Deck 10: Radioactivity and Nuclear Processes
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/93
Play
Full screen (f)
Deck 10: Radioactivity and Nuclear Processes
1
Which is the explanation for the difference between the two isotopes of carbon, C-12 and C-14?
A)C-14 contains 2 more protons than does C-12.
B)C-14 contains 1 proton and 1 neutron more than does C-12.
C)C-14 contains 2 more neutrons than does C-12.
D)C-14 contains 2 more electrons than does C-12.
A)C-14 contains 2 more protons than does C-12.
B)C-14 contains 1 proton and 1 neutron more than does C-12.
C)C-14 contains 2 more neutrons than does C-12.
D)C-14 contains 2 more electrons than does C-12.
C-14 contains 2 more neutrons than does C-12.
2
The number of neutrons in the nucleus of the isotope 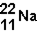 is _____ .
is _____ .
A)11
B)22
C)12
D)32
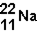 is _____ .
is _____ .A)11
B)22
C)12
D)32
11
3
Which of the following consists of a stream of charged particles?
A)alpha rays
B)beta rays
C)gamma rays
D)More than one response is correct.
A)alpha rays
B)beta rays
C)gamma rays
D)More than one response is correct.
More than one response is correct.
4
Sodium-24 has a half-life of 15.0 hours. Suppose you had a sample containing 0.010 moles of Na-24. How many hours would be required to reduce your sample to 6.25 × 10 − 4 moles?
A)120
B)60
C)45
D)30
A)120
B)60
C)45
D)30

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
5
Which of the following is not a nuclear reaction?
A)fusion
B)radioactive decay
C)fission
D)redox
A)fusion
B)radioactive decay
C)fission
D)redox

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
6
What is the nuclear composition of 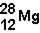 ?
?
A)12 neutrons and 28 protons
B)12 neutrons and 16 protons
C)12 protons and 28 neutrons
D)12 protons and 16 neutrons
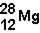 ?
?A)12 neutrons and 28 protons
B)12 neutrons and 16 protons
C)12 protons and 28 neutrons
D)12 protons and 16 neutrons

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
7
Carbon-14 dating is a useful tool for determining the age of the artifacts of ancient civilizations. However, when future archeologists study ruins of our civilization, they obtain confusing results -- with some artifacts dating MUCH older than others. Which of the following would be an explanation for these results?
A)Variations in the Sun's intensity and production of cosmic rays.
B)Ecosystem changes resulting in a reduction in carbon based life.
C)The use of petroleum based synthetic materials.
D)None of the choices.
A)Variations in the Sun's intensity and production of cosmic rays.
B)Ecosystem changes resulting in a reduction in carbon based life.
C)The use of petroleum based synthetic materials.
D)None of the choices.

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
8
The greatest threat posed by a nuclear (fission)power plant is
A)release of radioactive isotopes when the molten core reaches the water table.
B)fish kills resulting from use of local rivers for cooling.
C)having the core melt, ultimately reaching China.
D)thermonuclear detonation.
A)release of radioactive isotopes when the molten core reaches the water table.
B)fish kills resulting from use of local rivers for cooling.
C)having the core melt, ultimately reaching China.
D)thermonuclear detonation.

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
9
Which of the responses represent the missing particle in the following reaction? 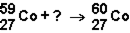
A)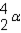
B)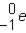
C)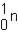
D)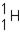
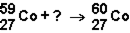
A)
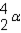
B)
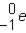
C)
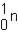
D)
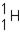

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
10
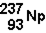 is an alpha emitter. What is the element produced by the decay of Np-237?
is an alpha emitter. What is the element produced by the decay of Np-237?A)U
B)Pa
C)Th
D)Pu

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
11
Which of the following are made up of particles identical to electrons?
A)alpha rays
B)beta rays
C)gamma rays
D)more than one response is correct
A)alpha rays
B)beta rays
C)gamma rays
D)more than one response is correct

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
12
Nuclear emissions are passed through a tunnel that has a positive plate on the top and a negative plate on the bottom. Which of the following emissions is deflected the greatest by the electrical field?
A)α particles
B)β particles
C)neutrons
D)γ rays
A)α particles
B)β particles
C)neutrons
D)γ rays

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
13
Which of the following would have the least penetrating power into matter?
A)alpha particles
B)beta particles
C)gamma rays
D)neutrons
A)alpha particles
B)beta particles
C)gamma rays
D)neutrons

