Deck 13: Condensation and Hydrolysis Reactions
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/72
Play
Full screen (f)
Deck 13: Condensation and Hydrolysis Reactions
1
An amine is a stronger base than an amide.
True
2
A carbohydrate is a polymer composed of simple sugar units.
True
3
If two molecules of a 1-butanol undergo a condensation reaction, the product of the reaction must be dibutyl ether.
True
4
The bond enclosed in the circle forms during an esterification reaction. 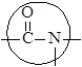


Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
5
Consider the following reaction. 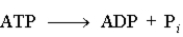 This reaction is a hydrolysis reaction.
This reaction is a hydrolysis reaction.
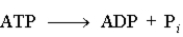 This reaction is a hydrolysis reaction.
This reaction is a hydrolysis reaction.
Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
6
Like a thioester, an amide contains a carbonyl group.

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
7
The structure for dipropyl ether is: 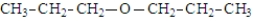
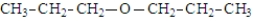

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
8
Consider the following reaction.  If the energy used for the reaction is obtained from ATP, the reaction will go to completion.
If the energy used for the reaction is obtained from ATP, the reaction will go to completion.
 If the energy used for the reaction is obtained from ATP, the reaction will go to completion.
If the energy used for the reaction is obtained from ATP, the reaction will go to completion.
Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
9
When three molecules of the amino acid alanine, shown below, react 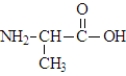 the polymer segment formed would be:
the polymer segment formed would be: 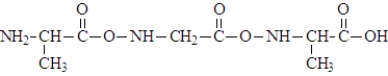
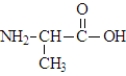 the polymer segment formed would be:
the polymer segment formed would be: 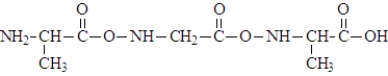

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
10
Amidation reactions would be part of a catabolic pathway.

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
11
ATP provides the metabolic link between anabolism and catabolism.

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
12
pH has little effect on the products of hydrolysis reactions.

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
13
The following type of reaction is utilized by biological systems in obtaining large amounts of energy from food. 


Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
14
When the following "protein" formed, five molecules of water were produced. 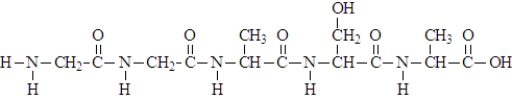
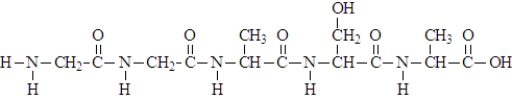

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
15
The formation of a copolymer requires that three different molecules react to form the polymer.

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
16
During dehydration reactions, the hydrogen atom maybe removed from an oxygen atom whereas in a condensation reaction the hydrogen is removed from a carbon atom.

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
17
Consider the structure of propyl myristate. 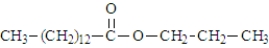 Saponification using KOH would produce the "soap" shown below.
Saponification using KOH would produce the "soap" shown below. 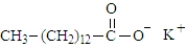
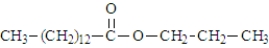 Saponification using KOH would produce the "soap" shown below.
Saponification using KOH would produce the "soap" shown below. 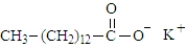

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
18
The following molecule was formed from the reaction of four alcohol molecules. 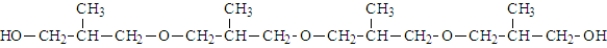
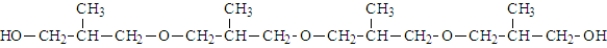

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
19
The following is an example of a phosphodiester. 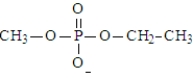
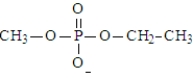

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
20
Alkaline phosphatase is an enzyme involved in some of the hydrolysis reactions in the human body.

