Deck 15: The Chemistry of Household Products
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/68
Play
Full screen (f)
Deck 15: The Chemistry of Household Products
1
Which of these represent(s) characteristic(s) of LAS detergents but not of ABS detergents?
I. They are biodegradable.
II. They form branched alkyl chains.
III. They are anionic detergents.
A) I only
B) II only
C) I and II
D) I and III
E) II and III
I. They are biodegradable.
II. They form branched alkyl chains.
III. They are anionic detergents.
A) I only
B) II only
C) I and II
D) I and III
E) II and III
I only
2
A mixture of one substance dispersed in a finely divided state throughout another is known as a _____.
A) polymer
B) surfactant
C) micelle
D) colloidal suspension
E) soap
A) polymer
B) surfactant
C) micelle
D) colloidal suspension
E) soap
colloidal suspension
3
Identify the characteristic(s) of a soap.
I. It acts at surfaces.
II. It contains a polar hydrocarbon tail.
III. Its abundance in water results in micelles.
A) I only
B) II only
C) I and II
D) II and III
E) I, II, and III
I. It acts at surfaces.
II. It contains a polar hydrocarbon tail.
III. Its abundance in water results in micelles.
A) I only
B) II only
C) I and II
D) II and III
E) I, II, and III
II only
4
When soapy water is added to grease or oil, the soap molecule's _____ interacts with nonpolar grease and its _____ interacts with polar water.
A) hydrocarbon tail; ionic head
B) ionic head; hydrocarbon tail
C) micelle; hydrocarbon tail
D) hydrocarbon tail; micelle
E) ionic head; micelle
A) hydrocarbon tail; ionic head
B) ionic head; hydrocarbon tail
C) micelle; hydrocarbon tail
D) hydrocarbon tail; micelle
E) ionic head; micelle

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
5
Soap molecules cluster in structures called _____.
A) polymers.
B) detergents.
C) micelles.
D) colloids.
E) surfactants.
A) polymers.
B) detergents.
C) micelles.
D) colloids.
E) surfactants.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
6
Which of the following is minimized when a group of soap molecules form micelles?
A) Interaction between the hydrocarbon tail and grease
B) Interaction between the hydrocarbon tail and water molecules
C) Interaction between the ionic head and water molecules
D) Interaction between the ionic head and grease
E) None of these
A) Interaction between the hydrocarbon tail and grease
B) Interaction between the hydrocarbon tail and water molecules
C) Interaction between the ionic head and water molecules
D) Interaction between the ionic head and grease
E) None of these

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
7
Which of these ions found in hard water reduces the effectiveness of a soap?
A) Ca2+
B) C4 −
C) F −
D) Ag+
E) Hg2+
A) Ca2+
B) C4 −
C) F −
D) Ag+
E) Hg2+

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
8
Which of these is the primary problem with alkylbenzenesufonates (ABS) detergents?
A) They are non-biodegradable.
B) They are expensive.
C) They have limited effect on fabric.
D) They form curd on bathtubs.
E) None of these
A) They are non-biodegradable.
B) They are expensive.
C) They have limited effect on fabric.
D) They form curd on bathtubs.
E) None of these

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
9
Which of these was/were the first synthetic detergent designed to combat the problem of hard water?
A) Nonionic detergents
B) LAS
C) Cationic detergents
D) ABS
E) LBS
A) Nonionic detergents
B) LAS
C) Cationic detergents
D) ABS
E) LBS

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
10
A soap is classified as a(n) _____.
A) lipid.
B) oil.
C) surfactant.
D) plastic.
E) enzyme.
A) lipid.
B) oil.
C) surfactant.
D) plastic.
E) enzyme.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
11
Which of these substances is trapped in the center of a micelle when it is formed?
A) Soap
B) Grease
C) Water
D) Bleach
E) None of these
A) Soap
B) Grease
C) Water
D) Bleach
E) None of these

