Deck 8: Alcohols, Phenols, Ethers, and Their Sulfur Analogs
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/36
Play
Full screen (f)
Deck 8: Alcohols, Phenols, Ethers, and Their Sulfur Analogs
1
Instructions: Draw structures corresponding to each of the following IUPAC names.
Draw:
Dicyclohexyl sulfide
Draw:
Dicyclohexyl sulfide
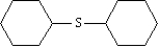
2
Instructions: Provide correct IUPAC names for each of the structures below.
Name: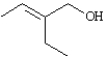
Name:

(E)-2-ethyl-but-2-en-1-ol
3
Instructions: Draw structures corresponding to each of the following IUPAC names.
Draw:
cyclopropyl ethyl sulfide
Draw:
cyclopropyl ethyl sulfide
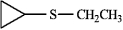
4
Instructions: Draw structures corresponding to each of the following IUPAC names.
Draw:
2-phenylpropan-2-ol
Draw:
2-phenylpropan-2-ol

Unlock Deck
Unlock for access to all 36 flashcards in this deck.
Unlock Deck
k this deck
5
Which of the following would not react with either Na2Cr2O7 or periodinane? 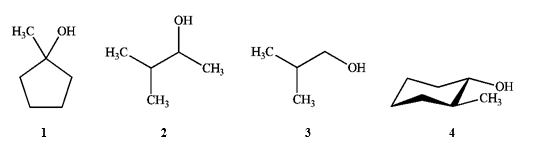
A) only 1
B) only 3
C) only 2 and 4
D) 1, 2, 3 and 4

A) only 1
B) only 3
C) only 2 and 4
D) 1, 2, 3 and 4

Unlock Deck
Unlock for access to all 36 flashcards in this deck.
Unlock Deck
k this deck
6
Instructions: Consider the reaction below to answer the following question. 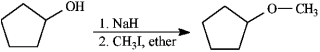
Refer to instructions. Mechanistically, the Williamson ether synthesis outlined above is:
A) an E1 process
B) an SN1 process
C) an E2 process
D) an SN2 process

Refer to instructions. Mechanistically, the Williamson ether synthesis outlined above is:
A) an E1 process
B) an SN1 process
C) an E2 process
D) an SN2 process

Unlock Deck
Unlock for access to all 36 flashcards in this deck.
Unlock Deck
k this deck
7
Instructions: Consider the Grignard reaction below to answer the following question(s). 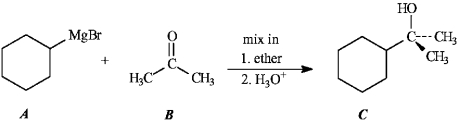
Refer to instructions. The nucleophile in this reaction is indicated by letter _____.

Refer to instructions. The nucleophile in this reaction is indicated by letter _____.

Unlock Deck
Unlock for access to all 36 flashcards in this deck.
Unlock Deck
k this deck
8
Instructions: Consider the Grignard reaction below to answer the following question(s). 
Refer to instructions. The electrophile in this reaction is indicated by letter _____.

Refer to instructions. The electrophile in this reaction is indicated by letter _____.

Unlock Deck
Unlock for access to all 36 flashcards in this deck.
Unlock Deck
k this deck
9
Instructions: To answer the following question(s), consider the reaction below. 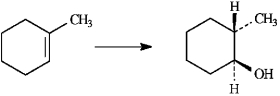
Refer to instructions. On the templates provided below, draw both conformations of the alcohol product. Circle the least stable conformation.

Refer to instructions. On the templates provided below, draw both conformations of the alcohol product. Circle the least stable conformation.


Unlock Deck
Unlock for access to all 36 flashcards in this deck.
Unlock Deck
k this deck
10
Instructions: To answer the following question(s), consider the reaction below. 
Refer to instructions. Provide the IUPAC name for the product alcohol.

Refer to instructions. Provide the IUPAC name for the product alcohol.

Unlock Deck
Unlock for access to all 36 flashcards in this deck.
Unlock Deck
k this deck
11
Instructions: Refer to the data below to answer the following question(s).
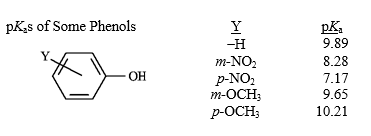
Refer to instructions.
a)The weakest acid in the table is _____.
b)Which of the acids in the table has the weakest conjugate base?

Refer to instructions.
a)The weakest acid in the table is _____.
b)Which of the acids in the table has the weakest conjugate base?

Unlock Deck
Unlock for access to all 36 flashcards in this deck.
Unlock Deck
k this deck
12
Instructions: Draw structures corresponding to each of the following IUPAC names.
Draw:
3-methylbut-2-en-1-ol
Draw:
3-methylbut-2-en-1-ol

Unlock Deck
Unlock for access to all 36 flashcards in this deck.
Unlock Deck
k this deck
13
Instructions: Consider the Grignard reaction below to answer the following question(s). 
Refer to instructions. If a secondary alcohol were desired as a product of the reaction, B should be replaced with
A) an ester
B) an aldehyde
C) formaldehyde (methanal)
D) a primary alcohol

Refer to instructions. If a secondary alcohol were desired as a product of the reaction, B should be replaced with
A) an ester
B) an aldehyde
C) formaldehyde (methanal)
D) a primary alcohol

Unlock Deck
Unlock for access to all 36 flashcards in this deck.
Unlock Deck
k this deck
14
A useful and general method for the synthesis of alcohols is the addition of Grignard reagents to carbonyl compounds. Show what Grignard reagent and what carbonyl compound you would start with to prepare each alcohol below. List all possibilities. 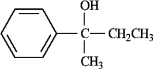


Unlock Deck
Unlock for access to all 36 flashcards in this deck.
Unlock Deck
k this deck
15
Instructions: Draw structures corresponding to each of the following IUPAC names.
Draw:
3-methylbutane-1-thiol
Draw:
3-methylbutane-1-thiol

Unlock Deck
Unlock for access to all 36 flashcards in this deck.
Unlock Deck
k this deck
16
Instructions: Provide correct IUPAC names for each of the structures below.
Name: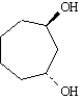
Name:


Unlock Deck
Unlock for access to all 36 flashcards in this deck.
Unlock Deck
k this deck
17
Instructions: To answer the following question(s), consider the reaction below. 
-Refer to instructions. The alcohol product is classified as a:
A) 1 alcohol
B) 2 alcohol
C) 3 alcohol
D) 4 alcohol

-Refer to instructions. The alcohol product is classified as a:
A) 1 alcohol
B) 2 alcohol
C) 3 alcohol
D) 4 alcohol

Unlock Deck
Unlock for access to all 36 flashcards in this deck.
Unlock Deck
k this deck
18
Instructions: Consider the reaction below to answer the following question. 
Refer to instructions. On the structures provided below draw arrows showing the mechanism of step two of this reaction.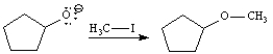

Refer to instructions. On the structures provided below draw arrows showing the mechanism of step two of this reaction.
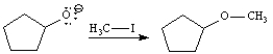

Unlock Deck
Unlock for access to all 36 flashcards in this deck.
Unlock Deck
k this deck
19
Instructions: Provide correct IUPAC names for each of the structures below.
Name: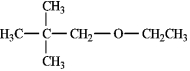
Name:
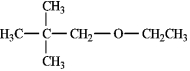

Unlock Deck
Unlock for access to all 36 flashcards in this deck.
Unlock Deck
k this deck
20
Instructions: Draw structures corresponding to each of the following IUPAC names.
Draw:
2, 4, 6-trinitrophenol
Draw:
2, 4, 6-trinitrophenol

Unlock Deck
Unlock for access to all 36 flashcards in this deck.
Unlock Deck
k this deck
21
Instructions:
Give the major organic product(s) of the following reactions or sequences of reactions. Show all relevant stereochemistry.
Write the product(s):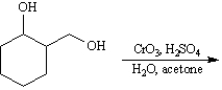
Give the major organic product(s) of the following reactions or sequences of reactions. Show all relevant stereochemistry.
Write the product(s):


Unlock Deck
Unlock for access to all 36 flashcards in this deck.
Unlock Deck
k this deck
22
Choose the best reagent(s) for carrying out the following conversions from the list provided below. Place the letter of the best choice in the blank. Reagents may be used more than once.
Refer to instructions. _____
A)CrO3, H2SO4, H2O
B)1. NaBH4, ethanol
2. H3O+
C)1. LiAl4, ether
2. H3O+
D)periodinane
Refer to instructions. _____

A)CrO3, H2SO4, H2O
B)1. NaBH4, ethanol
2. H3O+
C)1. LiAl4, ether
2. H3O+
D)periodinane

Unlock Deck
Unlock for access to all 36 flashcards in this deck.
Unlock Deck
k this deck
23
Choose the best reagent for carrying out the following reactions from the list below. Place the letter of the reagent(s) in the box over the reaction arrow. Only one letter per box. 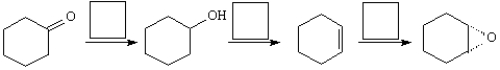
A)m-ClC6H4CO3H
B)H2/Pd
C)warm H2SO4/H2O
D)PCC, CH2Cl2
E)LiAlH4 in ether, then H3O+
F)NaOH, heat

A)m-ClC6H4CO3H
B)H2/Pd
C)warm H2SO4/H2O
D)PCC, CH2Cl2
E)LiAlH4 in ether, then H3O+
F)NaOH, heat

Unlock Deck
Unlock for access to all 36 flashcards in this deck.
Unlock Deck
k this deck
24
Instructions:
Give the major organic product(s) of the following reactions or sequences of reactions. Show all relevant stereochemistry.
Write the product(s):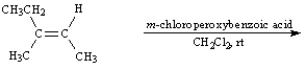
Give the major organic product(s) of the following reactions or sequences of reactions. Show all relevant stereochemistry.
Write the product(s):
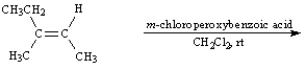

Unlock Deck
Unlock for access to all 36 flashcards in this deck.
Unlock Deck
k this deck
25
Instructions:
Give the major organic product(s) of the following reactions or sequences of reactions. Show all relevant stereochemistry.
Write the product(s):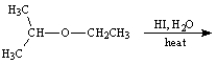
Give the major organic product(s) of the following reactions or sequences of reactions. Show all relevant stereochemistry.
Write the product(s):
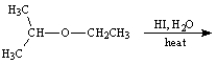

Unlock Deck
Unlock for access to all 36 flashcards in this deck.
Unlock Deck
k this deck
26
Instructions:
Give the major organic product(s) of the following reactions or sequences of reactions. Show all relevant stereochemistry.
Write the product(s):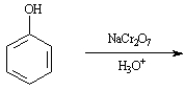
Give the major organic product(s) of the following reactions or sequences of reactions. Show all relevant stereochemistry.
Write the product(s):


Unlock Deck
Unlock for access to all 36 flashcards in this deck.
Unlock Deck
k this deck
27
Choose the best reagent(s) for carrying out the following conversions from the list provided below. Place the letter of the best choice in the blank. Reagents may be used more than once.
Refer to instructions. _____
A)CrO3, H2SO4, H2O
B)1. NaBH4, ethanol
2. H3O+
C)1. LiAl4, ether
2. H3O+
D)periodinane
Refer to instructions. _____

A)CrO3, H2SO4, H2O
B)1. NaBH4, ethanol
2. H3O+
C)1. LiAl4, ether
2. H3O+
D)periodinane

Unlock Deck
Unlock for access to all 36 flashcards in this deck.
Unlock Deck
k this deck
28
Choose the best reagent(s) for carrying out the following conversions from the list provided below. Place the letter of the best choice in the blank. Reagents may be used more than once.
Refer to instructions: ______
A)CrO3, H2SO4, H2O
B)1. NaBH4, ethanol
2. H3O+
C)1. LiAl4, ether
2. H3O+
D)periodinane
Refer to instructions: ______

A)CrO3, H2SO4, H2O
B)1. NaBH4, ethanol
2. H3O+
C)1. LiAl4, ether
2. H3O+
D)periodinane

Unlock Deck
Unlock for access to all 36 flashcards in this deck.
Unlock Deck
k this deck
29
What is the IUPAC name of the following compound? 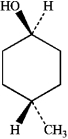
A) cis-4-methylcyclohexanol
B) trans-4-methylcyclohexanol
C) cis-4-hydroxy-1-methylcyclohexane
D) trans-4-hydroxy-1-methylcyclohexane

A) cis-4-methylcyclohexanol
B) trans-4-methylcyclohexanol
C) cis-4-hydroxy-1-methylcyclohexane
D) trans-4-hydroxy-1-methylcyclohexane

Unlock Deck
Unlock for access to all 36 flashcards in this deck.
Unlock Deck
k this deck
30
What is the IUPAC name of the following compound? 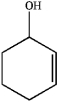
A) cyclohexen-3-ol
B) cyclohexen-2-ol
C) cyclohex-1-en-3-ol
D) cyclohex-2-en-1-ol

A) cyclohexen-3-ol
B) cyclohexen-2-ol
C) cyclohex-1-en-3-ol
D) cyclohex-2-en-1-ol

Unlock Deck
Unlock for access to all 36 flashcards in this deck.
Unlock Deck
k this deck
31
Instructions:
Give the major organic product(s) of the following reactions or sequences of reactions. Show all relevant stereochemistry.
Write the product(s):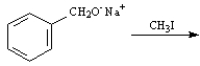
Give the major organic product(s) of the following reactions or sequences of reactions. Show all relevant stereochemistry.
Write the product(s):
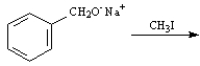

Unlock Deck
Unlock for access to all 36 flashcards in this deck.
Unlock Deck
k this deck
32
Instructions:
Give the major organic product(s) of the following reactions or sequences of reactions. Show all relevant stereochemistry.
Write the product(s):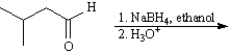
Give the major organic product(s) of the following reactions or sequences of reactions. Show all relevant stereochemistry.
Write the product(s):
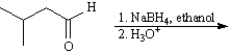

Unlock Deck
Unlock for access to all 36 flashcards in this deck.
Unlock Deck
k this deck
33
What is the best way to make the following ether by a Williamson ether synthesis? 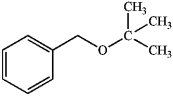


Unlock Deck
Unlock for access to all 36 flashcards in this deck.
Unlock Deck
k this deck
34
What is the IUPAC name of the following compound? 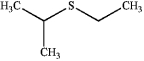
A) ethyl isopropyl thiol
B) 2-methylsulfanylpropane
C) ethyl isopropyl disulfide
D) ethyl isopropyl sulfide

A) ethyl isopropyl thiol
B) 2-methylsulfanylpropane
C) ethyl isopropyl disulfide
D) ethyl isopropyl sulfide

Unlock Deck
Unlock for access to all 36 flashcards in this deck.
Unlock Deck
k this deck
35
Which of the following are ethers? 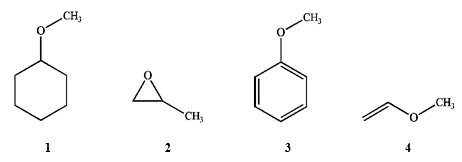
A) only 1 and 2
B) only 1 and 4
C) only 1, 2 and 3
D) all of these are ethers

A) only 1 and 2
B) only 1 and 4
C) only 1, 2 and 3
D) all of these are ethers

Unlock Deck
Unlock for access to all 36 flashcards in this deck.
Unlock Deck
k this deck
36
Instructions:
Give the major organic product(s) of the following reactions or sequences of reactions. Show all relevant stereochemistry.
Write the product(s):
Give the major organic product(s) of the following reactions or sequences of reactions. Show all relevant stereochemistry.
Write the product(s):


Unlock Deck
Unlock for access to all 36 flashcards in this deck.
Unlock Deck
k this deck