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
21
The following reaction would be coupled to anabolic reactions. ATP  ADP + P
ADP + P
 ADP + P
ADP + P
Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
22
The following reaction could be used to form ATP form ADP. 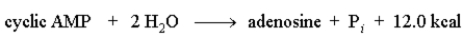
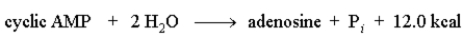

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
23
When an alcohol undergoes a condensation reaction, which of the following class of compounds might form?
A) ether
B) ketone
C) aldehyde
D) alkene
A) ether
B) ketone
C) aldehyde
D) alkene

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
24
The name of the following compound is: 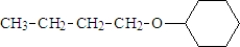
A) butyl hexyl ether
B) decyl ether
C) cyclohexyl butyl ether
D) butyl cyclohexyl ether
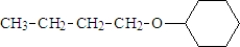
A) butyl hexyl ether
B) decyl ether
C) cyclohexyl butyl ether
D) butyl cyclohexyl ether

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
25
Consider the polymer formed from equal numbers of the two amino acids glycine and alanine. Which of the following is not a property of this polymer?
A) copolymer
B) contains amide bonds
C) formed by an esterification reaction
D) might be part of a protein
A) copolymer
B) contains amide bonds
C) formed by an esterification reaction
D) might be part of a protein

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
26
What is the organic product of the reaction of 1-butanol with propanoic acid?
A)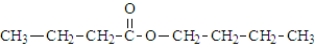
B)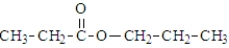
C)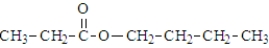
D)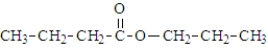
A)
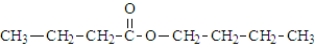
B)
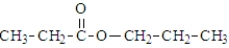
C)
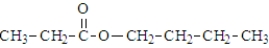
D)
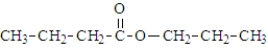

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
27
If oxalic acid which contains two carboxylic acids groups and ethylene diamine which contains two amine groups react, the type of bond formed in the polymers is
A) an ester.
B) an ether.
C) an amide.
D) a phosphoester.
A) an ester.
B) an ether.
C) an amide.
D) a phosphoester.

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
28
Consider the following structure: 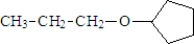 This compound could be formed by the reaction of:
This compound could be formed by the reaction of:
A) 2-propanol and cyclopentanol
B) 1-propanol and cyclopentanol
C) 2-propanol and cyclohexanol
D) 1-propanol and cyclohexanol
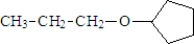 This compound could be formed by the reaction of:
This compound could be formed by the reaction of:A) 2-propanol and cyclopentanol
B) 1-propanol and cyclopentanol
C) 2-propanol and cyclohexanol
D) 1-propanol and cyclohexanol

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
29
Which of the following functional groups does not contain a carbonyl group?
A) ester
B) amide
C) ether
D) All contain a carbonyl group.
A) ester
B) amide
C) ether
D) All contain a carbonyl group.

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
30
Predict the products of the following reaction. Assume the pH is 7. 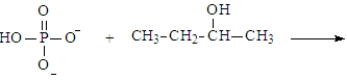
A)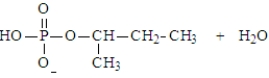
B)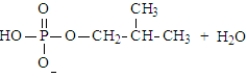
C)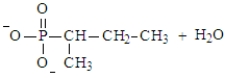
D)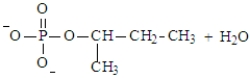
E) Both a and d could form at pH 7.
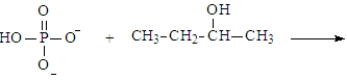
A)
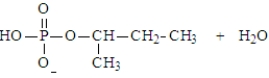
B)
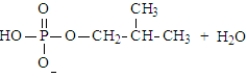
C)
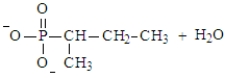
D)
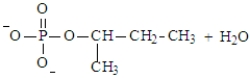
E) Both a and d could form at pH 7.

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
31
In addition to water, what is(are) the product(s) of the following reaction? 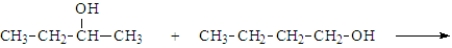
A)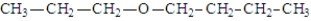
B)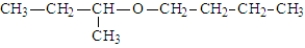
C)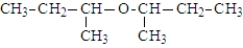
D)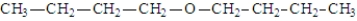
E) All of the above are possible products.
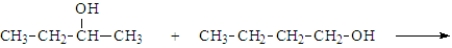
A)
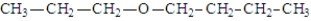
B)
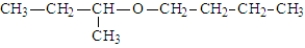
C)
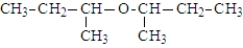
D)
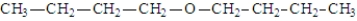
E) All of the above are possible products.