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
12
Colloidal suspensions are a type of _____.
A) emulsions
B) polymers
C) lipids
D) surfactants
E) reducing agents
A) emulsions
B) polymers
C) lipids
D) surfactants
E) reducing agents

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
13
Which of these substances dissolves easily in water and can be removed from fabric?
A) Grease
B) Syrup
C) Coffee
D) Wine
E) Ink
A) Grease
B) Syrup
C) Coffee
D) Wine
E) Ink

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
14
Which of these is not a problem associated with soap in hard water?
A) Formation of non-biodegradable products
B) Formation of bathtub rings
C) Formation of insoluble precipitates
D) Formation of gray films on dishes and clothes
E) None of these
A) Formation of non-biodegradable products
B) Formation of bathtub rings
C) Formation of insoluble precipitates
D) Formation of gray films on dishes and clothes
E) None of these

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
15
Which of these properties of a soap helps dissolve grease in water?
A) Acidity
B) Oxidizing ability
C) Polymerization
D) Molecular flexibility
E) Polar-nonpolar nature
A) Acidity
B) Oxidizing ability
C) Polymerization
D) Molecular flexibility
E) Polar-nonpolar nature

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
16
Which of these represent(s) characteristic(s) of ABS detergents?
I. Biodegradable
II. Branched alkyl chain
III. Cationic detergent
A) I only
B) II only
C) I and II
D) I and III
E) II and III
I. Biodegradable
II. Branched alkyl chain
III. Cationic detergent
A) I only
B) II only
C) I and II
D) I and III
E) II and III

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
17
Which of these is/are characteristic(s) of a soap?
I. It acts at surfaces.
II. It contains a polar ionic head.
III. Micelles of a soap are neutral and therefore react with grease effectively.
A) I only
B) II only
C) I and II
D) II and III
E) I, II, and III.
I. It acts at surfaces.
II. It contains a polar ionic head.
III. Micelles of a soap are neutral and therefore react with grease effectively.
A) I only
B) II only
C) I and II
D) II and III
E) I, II, and III.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
18
Identify the substance(s) that act as the emulsifying agent(s) in a mixture of soap, water, and grease.
A) Water
B) Grease
C) Soap
D) Grease and soap
E) Water and grease
A) Water
B) Grease
C) Soap
D) Grease and soap
E) Water and grease

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
19
Which of these detergents was the first developed in response to the problems associated with ABS detergents?
A) Cationic detergents
B) Nonionic detergents
C) LAS
D) LBS
E) None of these
A) Cationic detergents
B) Nonionic detergents
C) LAS
D) LBS
E) None of these

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
20
Identify the substance used to oxidize tough stains on fabrics.
A) Grease
B) Surfactants
C) Polymers
D) Micelles
E) Bleaches
A) Grease
B) Surfactants
C) Polymers
D) Micelles
E) Bleaches

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
21
Any substance that increases the effectiveness of a surfactant is known as a(n) _____.
A) ionizer
B) emulsifier
C) builder
D) dissolver
E) oxidizer
A) ionizer
B) emulsifier
C) builder
D) dissolver
E) oxidizer

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
22
Which of these processes occur(s) when phosphates are introduced into natural waters?
I. Fish and marine life undergo mutation.
II. Fertilized algae grow into extremely large blooms.
III. A large number of oxidation reactions deplete the oxygen supply.
A) I only
B) II only
C) I and III
D) II and III
E) I and II
I. Fish and marine life undergo mutation.
II. Fertilized algae grow into extremely large blooms.
III. A large number of oxidation reactions deplete the oxygen supply.
A) I only
B) II only
C) I and III
D) II and III
E) I and II

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
23
Which of these substances are not found in ordinary laundry detergents?
A) Bleaches
B) Sebum
C) Enzymes
D) Zeolites
E) Builders
A) Bleaches
B) Sebum
C) Enzymes
D) Zeolites
E) Builders