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
14
Tritium has a half-life of 12.5 years. If you had a sample of 8.00 grams of tritium today, how many grams of tritium would you have in 37.5 years?
A)8.00 g
B)4.00 g
C)2.00 g
D)1.00 g
A)8.00 g
B)4.00 g
C)2.00 g
D)1.00 g

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
15
You inject a 160 lb male patient with 1.0 ml of a saline solution containing a radioactive form of sodium. It has an activity of 5.0 × 104 dpm. After allowing sufficient time for the solution to mix, you remove a 1.0 ml sample of blood and measure its radioactivity. You discover it to have an activity of 11 dpm. What is the patient's blood volume? (Hint: this is similar to a M1V1 = M2V2 type problem.)
A)2.2 liters
B)3.5 liters
C)4.6 liters
D)7.0 liters
A)2.2 liters
B)3.5 liters
C)4.6 liters
D)7.0 liters

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
16
Which of the nuclear emissions is of the greatest mass?
A)α particles
B)β particles
C)neutrons
D)positrons
A)α particles
B)β particles
C)neutrons
D)positrons

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
17
Which of the following is not attracted or repelled by electrical charges?
A)alpha rays
B)beta rays
C)gamma rays
D)More than one response is correct.
A)alpha rays
B)beta rays
C)gamma rays
D)More than one response is correct.

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
18
Which of the following types of radiation is composed of particles which carry a negative charge?
A)alpha
B)beta
C)gamma
D)positron
A)alpha
B)beta
C)gamma
D)positron

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
19
An individual would have to work from behind a special, thick protective wall when which kind of an emitter is involved?
A)alpha
B)beta
C)gamma
D)free radicals
A)alpha
B)beta
C)gamma
D)free radicals

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
20
The decay of 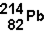 to
to 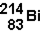 occurs through the emission of
occurs through the emission of
A)an alpha particle.
B)a beta particle.
C)a positron.
D)a proton.
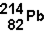 to
to 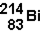 occurs through the emission of
occurs through the emission ofA)an alpha particle.
B)a beta particle.
C)a positron.
D)a proton.

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
21
In nuclear reactions, the principle change occurs in the ______.
A)molecular bonds
B)electron arrangement
C)physical state of the matter
D)nucleus of the atom
A)molecular bonds
B)electron arrangement
C)physical state of the matter
D)nucleus of the atom

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
22
What is a desired characteristic of a diagnostic tracer?
A)short half-life
B)a beta emitter
C)be soluble in water
D)all of these responses are correct
A)short half-life
B)a beta emitter
C)be soluble in water
D)all of these responses are correct

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
23
Technetium-99m is used in medical diagnosis by injecting a solution and watch for the pattern of emissions. A 0.325 g sample was injected into a person, and the emission rate indicates that there are approximately 0.01016 grams of Tc-99 left. How much time has passed since the injection? Tc-99 has a half-life of 6 hours.
A)24 hours
B)30 hours
C)36 hours
D)Not enough data.
A)24 hours
B)30 hours
C)36 hours
D)Not enough data.

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
24
What is one of the advantages of food that has been irradiated to kill bacteria?
A)Some irradiated foods do not require refrigeration if in air-tight packaging.
B)Irradiation is a less expensive method of preservation than is canning.
C)Irradiation has the effect of changing the color from dull colors to vibrant colors which makes the food much more pleasing.
D)All of these responses are correct.
A)Some irradiated foods do not require refrigeration if in air-tight packaging.
B)Irradiation is a less expensive method of preservation than is canning.
C)Irradiation has the effect of changing the color from dull colors to vibrant colors which makes the food much more pleasing.
D)All of these responses are correct.

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
25
An isotope of zinc containing 36 neutrons is bombarded with and captures a proton. What isotope is produced as a product of the reaction?
A)zinc-67
B)gallium-66
C)kryton-83
D)copper-65
A)zinc-67
B)gallium-66
C)kryton-83
D)copper-65

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
26
What are some of the symptoms of radiation sickness?
A)hemorrhage
B)nausea
C)anemia
D)all of them
A)hemorrhage
B)nausea
C)anemia
D)all of them

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
27
Synthetic elements can be produced by
A)the application of extremely high voltages.
B)by the bombardment of light with electrons.
C)by the bombardment of an atom by high speed neutrons.
D)All of the responses are correct.
A)the application of extremely high voltages.
B)by the bombardment of light with electrons.
C)by the bombardment of an atom by high speed neutrons.
D)All of the responses are correct.