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
32
In the formation of a polymer, which of the following reaction types would not be involved?
A) hydrolysis
B) condensation
C) amidation
D) esterification
A) hydrolysis
B) condensation
C) amidation
D) esterification

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
33
Which of the following functional groups is not produced from a condensation reaction?
A) alcohol
B) amide
C) ester
D) ether
E) phosphoester
A) alcohol
B) amide
C) ester
D) ether
E) phosphoester

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
34
When a molecule with the following structure reacts with itself to from polymer, the type of functional group formed is a(n): 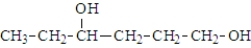
A) ether
B) ester
C) amide
D) phosphoester
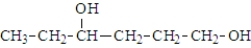
A) ether
B) ester
C) amide
D) phosphoester

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
35
All of the bonds that hold the units together to form a polymer are:
A) covalent bonds.
B) ionic bonds.
C) hydrogen bonds.
D) nonpolar bonds.
A) covalent bonds.
B) ionic bonds.
C) hydrogen bonds.
D) nonpolar bonds.

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
36
Lactic acid fermentation supplies less energy than glucose oxidation.

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
37
When the following segment of a polymer formed, how many different amino acids were used? 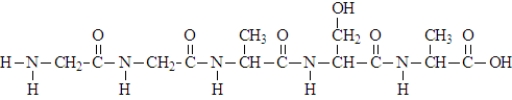
A) 1
B) 2
C) 3
D) 4
E) 5
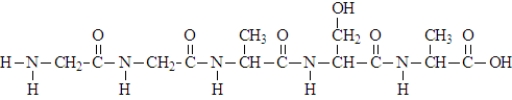
A) 1
B) 2
C) 3
D) 4
E) 5

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
38
During catabolism as nutrients are burned, the carbon atoms of the organic molecules end up as carbon dioxide and the hydrogen atoms end up as H+.

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
39
Consider the image shown below. The bond indicated by the arrow represents the bond that is broken when ADP forms . 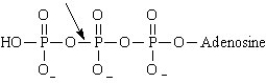
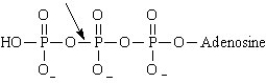

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
40
What is the organic product of the following reaction if carried out at 110 °C? 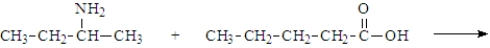
A)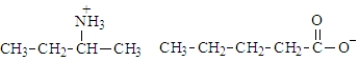
B)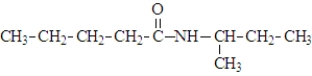
C)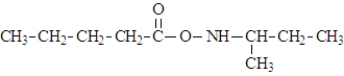
D)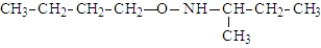
E)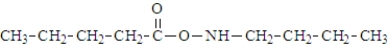
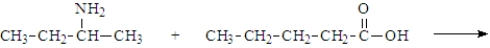
A)
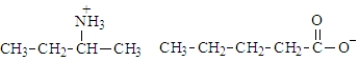
B)
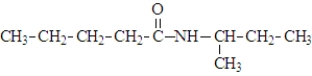
C)
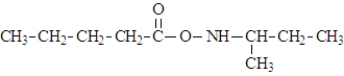
D)
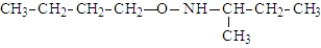
E)
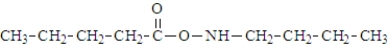

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
41
The following represent several common nonsteriodal, anti-inflammatory drugs (NSAIDs) available over the counter. 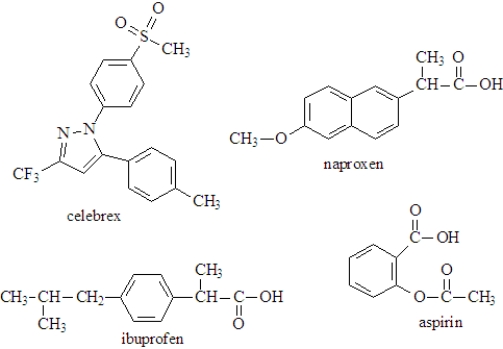 Fill the blanks with the appropriate term from the list below. More than one answer may be correct.
Fill the blanks with the appropriate term from the list below. More than one answer may be correct.
aspirin
ibuprofen
_____________________would not increase the H3O+ concentration in the stomach.
 Fill the blanks with the appropriate term from the list below. More than one answer may be correct.
Fill the blanks with the appropriate term from the list below. More than one answer may be correct. aspirin
ibuprofen
_____________________would not increase the H3O+ concentration in the stomach.