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
24
Which of these substances contains sodium ions that exchange places with calcium ions in hard water?
A) Phosphates
B) Sodium carbonate
C) Zeolites
D) Bleaches
E) Sodium tripolyphosphate
A) Phosphates
B) Sodium carbonate
C) Zeolites
D) Bleaches
E) Sodium tripolyphosphate

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
25
Which of these is not a class of detergents?
A) Hydrated
B) Cationic
C) Anionic
D) Nonionic
E) None of these
A) Hydrated
B) Cationic
C) Anionic
D) Nonionic
E) None of these

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
26
Which of these pairs represents the two functions of a builder in detergents?
A) Oxidize impurities in water , emulsify detergents
B) Reduce impurities in water , emulsify
C) Biodegrade waste products , oxidize impurities
D) Sequester waster products , suspend soil particles
E) Oxidize stains, suspend soil particles
A) Oxidize impurities in water , emulsify detergents
B) Reduce impurities in water , emulsify
C) Biodegrade waste products , oxidize impurities
D) Sequester waster products , suspend soil particles
E) Oxidize stains, suspend soil particles

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
27
Which of these are not molecular forces that hold hair strands together?
I. Disulfide linkages
II. Hydrogen bonds
III. Nonpolar hydrocarbon tails
A) I only
B) III only
C) I and II
D) I and III
E) I, II, and III
I. Disulfide linkages
II. Hydrogen bonds
III. Nonpolar hydrocarbon tails
A) I only
B) III only
C) I and II
D) I and III
E) I, II, and III

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
28
Which of these substances serve no laundering purpose in a detergent except to increase bulk?
A) Phosphates
B) Fillers
C) Enzymes
D) Zeolites
E) Builders
A) Phosphates
B) Fillers
C) Enzymes
D) Zeolites
E) Builders

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
29
What is the primary function of a builder in laundry detergents?
A) To act as an emulsifying agent
B) To remove grease and other nonpolar stains
C) To remove hard water ions such as Mg2+ and Ca2+
D) To increase the tensile strength of a fabric
E) To accelerate the natural biodegradation process of a surfactant
A) To act as an emulsifying agent
B) To remove grease and other nonpolar stains
C) To remove hard water ions such as Mg2+ and Ca2+
D) To increase the tensile strength of a fabric
E) To accelerate the natural biodegradation process of a surfactant

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
30
Which of these builders forms insoluble precipitates when it reacts with the ions found in hard water?
A) Phosphate
B) Sodium carbonate
C) Enzyme
D) Zeolite
E) Sodium tripolyphosphate
A) Phosphate
B) Sodium carbonate
C) Enzyme
D) Zeolite
E) Sodium tripolyphosphate

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
31
Which of these substances is secreted from the glands on the scalp to keep hair from drying out?
A) Bleaches
B) Buffers
C) Enzymes
D) Zeolites
E) Sebum
A) Bleaches
B) Buffers
C) Enzymes
D) Zeolites
E) Sebum

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
32
Which of these interactions that hold hair strands together is/are pH sensitive?
I. A salt bridge
II. A hydrogen bond
III. A disulfide linkage
A) I only
B) III only
C) I and II
D) I and III
E) I, II, and III
I. A salt bridge
II. A hydrogen bond
III. A disulfide linkage
A) I only
B) III only
C) I and II
D) I and III
E) I, II, and III

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
33
Which of these substances have been used as replacements for phosphates in laundry detergents?
A) Sodium carbonate
B) Zeolites
C) Enzymes
D) Bleaches
E) Both a and b
A) Sodium carbonate
B) Zeolites
C) Enzymes
D) Bleaches
E) Both a and b

