Deck 8: Molecular Shape and Structure: Life in Three Dimensions
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/21
Play
Full screen (f)
Deck 8: Molecular Shape and Structure: Life in Three Dimensions
1
Which of the following statements is true?
A) The atomic radius is the distance between two covalently bonded atoms of the same element.
B) The atomic radius is half the distance between two covalently bonded atoms of the same element.
C) The atomic radius is the distance between two covalently bonded atoms of different elements.
D) The atomic radius is half the distance between two covalently bonded atoms of different elements.
E) The atomic radius is the distance between two ionically bonded atoms of the same element.
A) The atomic radius is the distance between two covalently bonded atoms of the same element.
B) The atomic radius is half the distance between two covalently bonded atoms of the same element.
C) The atomic radius is the distance between two covalently bonded atoms of different elements.
D) The atomic radius is half the distance between two covalently bonded atoms of different elements.
E) The atomic radius is the distance between two ionically bonded atoms of the same element.
B
2
Place the following bonds in order of relative length, with 1 being the shortest and 3 being the longest.
a. Single bond = 3
b. Double bond = 2
c. Triple bond = 1
b. Double bond = 2
c. Triple bond = 1
3
A tetrahedral geometry typically exhibits which of the following bond angles?
A) 90
B) 180
C) 109.5
D) 106.5
E) 120
A) 90
B) 180
C) 109.5
D) 106.5
E) 120
109.5
4
A trigonal bipyramidal geometry exhibits two characteristic bond angles. Which bond angles are these?
A) 90
B) 109.5
C) 120
D) 180
E) 45
A) 90
B) 109.5
C) 120
D) 180
E) 45

Unlock Deck
Unlock for access to all 21 flashcards in this deck.
Unlock Deck
k this deck
5
Match the number of valence electron pairs with the type of geometry that they typically exhibit.
-Tetrahedral
A) 4
B) 3
C) 2
D) 6
-Tetrahedral
A) 4
B) 3
C) 2
D) 6

Unlock Deck
Unlock for access to all 21 flashcards in this deck.
Unlock Deck
k this deck
6
Match the number of valence electron pairs with the type of geometry that they typically exhibit.
-Trigonal planar
A) 5
B) 4
C) 3
D) 7
-Trigonal planar
A) 5
B) 4
C) 3
D) 7

Unlock Deck
Unlock for access to all 21 flashcards in this deck.
Unlock Deck
k this deck
7
Match the number of valence electron pairs with the type of geometry that they typically exhibit.
-Linear
A) 6
B) 5
C) 4
D) 8
-Linear
A) 6
B) 5
C) 4
D) 8

Unlock Deck
Unlock for access to all 21 flashcards in this deck.
Unlock Deck
k this deck
8
Match the number of valence electron pairs with the type of geometry that they typically exhibit.
-Octahedral
A) 7
B) 6
C) 5
D) 9
-Octahedral
A) 7
B) 6
C) 5
D) 9

Unlock Deck
Unlock for access to all 21 flashcards in this deck.
Unlock Deck
k this deck
9
Match the following geometries with the type of hybridized orbital that exhibits each geometry.
-Linear
A) sp
B) sp2
C) sp3
-Linear
A) sp
B) sp2
C) sp3

Unlock Deck
Unlock for access to all 21 flashcards in this deck.
Unlock Deck
k this deck
10
Match the following geometries with the type of hybridized orbital that exhibits each geometry.
-Trigonal planar
A) sp
B) sp2
C) sp3
-Trigonal planar
A) sp
B) sp2
C) sp3

Unlock Deck
Unlock for access to all 21 flashcards in this deck.
Unlock Deck
k this deck
11
Match the following geometries with the type of hybridized orbital that exhibits each geometry.
-Tetrahedral
A) sp
B) sp2
C) sp3
-Tetrahedral
A) sp
B) sp2
C) sp3

Unlock Deck
Unlock for access to all 21 flashcards in this deck.
Unlock Deck
k this deck
12
The 'R' in VSEPR theory denotes which the following words?
A) Resonance
B) Repulsion
C) Redistribution
D) Radius
E) Relationship
A) Resonance
B) Repulsion
C) Redistribution
D) Radius
E) Relationship