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
28
The term "nuclear energy" is most closely associated with which one of the following processes?
A)nuclear fusion
B)nuclear fission
C)cyclotron bombardment
D)radioactive decay
A)nuclear fusion
B)nuclear fission
C)cyclotron bombardment
D)radioactive decay

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
29
What characteristic is important in using radioisotope dating for very old items, such as rocks suspected to be over 100,000,000 years old?
A)The radioisotope cannot be soluble in water.
B)The radioisotope must give off light for easy measurement.
C)The radioisotope in question must decay to another radioisotope.
D)The radioisotope should have a very long half-life.
A)The radioisotope cannot be soluble in water.
B)The radioisotope must give off light for easy measurement.
C)The radioisotope in question must decay to another radioisotope.
D)The radioisotope should have a very long half-life.

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
30
Which of the emissions can be the cause of radiation sickness?
A)α particles
B)β particles
C)neutrons
D)all of them
A)α particles
B)β particles
C)neutrons
D)all of them

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
31
Which emissions can be detected by a film badge?
A)X-rays
B)gamma
C)beta
D)all are detected
A)X-rays
B)gamma
C)beta
D)all are detected

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
32
What is the emission that is used to obtain a CAT scan?
A)alpha
B)beta
C)X-ray
D)none of these responses
A)alpha
B)beta
C)X-ray
D)none of these responses

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
33
Which form of radiation would not be deflected by a magnetic field?
A)protons
B)neutrons
C)beta particles
D)positrons
A)protons
B)neutrons
C)beta particles
D)positrons

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
34
One reason that irradiated food is not readily available in most grocery stores is that
A)the cost of the irradiated foods is much higher than normally offered foods.
B)the chance of purchasing food that is still radioactive is rather high.
C)most people have not learned enough to know that they are safe.
D)All of the responses are correct.
A)the cost of the irradiated foods is much higher than normally offered foods.
B)the chance of purchasing food that is still radioactive is rather high.
C)most people have not learned enough to know that they are safe.
D)All of the responses are correct.

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
35
Free radicals are dangerous because
A)they are found incorporated in any radioisotope, including those that can enter the body through the lungs as does radon
B)they can be taken into the body in the food eaten if that food has been grown in radioactive soil
C)they are substances that are missing some electrons and are extremely active chemically
D)All of these responses are correct.
A)they are found incorporated in any radioisotope, including those that can enter the body through the lungs as does radon
B)they can be taken into the body in the food eaten if that food has been grown in radioactive soil
C)they are substances that are missing some electrons and are extremely active chemically
D)All of these responses are correct.

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
36
Which technique of medical investigation presents no ionizing radiation risk to the patient?
A)X-ray
B)CAT scan
C)MRI
D)all of them
A)X-ray
B)CAT scan
C)MRI
D)all of them

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
37
Which of the following radioisotopes is not used as a diagnostic tracer?
A)I-131
B)Mg-24
C)Kr-81
D)Fe-59
A)I-131
B)Mg-24
C)Kr-81
D)Fe-59

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
38
There are some elements within the periodic table that do not occur in nature. One of the man-made elements is _____ .
A)Ta
B)Pt
C)Tc
D)La
A)Ta
B)Pt
C)Tc
D)La

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
39
What is the emission that is used to obtain an MRI?
A)alpha
B)beta
C)X-ray
D)none of them
A)alpha
B)beta
C)X-ray
D)none of them

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
40
Which measure of radiation is used to account for health differences of various types of radiation?
A)curie
B)rem
C)gray
D)rad
A)curie
B)rem
C)gray
D)rad

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
41
The intensity of a radiation source was measured by a Geiger-Müller counter to be 236 counts per minute when the counter was place 124 ft from the source. What will be the intensity when the counter is 248 ft from the source?
A)59.0 counts per minute
B)236 counts per minute
C)118 counts per minute
D)472 counts per minute
A)59.0 counts per minute
B)236 counts per minute
C)118 counts per minute
D)472 counts per minute

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
42
Why is radiation so harmful to living things?
A)creates free radicals which are highly reactive
B)reactions occur causing genetic mutations
C)turns the skin green
D)More than one response is correct.
A)creates free radicals which are highly reactive
B)reactions occur causing genetic mutations
C)turns the skin green
D)More than one response is correct.