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
34
Which of these statements about hair is incorrect ?
A) Protein strands in hair are dissolvable in NaOH.
B) Hair is primarily composed of proteins.
C) Hair strands interact via hydrogen bonds, disulfide linkages, and salt bridges.
D) Hydrogen bonds are modified in wet and dry hair to induce curling.
E) Hair is sensitive to pH because of the presence of hydrogen bonds.
A) Protein strands in hair are dissolvable in NaOH.
B) Hair is primarily composed of proteins.
C) Hair strands interact via hydrogen bonds, disulfide linkages, and salt bridges.
D) Hydrogen bonds are modified in wet and dry hair to induce curling.
E) Hair is sensitive to pH because of the presence of hydrogen bonds.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
35
Which of these substances serve as a "balling" reducer and an additional stain remover in detergents?
A) Phosphates
B) Fillers
C) Enzymes
D) Zeolites
E) Builders
A) Phosphates
B) Fillers
C) Enzymes
D) Zeolites
E) Builders

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
36
Identify the type of detergent the given structure represents. 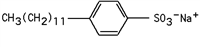
A) Cationic
B) Anionic
C) Nonionic
D) Hydrated
E) Neutral

A) Cationic
B) Anionic
C) Nonionic
D) Hydrated
E) Neutral

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
37
Permanently curled hair is achieved by applying a(n) ____ to the hair followed by a(n) _____ to reform the disulfide linkages.
A) mild acid; strong base
B) ionic salt; strong acid
C) mild oxidizing agent; mild reducing agent
D) mild reducing agent; mild oxidizing agent
E) strong acid; mild base
A) mild acid; strong base
B) ionic salt; strong acid
C) mild oxidizing agent; mild reducing agent
D) mild reducing agent; mild oxidizing agent
E) strong acid; mild base

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
38
Which of these processes is characterized by large algae blooms and may cause death to marine life in a lake or bayou?
A) Eutrophication
B) Reduction
C) Saponification
D) Emulsification
E) Corrosion
A) Eutrophication
B) Reduction
C) Saponification
D) Emulsification
E) Corrosion

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
39
Which of these substances is the active ingredient in a drain cleaner?
A) HCl
B) Bleach
C) NaOH
D) Sebum
E) Zeolite
A) HCl
B) Bleach
C) NaOH
D) Sebum
E) Zeolite

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
40
Which of these interactions between keratin strands is/are broken and reformed to "permanently" curl hair?
I. A salt bridge
II. A hydrogen bond
III. A disulfide linkage
A) I only
B) II only
C) III only
D) I and III
E) I, II, and III
I. A salt bridge
II. A hydrogen bond
III. A disulfide linkage
A) I only
B) II only
C) III only
D) I and III
E) I, II, and III

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
41
When nylon is produced, the small molecule lost during the condensation polymerization process is _____.
A) ammonia
B) hydrogen
C) water
D) carbon dioxide
E) carbon
A) ammonia
B) hydrogen
C) water
D) carbon dioxide
E) carbon

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
42
Which of the following are combined to produce nylon?
A) An alcohol and an amide
B) An alcohol and an amine
C) An acid and an amide
D) An acid and an amine
E) None of these
A) An alcohol and an amide
B) An alcohol and an amine
C) An acid and an amide
D) An acid and an amine
E) None of these

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
43
Which of these products are not an emulsion of oil and water?
I. Creams
II. Lotions
III. Foundations
IV. Perfumes
A) III only
B) IV only
C) I and II
D) III and IV
E) I, II, and III
I. Creams
II. Lotions
III. Foundations
IV. Perfumes
A) III only
B) IV only
C) I and II
D) III and IV
E) I, II, and III

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
44
Which of these substances can be used to lighten hair?
A) Hydrogen peroxide
B) Melanin
C) Phaeomelanin
D) Surfactants
E) Builders
A) Hydrogen peroxide
B) Melanin
C) Phaeomelanin
D) Surfactants
E) Builders

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
45
A polymer formed by attaching monomer units one after another without the loss of any atoms is known as a(n) _____.
A) thermopolymer
B) condensation polymer
C) copolymer
D) addition polymer
E) elastomer
A) thermopolymer
B) condensation polymer
C) copolymer
D) addition polymer
E) elastomer