Unlock Deck
Unlock for access to all 21 flashcards in this deck.
Unlock Deck
k this deck
13
Which of the following are taken into account when applying VSEPR theory? Select all that apply.
A) Sigma-bonding pairs
B) Pi-bonding pairs
C) Anti-bonding pairs
D) Non-bonding pairs
E) Unpaired valence electrons
A) Sigma-bonding pairs
B) Pi-bonding pairs
C) Anti-bonding pairs
D) Non-bonding pairs
E) Unpaired valence electrons

Unlock Deck
Unlock for access to all 21 flashcards in this deck.
Unlock Deck
k this deck
14
Which of the following statements about non-bonding pairs is true?
A) Non-bonding pairs increase the typical bond angles associated with a particular geometry as predicted by VSEPR theory.
B) Non-bonding pairs decrease the typical bond angles associated with a particular geometry as predicted by VSEPR theory.
C) Non-bonding pairs have no effect on the typical bond angles associated with a particular geometry as predicted by VSEPR theory.
A) Non-bonding pairs increase the typical bond angles associated with a particular geometry as predicted by VSEPR theory.
B) Non-bonding pairs decrease the typical bond angles associated with a particular geometry as predicted by VSEPR theory.
C) Non-bonding pairs have no effect on the typical bond angles associated with a particular geometry as predicted by VSEPR theory.

Unlock Deck
Unlock for access to all 21 flashcards in this deck.
Unlock Deck
k this deck
15
Bond rotation is possible about which of the following types of covalent bond?
A) Single bonds only
B) Double bonds only
C) Triple bonds only
D) None of these
E) Single bonds and double bonds
A) Single bonds only
B) Double bonds only
C) Triple bonds only
D) None of these
E) Single bonds and double bonds

Unlock Deck
Unlock for access to all 21 flashcards in this deck.
Unlock Deck
k this deck
16
Which of the following statements are false? Select all that apply.
A) sp3 hybridization generates three sp3 hybrid orbitals.
B) sp2 hybridization leaves one 2p orbital unhybridized.
C) sp hybridization results from the hybridization of two 2p orbitals.
D) Hybridization occurs between atomics orbitals of the lowest-possible energy.
E) Hybridization only occurs in molecules possessing single covalent bonds.
A) sp3 hybridization generates three sp3 hybrid orbitals.
B) sp2 hybridization leaves one 2p orbital unhybridized.
C) sp hybridization results from the hybridization of two 2p orbitals.
D) Hybridization occurs between atomics orbitals of the lowest-possible energy.
E) Hybridization only occurs in molecules possessing single covalent bonds.

Unlock Deck
Unlock for access to all 21 flashcards in this deck.
Unlock Deck
k this deck
17
Rotation is possible about which of the following bonds along the backbone of a polypeptide chain? Select any that apply.
A) C -C
B) C -N
C) C-N (peptide)
A) C -C
B) C -N
C) C-N (peptide)

Unlock Deck
Unlock for access to all 21 flashcards in this deck.
Unlock Deck
k this deck
18
Propane has the structural formula shown below. How many different conformations can propane exist in?
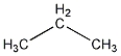
A) One
B) Two
C) Three
D) Four
E) An infinite number

A) One
B) Two
C) Three
D) Four
E) An infinite number

Unlock Deck
Unlock for access to all 21 flashcards in this deck.
Unlock Deck
k this deck
19
A molecule of water adopts which one of the following geometries?
A) Linear
B) Trigonal planar
C) Octahedral
D) Tetrahedral
E) Trigonal bipyramidal
A) Linear
B) Trigonal planar
C) Octahedral
D) Tetrahedral
E) Trigonal bipyramidal

Unlock Deck
Unlock for access to all 21 flashcards in this deck.
Unlock Deck
k this deck
20
Carbon, hydrogen, nitrogen, and oxygen have the following atomic radii (in picometres): C = 77; H = 37; N = 74; O = 73. Which of the following covalent bonds is the shortest?
A) C-H
B) N-H
C) C-O
D) C-C
E) O-H
A) C-H
B) N-H
C) C-O
D) C-C
E) O-H

Unlock Deck
Unlock for access to all 21 flashcards in this deck.
Unlock Deck
k this deck
21
Carbon, hydrogen, nitrogen, and oxygen have the following atomic radii (in picometres): C = 77; H = 37; O = 73. Which of the following covalent bonds is the longest?
A) C-C
B) C-O
C) C=C
D) C-H
E) C C
A) C-C
B) C-O
C) C=C
D) C-H
E) C C

Unlock Deck
Unlock for access to all 21 flashcards in this deck.
Unlock Deck
k this deck