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
42
What was the structure of the repeating unit in this polymer? 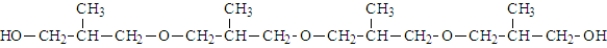
A)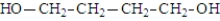
B)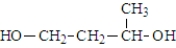
C)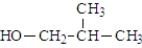
D)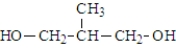
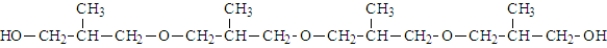
A)
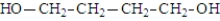
B)
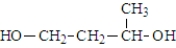
C)
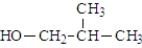
D)
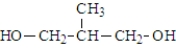

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
43
Consider the following compounds. 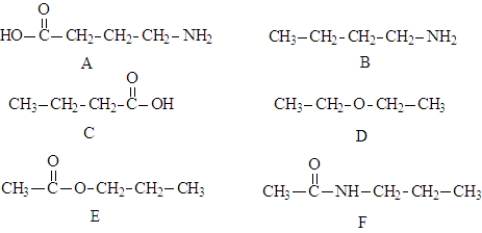 These compound may be involved in the following types of reaction.
These compound may be involved in the following types of reaction.
amidation
esterification
saponification
condensation
hydrolysis
polymerization
Fill in the blanks with the appropriate term from the list. More than one term may be correct.
Molecules of structure A could react with other molecules of A in a(n) ________________reaction to produce a very large molecule.
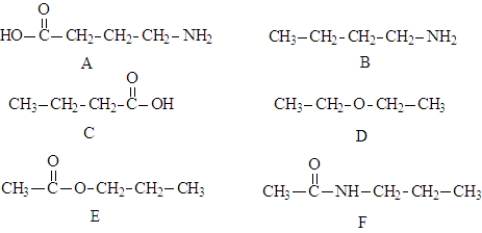 These compound may be involved in the following types of reaction.
These compound may be involved in the following types of reaction. amidation
esterification
saponification
condensation
hydrolysis
polymerization
Fill in the blanks with the appropriate term from the list. More than one term may be correct.
Molecules of structure A could react with other molecules of A in a(n) ________________reaction to produce a very large molecule.

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
44
Upon hydrolysis, a thioester will produce:
A) an alcohol and an acid
B) an amine and an acid
C) a thiol and an acid
D) two alcohols
E) a phosphate and an alcohol
A) an alcohol and an acid
B) an amine and an acid
C) a thiol and an acid
D) two alcohols
E) a phosphate and an alcohol

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
45
What are the products of the hydrolysis of the following compound at physiological pH? 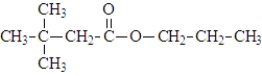
A)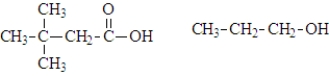
B)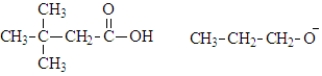
C)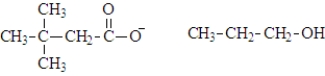
D)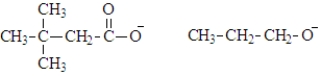
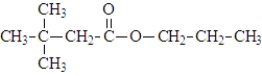
A)
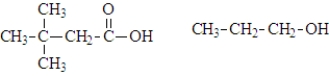
B)
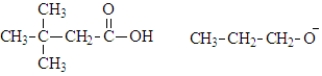
C)
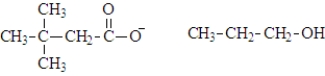
D)
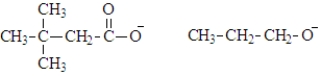