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
46
The ideal moisture content of skin is _____.
A) 0%
B) 10%
C) 20%
D) 30%
E) 40%
A) 0%
B) 10%
C) 20%
D) 30%
E) 40%

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
47
Which of these substances is a common UV absorber found in sunscreens?
A) Zeolite
B) Melanin
C) Sebum
D) PABA
E) Sodium lauryl sulfate
A) Zeolite
B) Melanin
C) Sebum
D) PABA
E) Sodium lauryl sulfate

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
48
PVC stands for polyvinyl _____.
A) carbon
B) carbonate
C) calcium
D) cations
E) chloride
A) carbon
B) carbonate
C) calcium
D) cations
E) chloride

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
49
Which of these substances are not a component in shampoo?
A) Cationic surfactants
B) Thickeners
C) Fragrances
D) Sodium lauryl sulfate
E) Quaternary ammonium compounds
A) Cationic surfactants
B) Thickeners
C) Fragrances
D) Sodium lauryl sulfate
E) Quaternary ammonium compounds

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
50
Which of these is not a substituted polyethylene polymer?
A) Polypropylene
B) Polyvinyl chloride
C) Polystyrene
D) Polyisobutylene
E) All of these are substituted polyethylenes
A) Polypropylene
B) Polyvinyl chloride
C) Polystyrene
D) Polyisobutylene
E) All of these are substituted polyethylenes

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
51
Which of these classes of polymers describe(s) polyethylene?
I. Thermoplastic
II. Copolymer
III. Addition polymer
A) I only
B) II only
C) I and III
D) II and III
E) I, II, and III
I. Thermoplastic
II. Copolymer
III. Addition polymer
A) I only
B) II only
C) I and III
D) II and III
E) I, II, and III

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
52
Polymers composed of alternating monomer units are known as _____.
A) copolymers
B) condensation polymers
C) thermopolymers
D) addition polymers
E) elastomers
A) copolymers
B) condensation polymers
C) thermopolymers
D) addition polymers
E) elastomers

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
53
A middle note in a perfume results from molecules that are _____ and _____ volatile than molecules that produce top notes in a perfume.
A) smaller; more
B) smaller; less
C) larger; more
D) thicker; more
A) smaller; more
B) smaller; less
C) larger; more
D) thicker; more

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
54
Which of these statements about deodorants is incorrect ?
A) Deodorants contain antibacterial substances.
B) Deodorants function by killing the bacteria that cause odor.
C) Deodorants reduce the amount of perspiration that a sweat gland produces.
D) Deodorants are designed to mask or eliminate body odor.
E) Deodorants mostly contain the chemical compound called triclosan.
A) Deodorants contain antibacterial substances.
B) Deodorants function by killing the bacteria that cause odor.
C) Deodorants reduce the amount of perspiration that a sweat gland produces.
D) Deodorants are designed to mask or eliminate body odor.
E) Deodorants mostly contain the chemical compound called triclosan.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
55
Which two substances are responsible for the natural color of hair?
A) Sebum and melanin
B) Sebum and peroxides
C) Melanin and phaeomelanin
D) Anionic surfactants and peroxides
E) Ammonium compounds and sulfide compounds
A) Sebum and melanin
B) Sebum and peroxides
C) Melanin and phaeomelanin
D) Anionic surfactants and peroxides
E) Ammonium compounds and sulfide compounds

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
56
Which of the following is most likely to be found in the monomer of an addition polymer?
A) An alcohol group
B) A carbon - carbon double bond
C) A nitrogen atom
D) A carbon - oxygen double bond
E) None of these
A) An alcohol group
B) A carbon - carbon double bond
C) A nitrogen atom
D) A carbon - oxygen double bond
E) None of these

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
57
Polymers that produce water as a byproduct during their formation are known as _____.
A) copolymers
B) condensation polymers
C) thermopolymers
D) addition polymers
E) elastomers
A) copolymers
B) condensation polymers
C) thermopolymers
D) addition polymers
E) elastomers