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
43
How much of a fissionable material is required to have a self-sustaining reaction?
A)A mass of the fissionable element that is three times its atomic weight is required.
B)A mass of the fissionable element that is greater than the mass of the control rods.
C)A supercritical mass of fissionable material is required and causes branching.
D)A critical mass of fissionable material is required and varies depending on the identity of the fissionable material.
A)A mass of the fissionable element that is three times its atomic weight is required.
B)A mass of the fissionable element that is greater than the mass of the control rods.
C)A supercritical mass of fissionable material is required and causes branching.
D)A critical mass of fissionable material is required and varies depending on the identity of the fissionable material.

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
44
History's first critical chain reaction took place in an atomic pile. The neutrons from fission reactions caused other fission reactions to occur. What would be the most effective method to stop the fission reaction from proceeding?
A)throw water on the pile to cool the reaction
B)add more neutrons to the reaction
C)place strong neutron absorbers into the pile
D)none of the choices
A)throw water on the pile to cool the reaction
B)add more neutrons to the reaction
C)place strong neutron absorbers into the pile
D)none of the choices

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
45
Nuclear power plants have control rods that absorb neutrons to control the reaction. What is the composition of the control rods?
A)steel
B)concrete
C)U-238
D)carbon
A)steel
B)concrete
C)U-238
D)carbon

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
46
What is the nature of the reactions that occur in a star?
A)They are extremely fast chemical reactions
B)They are fission reactions
C)They are a mixture of chemical & nuclear reactions
D)They are fusion reactions
A)They are extremely fast chemical reactions
B)They are fission reactions
C)They are a mixture of chemical & nuclear reactions
D)They are fusion reactions

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
47
If you are a medical professional and have a chance to be exposed to many sources of radiation, which of the following units to measure biological radiation will most likely be used to express your level of total exposure?
A)roentgen
B)rad
C)gray
D)rem
A)roentgen
B)rad
C)gray
D)rem

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
48
Which of the following would be the correct symbol for an americium-243 nucleus using the 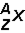 symbolism?
symbolism?
A)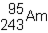
B)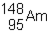
C)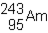
D)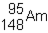
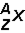 symbolism?
symbolism?A)
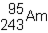
B)
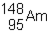
C)
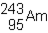
D)
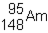

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
49
Consider the figure below. Which form of emitted radiation would follow the path shown by the arrow labeled 1? 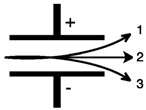
A)α
B)β−
C)γ
D)β+

A)α
B)β−
C)γ
D)β+

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
50
Manganese can undergo several types of radioactive emission. In which type of decay does the following reaction occur? 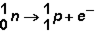
A)alpha
B)beta
C)positron
D)gamma
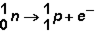
A)alpha
B)beta
C)positron
D)gamma

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
51
A patient that works at a nuclear power plant comes to you complaining of nausea and fatigue. You find out that he might have been exposed to a radiation source; what would you recommend be done?
A)Nothing, If he is still alive, there is no real problem.
B)Send the patient home to rest because nausea and fatigue are not signs of a serious exposure.
C)Have additional tests ran to find out the level of exposure.
D)Tell the patient to take two aspirins and call you in the morning, he probably has the flu.
A)Nothing, If he is still alive, there is no real problem.
B)Send the patient home to rest because nausea and fatigue are not signs of a serious exposure.
C)Have additional tests ran to find out the level of exposure.
D)Tell the patient to take two aspirins and call you in the morning, he probably has the flu.

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
52
Sn-108 undergoes electron capture. The product of this reaction would be _____ .
A)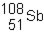
B)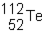
C)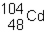
D)
A)
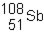
B)
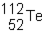
C)
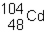
D)


Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
53
Which of the following is a physical measurement of radioactivity?
A)gray
B)becquerel
C)rad
D)roentgen
A)gray
B)becquerel
C)rad
D)roentgen

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
54
A radioactive isotope of a metal produced a reading of 188 Bq. Another reading of the activity was taken 4 hours later and the reading was 47.0 Bq. What is the half-life of this isotope?
A)4 hr
B)2 hr
C)1 hr
D)30 min
A)4 hr
B)2 hr
C)1 hr
D)30 min

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
55
Uranium-235, under the proper circumstances, will undergo nuclear decay releasing 3 high speed neutrons in the process.
A)The neutrons released slow down with time and are not functional.
B)The neutrons released can be used to cause fusion reactions.
C)The neutrons released can cause a chain reaction with other U-235 atoms.
D)The neutrons are recaptured by the nucleus to produce heavier atoms.
A)The neutrons released slow down with time and are not functional.
B)The neutrons released can be used to cause fusion reactions.
C)The neutrons released can cause a chain reaction with other U-235 atoms.
D)The neutrons are recaptured by the nucleus to produce heavier atoms.