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
46
What are the products formed when the following substance undergoes hydrolysis? 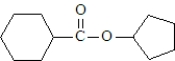
A)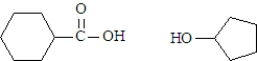
B)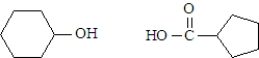
C)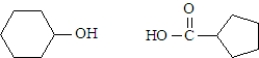
D)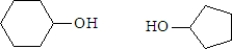
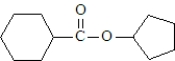
A)
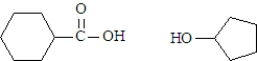
B)
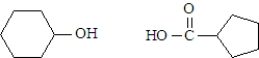
C)
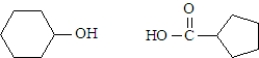
D)
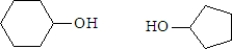

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
47
The hydrolysis of the following compounds produce energy as given in the parenthesis. Which of these reactions can drive the formation of ATP?
A) glycerol-1-phosphate (2.2 kcal/mol)
B) fructose-6-phosphate (3.8 kcal/mol)
C) glucose-6-phosphate (3.3 kcal/mol)
D) None of the reactions evolves enough energy.
A) glycerol-1-phosphate (2.2 kcal/mol)
B) fructose-6-phosphate (3.8 kcal/mol)
C) glucose-6-phosphate (3.3 kcal/mol)
D) None of the reactions evolves enough energy.

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
48
The following represent several common nonsteriodal, anti-inflammatory drugs (NSAIDs) available over the counter.  Fill the blanks with the appropriate term from the list below. More than one answer may be correct.
Fill the blanks with the appropriate term from the list below. More than one answer may be correct.
aspirin
ibuprofen
If taken together, ibuprofen could under go an acid-base reaction with __________________.
 Fill the blanks with the appropriate term from the list below. More than one answer may be correct.
Fill the blanks with the appropriate term from the list below. More than one answer may be correct. aspirin
ibuprofen
If taken together, ibuprofen could under go an acid-base reaction with __________________.

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
49
The following represent several common nonsteriodal, anti-inflammatory drugs (NSAIDs) available over the counter.  Fill the blanks with the appropriate term from the list below. More than one answer may be correct.
Fill the blanks with the appropriate term from the list below. More than one answer may be correct.
aspirin
ibuprofen
At physiological pH, ___________________ would exist as a negative ion.
 Fill the blanks with the appropriate term from the list below. More than one answer may be correct.
Fill the blanks with the appropriate term from the list below. More than one answer may be correct. aspirin
ibuprofen
At physiological pH, ___________________ would exist as a negative ion.

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
50
ATP plays a role in all of the following processes except which one?
A) phosphorylation reactions
B) exothermic reactions
C) membrane transport
D) muscle contraction
A) phosphorylation reactions
B) exothermic reactions
C) membrane transport
D) muscle contraction

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
51
What are the products of the hydrolysis of the following compound when carried out at a physiological pH? 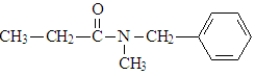
A)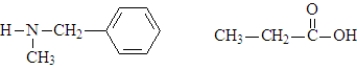
B)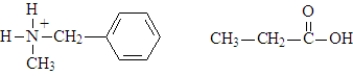
C)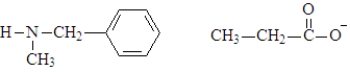
D)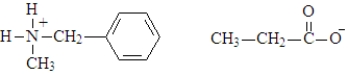
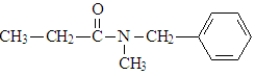
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
52
Which of the following would produce ethanol on hydrolysis?
A)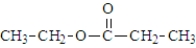
B)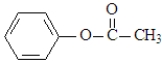
C)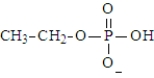
D)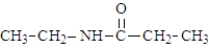
E) both a and c
F)All produce ethanol on hydrolysis.
A)
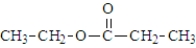
B)

C)
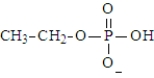
D)
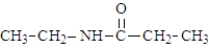
E) both a and c
F)All produce ethanol on hydrolysis.

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
53
The following represent several common nonsteriodal, anti-inflammatory drugs (NSAIDs) available over the counter.  Fill the blanks with the appropriate term from the list below. More than one answer may be correct.
Fill the blanks with the appropriate term from the list below. More than one answer may be correct.
aspirin
ibuprofen
_________________contains an ether functional group.
 Fill the blanks with the appropriate term from the list below. More than one answer may be correct.
Fill the blanks with the appropriate term from the list below. More than one answer may be correct. aspirin
ibuprofen
_________________contains an ether functional group.