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
58
Small volatile molecules are responsible for the scent in the_____ note of a perfume.
A) top
B) middle
C) bottom
D) lower
A) top
B) middle
C) bottom
D) lower

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
59
How many chemical receptors does the human nose contain?
A) 50
B) 50,000
C) 50,000,000
D) 50,000,000,000
E) 50,000,000,000,000
A) 50
B) 50,000
C) 50,000,000
D) 50,000,000,000
E) 50,000,000,000,000

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
60
Which of these substances are the primary component in conditioners?
A) Anionic surfactants
B) Cationic surfactants
C) Fragrances
D) Nonionic surfactants
E) Sodium lauryl sulfates
A) Anionic surfactants
B) Cationic surfactants
C) Fragrances
D) Nonionic surfactants
E) Sodium lauryl sulfates

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
61
A polymer that softens when heated or hardens when cooled is known as a(n) _____.
A) copolymer
B) thermoplastic
C) condensation polymer
D) addition polymer
E) elastomer
A) copolymer
B) thermoplastic
C) condensation polymer
D) addition polymer
E) elastomer

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
62
Brighteners absorb _____ light and emit _____ light.
A) visible; ultraviolet
B) visible; infrared
C) ultraviolet; infrared
D) infrared; microwave
E) ultraviolet; visible
A) visible; ultraviolet
B) visible; infrared
C) ultraviolet; infrared
D) infrared; microwave
E) ultraviolet; visible

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
63
LAS detergents decompose over time forming _____, _____, and H2O.
A) Na+; NO3−
B) CH4; Ca2+
C) CO2; SO42−
D) NH3; SO3−
E) Mg2+; CO
A) Na+; NO3−
B) CH4; Ca2+
C) CO2; SO42−
D) NH3; SO3−
E) Mg2+; CO

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
64
Which of these processes is used to make rubber more elastic?
A) Condensation
B) Reduction
C) Addition
D) Vulcanization
E) Sublimation
A) Condensation
B) Reduction
C) Addition
D) Vulcanization
E) Sublimation

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
65
Which of the following statements about the pH of hair products holds true?
A) It should be strongly acidic.
B) It should be strongly alkaline.
C) It should be neutral to slightly alkaline.
D) It should be neutral to slightly acidic
E) It should be slightly alkaline.
A) It should be strongly acidic.
B) It should be strongly alkaline.
C) It should be neutral to slightly alkaline.
D) It should be neutral to slightly acidic
E) It should be slightly alkaline.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
66
Which of the following is/are true of colloidal solutions?
I. Groups of molecules clump together in colloidal solutions to form small particles.
II. A laser beam cannot be seen when it is passed through colloidal solutions.
III. A laser beam can be seen clearly when it is passed through colloidal solutions.
IV. Incident light passes through colloidal solutions without scattering/
A) II only
B) I and III
C) IV only
D) I and II
E) II and IV
I. Groups of molecules clump together in colloidal solutions to form small particles.
II. A laser beam cannot be seen when it is passed through colloidal solutions.
III. A laser beam can be seen clearly when it is passed through colloidal solutions.
IV. Incident light passes through colloidal solutions without scattering/
A) II only
B) I and III
C) IV only
D) I and II
E) II and IV

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
67
Which of these classes of polymers best describes rubber?
A) Copolymer
B) Condensation polymer
C) Thermopolymer
D) Addition polymer
E) Elastomer
A) Copolymer
B) Condensation polymer
C) Thermopolymer
D) Addition polymer
E) Elastomer

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
68
Which of the following type of hair will have the lowest amount of melanin?
A) Black hair
B) White hair
C) Red hair
D) Brown hair
E) Dark blonde hair
A) Black hair
B) White hair
C) Red hair
D) Brown hair
E) Dark blonde hair

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck



