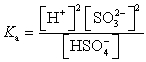
A)
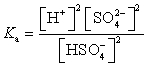
B)
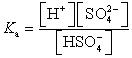
C)
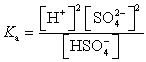
D)
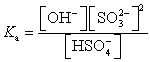
E)
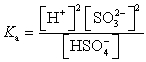

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
80
Explain the importance of the equilibrium constant.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck