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
54
The following represent several common nonsteriodal, anti-inflammatory drugs (NSAIDs) available over the counter.  Fill the blanks with the appropriate term from the list below. More than one answer may be correct.
Fill the blanks with the appropriate term from the list below. More than one answer may be correct.
aspirin
ibuprofen
______________________ can function as a proton acceptor.
 Fill the blanks with the appropriate term from the list below. More than one answer may be correct.
Fill the blanks with the appropriate term from the list below. More than one answer may be correct. aspirin
ibuprofen
______________________ can function as a proton acceptor.

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
55
Malonic acid and ethylene glycol react.  What is the product of the reaction of two molecules of malonic acid and one molecule of ethylene glycol?
What is the product of the reaction of two molecules of malonic acid and one molecule of ethylene glycol?
A)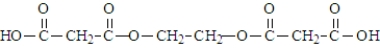
B)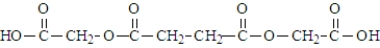
C)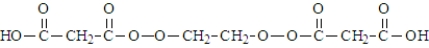
D)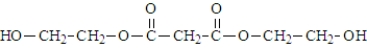
 What is the product of the reaction of two molecules of malonic acid and one molecule of ethylene glycol?
What is the product of the reaction of two molecules of malonic acid and one molecule of ethylene glycol?A)
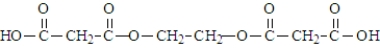
B)
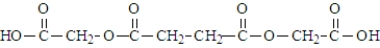
C)
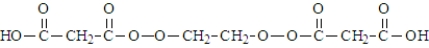
D)
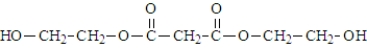

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
56
The following represent several common nonsteriodal, anti-inflammatory drugs (NSAIDs) available over the counter.  Fill the blanks with the appropriate term from the list below. More than one answer may be correct.
Fill the blanks with the appropriate term from the list below. More than one answer may be correct.
aspirin
ibuprofen
___________________ has the potential to be administered as a hydrochloride salt.
 Fill the blanks with the appropriate term from the list below. More than one answer may be correct.
Fill the blanks with the appropriate term from the list below. More than one answer may be correct. aspirin
ibuprofen
___________________ has the potential to be administered as a hydrochloride salt.

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
57
Consider the following "polymer". 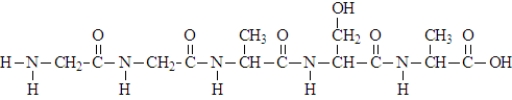 Which of the following characterizes this structure?
Which of the following characterizes this structure?
A) copolymer
B) formed by an amidation reaction
C) formed by a hydrolysis reaction
D) formed by an esterification reaction
E) both a and b
F) both a and c
G) both a and d
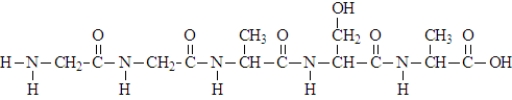 Which of the following characterizes this structure?
Which of the following characterizes this structure?A) copolymer
B) formed by an amidation reaction
C) formed by a hydrolysis reaction
D) formed by an esterification reaction
E) both a and b
F) both a and c
G) both a and d

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
58
The following reaction is driven by the breakdown of ATP, what is the single chemical equation that combines these two reactions? 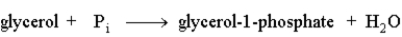

A)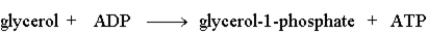
B)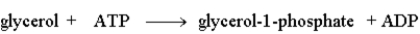
C)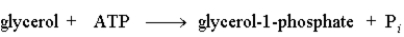
D)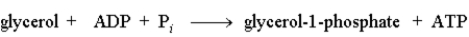
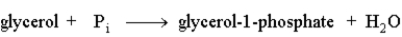

A)
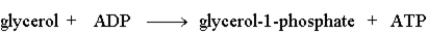
B)
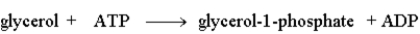
C)
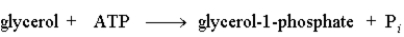
D)
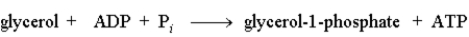