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
56
Consider the figure below. Which form of emitted radiation would follow the path shown by the arrow labeled 3? 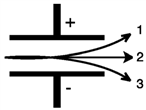
A)α
B)β−
C)γ
D)n

A)α
B)β−
C)γ
D)n

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
57
5.6 mCi would be equal to _____ .
A)3.7 × 1010 disintegrations\sec
B)2.1 × 1011 disintegrations\sec
C)2.1 × 108 disintegrations\sec
D)1.5 × 10-13 disintegrations\sec
A)3.7 × 1010 disintegrations\sec
B)2.1 × 1011 disintegrations\sec
C)2.1 × 108 disintegrations\sec
D)1.5 × 10-13 disintegrations\sec

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
58
The new nuclei produced when unstable nuclei undergo radioactive decay is referred to as the _____ .
A)father nuclei
B)daughter nuclei
C)mother nuclei
D)neighbor nuclei
A)father nuclei
B)daughter nuclei
C)mother nuclei
D)neighbor nuclei

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
59
A good radioisotope tracer for medical use should not have the following characteristic.
A)have a long half-life
B)produce penetrating gamma radiation
C)decay to a nontoxic form
D)undergo the same reactions as the nonradioactive element
A)have a long half-life
B)produce penetrating gamma radiation
C)decay to a nontoxic form
D)undergo the same reactions as the nonradioactive element

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
60
Consider the figure below. Which form of emitted radiation would follow the path shown by the arrow labeled 2? 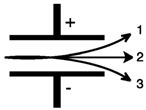
A)α
B)β−
C)γ
D)β+

A)α
B)β−
C)γ
D)β+

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
61
Film badges will detect only X-rays.

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
62
Ionizing radiation produced fee radicals in living tissue.

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
63
Carbon-14 is only effective in dating an object up to approximately 10 half-lives. The reason is that the speed with which C-14 decays increases after 10 half-lives.

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
64
A rem is a biological radiation measurement which is independent of the type of radiation.

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
65
The reasons why I-131 is not suitable for use as a tracer are the same reasons why I-131 is an appropriate choice to treat thyroid cancer.

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
66
A patient undergoes external irradiation with Co-60 for treatment of cancer. Which of the following would you NOT expect to observe as a result of this type of treatment?
A)diarrhea
B)hair loss
C)bone marrow destruction
D)altered blood chemistry
A)diarrhea
B)hair loss
C)bone marrow destruction
D)altered blood chemistry

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
67
A desirable characteristic of a radioisotope used as a tracer is that it produces a radioactive daughter.

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
68
A nuclear reaction in which beta particles are emitted yields an atom that weighs 2 μ less than the starting element.

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
69
Gamma emissions can be stopped with the use of an electromagnetic field because of their dense charge and their mass.

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
70
Which of the following would be associated with medical testing?
A)breeder reactor
B)carbon dating
C)geochronology
D)radioimmunoassay
A)breeder reactor
B)carbon dating
C)geochronology
D)radioimmunoassay

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
71
Nuclear power generation relies on what type of reaction?
A)subcritical
B)critical
C)supercritical
D)thermonuclear
A)subcritical
B)critical
C)supercritical
D)thermonuclear

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
72
Radioisotopes which emit alpha rays make the best diagnostic tracers.

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
73
Diagnostic tracers form cool spots when they are accumulated in diseased tissue.

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
74
A wooden box found in an Egyptian tomb could have its age determined using carbon-14.

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
75
Increased distance between the organism and the radiation source reduces the effect of radiation.

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
76
The intensity of radiation is 100 units 15 feet from the source. The intensity would then be 300 units 5 feet from the source.

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
77
A dose of 600 rem is nearly always fatal.

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
78
A major problem with attempting to measure the effect of an exposure to emissions is that each different emission has a different effect on tissue due to the differences in penetration.

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
79
A gold artifact from Peru could have its age determined using carbon-14.

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
80
A common use of radioisotopes is to speed up chemical reactions.

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck



