Deck 3: Alkenes and Alkynes: the Nature of Organic Reactions
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/40
Play
Full screen (f)
Deck 3: Alkenes and Alkynes: the Nature of Organic Reactions
1
Instructions: Identify the functional groups present in each compound below, and predict the direction of polarity in each.
-Identify and predict:
mustard gas Cl-CH2CH2-CH2CH2-Cl
-Identify and predict:
mustard gas Cl-CH2CH2-CH2CH2-Cl
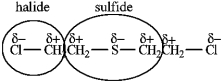
2
Instructions: Identify and label the nucleophile and electrophile in each reaction below.
Identify and label: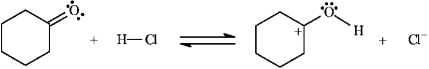
Identify and label:

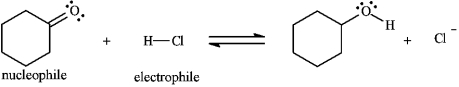
3
Instructions: Identify and label the nucleophile and electrophile in each reaction below.
Identify and label: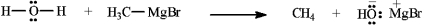
Identify and label:
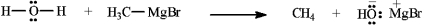
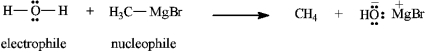
4
Match each definition to one of the terms below.
The energy needed by reactants to reach the transition state.
A)transition state
B)endergonic reaction
C)activation energy
D)Gibbs free energy change
E)exergonic reaction
F)reaction intermediate
The energy needed by reactants to reach the transition state.
A)transition state
B)endergonic reaction
C)activation energy
D)Gibbs free energy change
E)exergonic reaction
F)reaction intermediate

Unlock Deck
Unlock for access to all 40 flashcards in this deck.
Unlock Deck
k this deck
5
Match each definition to one of the terms below.
A species that lies at an energy maximum during an individual step in a reaction.
A)transition state
B)endergonic reaction
C)activation energy
D)Gibbs free energy change
E)exergonic reaction
F)reaction intermediate
A species that lies at an energy maximum during an individual step in a reaction.
A)transition state
B)endergonic reaction
C)activation energy
D)Gibbs free energy change
E)exergonic reaction
F)reaction intermediate

Unlock Deck
Unlock for access to all 40 flashcards in this deck.
Unlock Deck
k this deck
6
Instructions: Identify the functional groups present in each compound below, and predict the direction of polarity in each.
Identify and predict:
amphetamine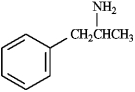
Identify and predict:
amphetamine


Unlock Deck
Unlock for access to all 40 flashcards in this deck.
Unlock Deck
k this deck
7
Instructions: Classify each structure below as a nucleophile or electrophile, and briefly explain your choice.
Classify and explain:
azide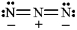
Classify and explain:
azide
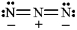

Unlock Deck
Unlock for access to all 40 flashcards in this deck.
Unlock Deck
k this deck
8
Which of the following is a characteristic of a polar reaction?
A) symmetrical bond making and breaking
B) one electron from each reactant forms the bond
C) involves a neutral species with an unpaired electron
D) are more common that radical reactions
E) all of these
A) symmetrical bond making and breaking
B) one electron from each reactant forms the bond
C) involves a neutral species with an unpaired electron
D) are more common that radical reactions
E) all of these

Unlock Deck
Unlock for access to all 40 flashcards in this deck.
Unlock Deck
k this deck
9
Instructions: Classify each structure below as a nucleophile or electrophile, and briefly explain your choice.
Classify and explain:
phenol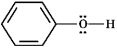
Classify and explain:
phenol


Unlock Deck
Unlock for access to all 40 flashcards in this deck.
Unlock Deck
k this deck
10
Instructions: Consider the reaction of 2-bromo-2-methylpropane with water, shown below, to answer the following question(s). 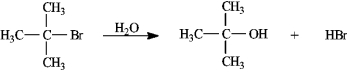
Refer to instructions. This reaction is an example of:
A) a substitution reaction.
B) a rearrangement reaction.
C) an elimination reaction.
D) an addition reaction.

Refer to instructions. This reaction is an example of:
A) a substitution reaction.
B) a rearrangement reaction.
C) an elimination reaction.
D) an addition reaction.

Unlock Deck
Unlock for access to all 40 flashcards in this deck.
Unlock Deck
k this deck
11
Instructions: In the reaction below:
a)Label the nucleophile (Nu) and the electrophile (E).
b)Draw arrows on the structures showing electron flow in the reaction.
Label and indicate flow: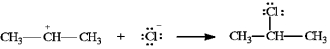
a)Label the nucleophile (Nu) and the electrophile (E).
b)Draw arrows on the structures showing electron flow in the reaction.
Label and indicate flow:
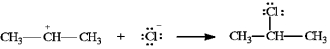

Unlock Deck
Unlock for access to all 40 flashcards in this deck.
Unlock Deck
k this deck
12
Instructions: Add curved arrows to the following reaction(s) to indicate the flow of electrons in each.
Indicate flow: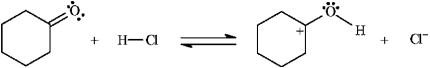
Indicate flow:


Unlock Deck
Unlock for access to all 40 flashcards in this deck.
Unlock Deck
k this deck
13
Match each definition to one of the terms below.
A reaction that involves a species with an unpaired electron.
A)polarization
B)addition reaction
C)radical reaction
D)electrophile
E)polar reaction
F)substitution
G)nucleophile
H)elimination reaction
A reaction that involves a species with an unpaired electron.
A)polarization
B)addition reaction
C)radical reaction
D)electrophile
E)polar reaction
F)substitution
G)nucleophile
H)elimination reaction

Unlock Deck
Unlock for access to all 40 flashcards in this deck.
Unlock Deck
k this deck
14
Instructions: Use the reaction energy diagram below to answer the following question(s). 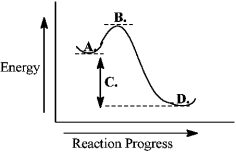
The products are found at _____ on the diagram.

The products are found at _____ on the diagram.

Unlock Deck
Unlock for access to all 40 flashcards in this deck.
Unlock Deck
k this deck
15
In an organic reaction, which of the following is most likely to function as only a nucleophile?
A)BF3
B)(CH3)2CH2NH2
C)Fe2+
D)CH3CH2S-
E) both a and c
A)BF3
B)(CH3)2CH2NH2
C)Fe2+
D)CH3CH2S-
E) both a and c

Unlock Deck
Unlock for access to all 40 flashcards in this deck.
Unlock Deck
k this deck
16
Match each definition to one of the terms below.
Another term used for a Lewis acid.
A)polarization
B)addition reaction
C)radical reaction
D)electrophile
E)polar reaction
F)substitution
G)nucleophile
H)elimination reaction
Another term used for a Lewis acid.
A)polarization
B)addition reaction
C)radical reaction
D)electrophile
E)polar reaction
F)substitution
G)nucleophile
H)elimination reaction

Unlock Deck
Unlock for access to all 40 flashcards in this deck.
Unlock Deck
k this deck
17
Instructions: Use the reaction energy diagram below to answer the following question(s). 
The free-energy change for the reaction is indicated at _____ on the diagram.

The free-energy change for the reaction is indicated at _____ on the diagram.

Unlock Deck
Unlock for access to all 40 flashcards in this deck.
Unlock Deck
k this deck
18
Instructions: The reaction below is commonly used as a laboratory preparation of cyclohexene. Use this reaction to answer the following question(s). 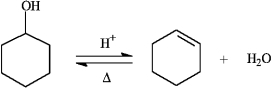
Refer to instructions. The forward and reverse reactions are classified, respectively, as:
A) addition, elimination
B) elimination, substitution
C) elimination, addition
D) elimination, rearrangement
E) substitution, addition

Refer to instructions. The forward and reverse reactions are classified, respectively, as:
A) addition, elimination
B) elimination, substitution
C) elimination, addition
D) elimination, rearrangement
E) substitution, addition

Unlock Deck
Unlock for access to all 40 flashcards in this deck.
Unlock Deck
k this deck
19
Instructions: Use the reaction energy diagram below to answer the following question(s). 
The transition state is found at _____ on the diagram.

The transition state is found at _____ on the diagram.

Unlock Deck
Unlock for access to all 40 flashcards in this deck.
Unlock Deck
k this deck
20
Instructions: Add curved arrows to the following reaction(s) to indicate the flow of electrons in each.
Indicate flow: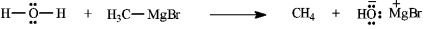
Indicate flow:
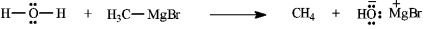

Unlock Deck
Unlock for access to all 40 flashcards in this deck.
Unlock Deck
k this deck
21
Which of the following substituents has the highest priority according to the Cahn-Ingold-Prelog system?
A)(-COOH)
B)(-CHO)
C)(-CH2OH)
D)(-CH3)
A)(-COOH)
B)(-CHO)
C)(-CH2OH)
D)(-CH3)

Unlock Deck
Unlock for access to all 40 flashcards in this deck.
Unlock Deck
k this deck
22
Name this compound. 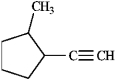


Unlock Deck
Unlock for access to all 40 flashcards in this deck.
Unlock Deck
k this deck
23
The following group is a substituent on a molecule. What is an accepted IUPAC name for this group? 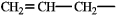
A) propenyl
B) allyl
C) vinyl
D) propylene
E) either a or b
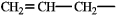
A) propenyl
B) allyl
C) vinyl
D) propylene
E) either a or b

Unlock Deck
Unlock for access to all 40 flashcards in this deck.
Unlock Deck
k this deck
24
Instructions: Draw structures corresponding to each name below.
Draw:
trans-4,4-dimethylpent-2-ene
Draw:
trans-4,4-dimethylpent-2-ene

Unlock Deck
Unlock for access to all 40 flashcards in this deck.
Unlock Deck
k this deck
25
Instructions: Assign E or Z configurations to each alkene below.
Assign: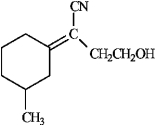
Assign:


Unlock Deck
Unlock for access to all 40 flashcards in this deck.
Unlock Deck
k this deck
26
Which of the following substituents has the highest priority according to the Cahn-Ingold-Prelog system?
A)(-NH2)
B)(-NHCH3)
C)(-CH2NH2)
D)(-CH2NHCH3)
A)(-NH2)
B)(-NHCH3)
C)(-CH2NH2)
D)(-CH2NHCH3)

Unlock Deck
Unlock for access to all 40 flashcards in this deck.
Unlock Deck
k this deck
27
Instructions: Provide names for each structure below. Be sure to include the cis,trans or E,Z designations where applicable.
Name and designate: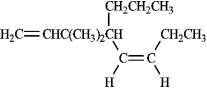
Name and designate:


Unlock Deck
Unlock for access to all 40 flashcards in this deck.
Unlock Deck
k this deck
28
Instructions: Pent-2-ene is an example of a disubstituted alkene. Use this alkene to answer the following question(s). 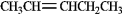
Refer to instructions. Draw the cis and trans isomers of pent-2-ene and label them.
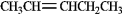
Refer to instructions. Draw the cis and trans isomers of pent-2-ene and label them.

Unlock Deck
Unlock for access to all 40 flashcards in this deck.
Unlock Deck
k this deck
29
What is the IUPAC name of the following compound? 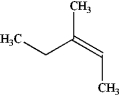
A)(E)-3-methylpent-3-ene
B)(Z)-3-methylpent-3-ene
C)(E)-3-methylpent-2-ene
D)(Z)-3-methylpent-2-ene

A)(E)-3-methylpent-3-ene
B)(Z)-3-methylpent-3-ene
C)(E)-3-methylpent-2-ene
D)(Z)-3-methylpent-2-ene

Unlock Deck
Unlock for access to all 40 flashcards in this deck.
Unlock Deck
k this deck
30
Instructions: Draw structures corresponding to each name below.
Draw:
(3E)-3,7-dimethylocta-1,3,6-triene
Draw:
(3E)-3,7-dimethylocta-1,3,6-triene

Unlock Deck
Unlock for access to all 40 flashcards in this deck.
Unlock Deck
k this deck
31
Instructions: Use the reaction energy diagram below to answer the following question(s). 
The reactants are found at _____ on the diagram.

The reactants are found at _____ on the diagram.

Unlock Deck
Unlock for access to all 40 flashcards in this deck.
Unlock Deck
k this deck
32
The structures below show the stepwise bond making and bond breaking in this reaction. Draw curved arrows to show the electron flow that has occurred in each step. 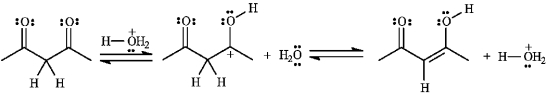


Unlock Deck
Unlock for access to all 40 flashcards in this deck.
Unlock Deck
k this deck
33
Instructions: Provide names for each structure below. Be sure to include the cis,trans or E,Z designations where applicable.
Name and designate: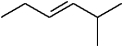
Name and designate:


Unlock Deck
Unlock for access to all 40 flashcards in this deck.
Unlock Deck
k this deck
34
Instructions: Provide names for each structure below. Be sure to include the cis,trans or E,Z designations where applicable.
Name and designate: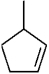
Name and designate:


Unlock Deck
Unlock for access to all 40 flashcards in this deck.
Unlock Deck
k this deck
35
Predict the product of the following reaction of Prostaglandin H2 by interpreting the flow of electrons as indicated by the curved arrows. 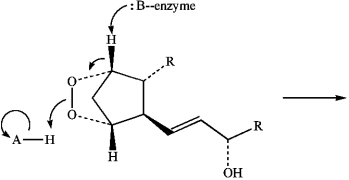


Unlock Deck
Unlock for access to all 40 flashcards in this deck.
Unlock Deck
k this deck
36
Choose substituents X and Y (listed in order below) for the following compound so as to make a Z isomer. 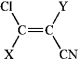
A)(-Br, -NHCH3)
B)(-F, -CHO)
C)(-I, -OCH3)
D)(-COOH, -CH2NH2)
E)(-Br, -COOH)

A)(-Br, -NHCH3)
B)(-F, -CHO)
C)(-I, -OCH3)
D)(-COOH, -CH2NH2)
E)(-Br, -COOH)

Unlock Deck
Unlock for access to all 40 flashcards in this deck.
Unlock Deck
k this deck
37
Instructions: Pent-2-ene is an example of a disubstituted alkene. Use this alkene to answer the following question(s). 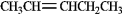
Refer to instructions. Circle the isomer of pent-2-ene that is most stable.
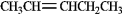
Refer to instructions. Circle the isomer of pent-2-ene that is most stable.

Unlock Deck
Unlock for access to all 40 flashcards in this deck.
Unlock Deck
k this deck
38
Rank each set of substituents using the Cahn-Ingold-Prelog sequence rules by numbering the highest priority substituent 1 and numbering the lowest priority substituent 4. Place the number in the blank below the substituent. 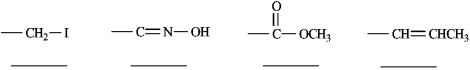
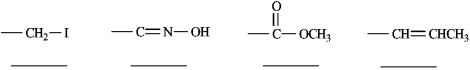

Unlock Deck
Unlock for access to all 40 flashcards in this deck.
Unlock Deck
k this deck
39
Below are all the chemical structures and intermediates involved in a reaction. On the structures provided, show all electron flow using the arrow formalism for the complete stepwise mechanism. 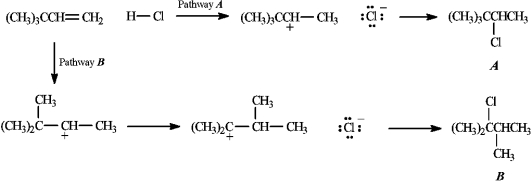


Unlock Deck
Unlock for access to all 40 flashcards in this deck.
Unlock Deck
k this deck
40
Instructions: Assign E or Z configurations to each alkene below.
Assign: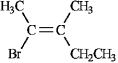
Assign:


Unlock Deck
Unlock for access to all 40 flashcards in this deck.
Unlock Deck
k this deck