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
59
When ethyl propyl ether is hydrolyzed, which of the following products form?
A) propanol and ethanol
B) propanoic acid and ethanoic acid
C) propanol and ethanoic acid
D) ethanol and propanoic acid
A) propanol and ethanol
B) propanoic acid and ethanoic acid
C) propanol and ethanoic acid
D) ethanol and propanoic acid

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
60
The following represent several common nonsteriodal, anti-inflammatory drugs (NSAIDs) available over the counter.  Fill the blanks with the appropriate term from the list below. More than one answer may be correct.
Fill the blanks with the appropriate term from the list below. More than one answer may be correct.
aspirin
ibuprofen
________________________could be classified as an ester.
 Fill the blanks with the appropriate term from the list below. More than one answer may be correct.
Fill the blanks with the appropriate term from the list below. More than one answer may be correct. aspirin
ibuprofen
________________________could be classified as an ester.

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
61
What mass in grams of ATP (C10H16N5O13P3) must be used to supply 4.26 kcal to the cells?

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
62
At her aerobics class, Rachelle notices a dull pain in her lower leg. When the pain does not go away after a couple of days, Rachelle decides to have her doctor check her leg. The doctor orders an X-ray, but the X-ray image shows no evidence of injury to the bone. To help him diagnose the cause of Rachelle's pain, the doctor orders a blood test to determine Rachelle's level of alkaline phosphatase (ALP).
a.) Alkaline phosphatase is an enzyme commonly found in ________________.
b.) Why would Rachelle's alkaline phosphatase level be significantly higher than normal given her stress fracture?
a.) Alkaline phosphatase is an enzyme commonly found in ________________.
b.) Why would Rachelle's alkaline phosphatase level be significantly higher than normal given her stress fracture?

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
63
Draw the structure of the product of the condensation reaction of the ethanol with benzoic acid (shown below). 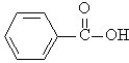


Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
64
Draw structure of the "polymer" of the condensation reaction of four molecules of ethylene glycol (HO-CH2-CH2-OH).

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
65
Draw the structure of the product of the reaction of cyclohexanol and 2-pentanol.

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
66
Draw the structure of the product of the condensation reaction of the following substances. acetic acid and diethylamine

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
67
How much energy in kilocalories is needed to convert 1.75 mol of ADP to ATP?

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
68
Consider the following compounds. 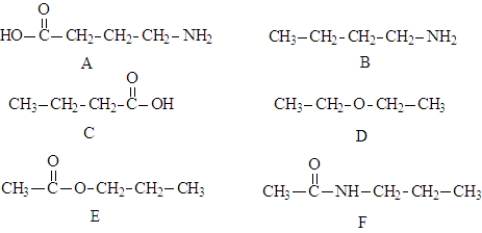 These compound may be involved in the following types of reaction.
These compound may be involved in the following types of reaction.
amidation
esterification
saponification
condensation
hydrolysis
polymerization
Fill in the blanks with the appropriate term from the list. More than one term may be correct.
Structure B and structure C could combine in a(n) __________________ reaction.
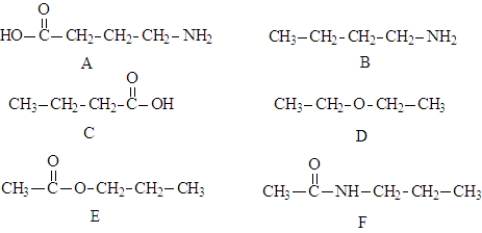 These compound may be involved in the following types of reaction.
These compound may be involved in the following types of reaction. amidation
esterification
saponification
condensation
hydrolysis
polymerization
Fill in the blanks with the appropriate term from the list. More than one term may be correct.
Structure B and structure C could combine in a(n) __________________ reaction.

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
69
Consider the following compounds. 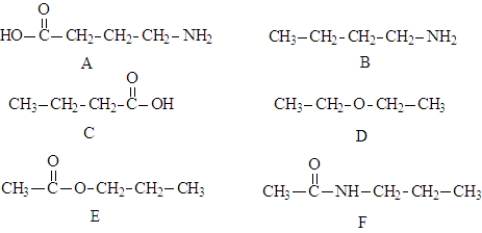 These compound may be involved in the following types of reaction.
These compound may be involved in the following types of reaction.
amidation
esterification
saponification
condensation
hydrolysis
polymerization
Fill in the blanks with the appropriate term from the list. More than one term may be correct.
Structure D is the product of the reaction of two alcohols in a(n) _______________________ reaction.
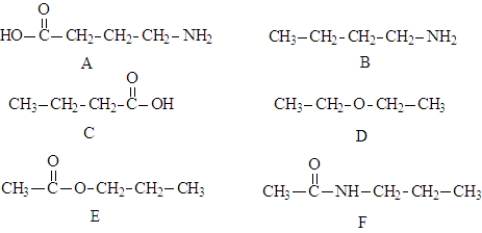 These compound may be involved in the following types of reaction.
These compound may be involved in the following types of reaction. amidation
esterification
saponification
condensation
hydrolysis
polymerization
Fill in the blanks with the appropriate term from the list. More than one term may be correct.
Structure D is the product of the reaction of two alcohols in a(n) _______________________ reaction.

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
70
Draw the structure of the product of the condensation reaction of the phosphate ion with cyclohexanol.

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
71
Consider the following compounds. 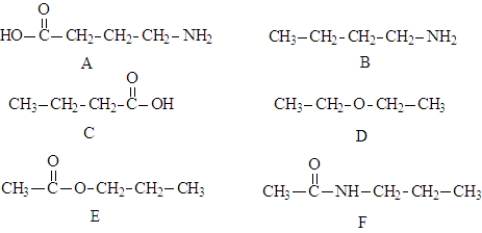 These compound may be involved in the following types of reaction.
These compound may be involved in the following types of reaction.
amidation
esterification
saponification
condensation
hydrolysis
polymerization
Fill in the blanks with the appropriate term from the list. More than one term may be correct.
Molecules of structure E can react with KOH in a(n) ___________________ reaction.
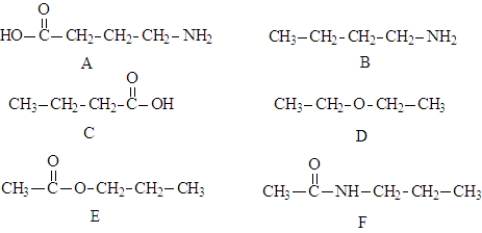 These compound may be involved in the following types of reaction.
These compound may be involved in the following types of reaction. amidation
esterification
saponification
condensation
hydrolysis
polymerization
Fill in the blanks with the appropriate term from the list. More than one term may be correct.
Molecules of structure E can react with KOH in a(n) ___________________ reaction.

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
72
Consider the following compounds. 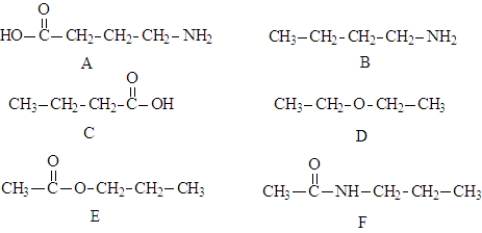 These compound may be involved in the following types of reaction.
These compound may be involved in the following types of reaction.
amidation
esterification
saponification
condensation
hydrolysis
polymerization
Fill in the blanks with the appropriate term from the list. More than one term may be correct.
Structures E and F are the products of a(n) ____________________ reaction.
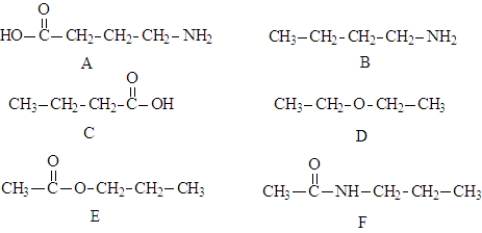 These compound may be involved in the following types of reaction.
These compound may be involved in the following types of reaction. amidation
esterification
saponification
condensation
hydrolysis
polymerization
Fill in the blanks with the appropriate term from the list. More than one term may be correct.
Structures E and F are the products of a(n) ____________________ reaction.

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck



