Deck 5: Moles, Concentrations, and Dilutions: Making Sense of Chemical Numbers
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/22
Play
Full screen (f)
Deck 5: Moles, Concentrations, and Dilutions: Making Sense of Chemical Numbers
1
You can have a mole of which of the following? Select any that apply.
A) Atoms
B) Ions
C) Molecules
D) Electrons
E) . Protons
A) Atoms
B) Ions
C) Molecules
D) Electrons
E) . Protons
A,B,C,D,E
2
Which of the following numbers represents the Avogadro constant?
A) 6* 1023
B) 1*1023
C) 6 *102
D) 23
E) 623
A) 6* 1023
B) 1*1023
C) 6 *102
D) 23
E) 623
6* 1023
3
Which of the following is the correct mass of one mole of 12C?
A) 1 g
B) 12.5 g
C) 6 * 1023 g
D) 72 g
E) 12 g
A) 1 g
B) 12.5 g
C) 6 * 1023 g
D) 72 g
E) 12 g
12 g
4
The molar mass of a compound has which of the following units?
A) mol
B) g mol-1
C) G
D) amu
E) mol g-1
A) mol
B) g mol-1
C) G
D) amu
E) mol g-1

Unlock Deck
Unlock for access to all 22 flashcards in this deck.
Unlock Deck
k this deck
5
Which of the following has the greatest mass, noting that the molar mass of carbon is 12; the molar mass of oxygen is 16; the molar mass of hydrogen is 1; and the molar mass of nitrogen is 15?
A) 10 mol H2
B) 2 mol CH4
C) 5 mol H2O
D) 3 mol NH3
E) 5 mol O2
A) 10 mol H2
B) 2 mol CH4
C) 5 mol H2O
D) 3 mol NH3
E) 5 mol O2

Unlock Deck
Unlock for access to all 22 flashcards in this deck.
Unlock Deck
k this deck
6
How many moles are there in 30 g of C?
A) 360 mol
B) 30 mol
C) 2.5 mol
D) 0.4 mol
E) 12 mol
A) 360 mol
B) 30 mol
C) 2.5 mol
D) 0.4 mol
E) 12 mol

Unlock Deck
Unlock for access to all 22 flashcards in this deck.
Unlock Deck
k this deck
7
What is the mass of 1.5 mol CH3OH?
A) 48 g
B) 21.333 g
C) 32 g
D) 0.05 g
E) 1.5 g
A) 48 g
B) 21.333 g
C) 32 g
D) 0.05 g
E) 1.5 g

Unlock Deck
Unlock for access to all 22 flashcards in this deck.
Unlock Deck
k this deck
8
2 moles of the amino acid glycine have a mass of 152
A) 152 g mol-1
B) 0.013 g mol-1
C) 2 g mol-1
D) 304 g mol-1
E) 76 g mol-1
A) 152 g mol-1
B) 0.013 g mol-1
C) 2 g mol-1
D) 304 g mol-1
E) 76 g mol-1

Unlock Deck
Unlock for access to all 22 flashcards in this deck.
Unlock Deck
k this deck
9
How many moles are there in 121.6 g of glycine?
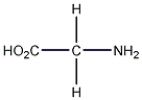
A) 1.6 mol
B) 0.625 mol
C) 121.6 mol
D) 8.76* 103 mol
E) 76 mol

A) 1.6 mol
B) 0.625 mol
C) 121.6 mol
D) 8.76* 103 mol
E) 76 mol

Unlock Deck
Unlock for access to all 22 flashcards in this deck.
Unlock Deck
k this deck
10
Which of the following two quantities are identical?
A) 1 dm3
B) 1 m3
C) 1 L
D) 100 mL
E) 1 cm3
A) 1 dm3
B) 1 m3
C) 1 L
D) 100 mL
E) 1 cm3

Unlock Deck
Unlock for access to all 22 flashcards in this deck.
Unlock Deck
k this deck
11
The concentration of a substance is defined as the number of moles in what volume?
A) 1 mL
B) 1 cm3
C) 1 L
D) 1 m3
E) 1 L
A) 1 mL
B) 1 cm3
C) 1 L
D) 1 m3
E) 1 L

Unlock Deck
Unlock for access to all 22 flashcards in this deck.
Unlock Deck
k this deck
12
What amount of hydrogen chloride is present in 500 mL of a solution with a concentration of 0.5 mol L-1?
A) 0.5 mol
B) 250 mol
C) 1000 mol
D) 0.001 mol
E) 0.25 mol
A) 0.5 mol
B) 250 mol
C) 1000 mol
D) 0.001 mol
E) 0.25 mol

Unlock Deck
Unlock for access to all 22 flashcards in this deck.
Unlock Deck
k this deck
13
How many moles are present in 10 mL of a sodium chloride (salt) solution, with a concentration of 2 mol L-1?
A) 0.02 mol
B) 20 mol
C) 0.2 mol
D) 5 mol
E) 0.1 mol
A) 0.02 mol
B) 20 mol
C) 0.2 mol
D) 5 mol
E) 0.1 mol

Unlock Deck
Unlock for access to all 22 flashcards in this deck.
Unlock Deck
k this deck
14
What is the concentration of 100 mL of a solution containing 2.5 moles of glucose?
A) 40 mol L-1
B) 0.025 mol L-1
C) 25 mol L-1
D) 250 mol L-1
E) 0.25 mol L-1
A) 40 mol L-1
B) 0.025 mol L-1
C) 25 mol L-1
D) 250 mol L-1
E) 0.25 mol L-1

Unlock Deck
Unlock for access to all 22 flashcards in this deck.
Unlock Deck
k this deck
15
How many moles of NaOH do we need to prepare 250 mL of a 2M solution?
A) 0.5 mol
B) 500 mol
C) 0.008 mol
D) 8 mol
E) 2 mol
A) 0.5 mol
B) 500 mol
C) 0.008 mol
D) 8 mol
E) 2 mol

Unlock Deck
Unlock for access to all 22 flashcards in this deck.
Unlock Deck
k this deck
16
How many moles of NH3 do we need to prepare 100 mL of a 0.5M solution?
A) 0.5 mol
B) 0.2 mol
C) 200 mol
D) 0.05 mol
E) 50 mol
A) 0.5 mol
B) 0.2 mol
C) 200 mol
D) 0.05 mol
E) 50 mol

Unlock Deck
Unlock for access to all 22 flashcards in this deck.
Unlock Deck
k this deck
17
We have 100 mL of a solution of NaCl with a concentration of 0.5 mol L-1 and want to dilute it to a concentration of 0.1 mol L-1. How much water would we need to add to achieve this?
A) 0.5 L
B) 0.4 L
C) 500 L
D) 0.005 L
E) 0.1 L
A) 0.5 L
B) 0.4 L
C) 500 L
D) 0.005 L
E) 0.1 L

Unlock Deck
Unlock for access to all 22 flashcards in this deck.
Unlock Deck
k this deck
18
A sample of a solution with concentration of 0.005 mol L-1 has a molar absorptivity of 150 L mol-1 cm-1 at 350 nm. What absorbance will be measured by a UV-visible spectrophotometer when the sample is measured in a cuvette with a path length of 1 cm at 350 nm?
A) 3.3 *10-3
B) 0.75
C) 30 000
D) 75
E) 0.075
A) 3.3 *10-3
B) 0.75
C) 30 000
D) 75
E) 0.075

Unlock Deck
Unlock for access to all 22 flashcards in this deck.
Unlock Deck
k this deck
19
A substance, X, has a molar absorptivity of 1250 L mol-1 cm-1 at 325 nm. A sample of X gives an absorbance of 0.8 when measured in a UV-visible spectrophotometer at 325 nm with a path length of 1 cm. What is the concentration of the sample of X?
A) 1000 mol L-1
B) 1 mol L-1
C) 1563 mol L-1
D) 6.4*10-4 mol L-1
E) 1.56 mol L-1
A) 1000 mol L-1
B) 1 mol L-1
C) 1563 mol L-1
D) 6.4*10-4 mol L-1
E) 1.56 mol L-1

Unlock Deck
Unlock for access to all 22 flashcards in this deck.
Unlock Deck
k this deck
20
Two compounds, A and B, react with the stoichiometry 1:3, i.e. A + 3B. If there are 0.1 moles of A present in a sample initially, how many moles of B must be added for A to be completely consumed?
A) 0.1 mol
B) 0.3 mol
C) 0.0333 mol
D) 3 mol
E) 1 mol
A) 0.1 mol
B) 0.3 mol
C) 0.0333 mol
D) 3 mol
E) 1 mol

Unlock Deck
Unlock for access to all 22 flashcards in this deck.
Unlock Deck
k this deck
21
A solution containing red blood cells at a concentration of 2000 cells per mL undergoes three serial dilution steps, whereby 1 mL of starting solution is diluted with 9 mL of solvent at each step. What is the concentration of red blood cells after the final dilution step?
A) 666 cells mL-1
B) 2.47 cells mL-1
C) 2 cells mL-1
D) 20 cells mL-1
E) 74.1 cells mL-1
A) 666 cells mL-1
B) 2.47 cells mL-1
C) 2 cells mL-1
D) 20 cells mL-1
E) 74.1 cells mL-1

Unlock Deck
Unlock for access to all 22 flashcards in this deck.
Unlock Deck
k this deck
22
25 mL of a growth medium containing bacteria at a concentration of 3 * 105 cells per litre is diluted to a volume of 0.05 L. What is the concentration of bacteria after dilution?
A) 1.5 * 106 cells L-1
B) 1.75 * 108 cells L-1
C) 6.7 * 10-6 cells L-1
D) 6 * 105 cells L-1
E) 1.5* 105 cells L-1
A) 1.5 * 106 cells L-1
B) 1.75 * 108 cells L-1
C) 6.7 * 10-6 cells L-1
D) 6 * 105 cells L-1
E) 1.5* 105 cells L-1

Unlock Deck
Unlock for access to all 22 flashcards in this deck.
Unlock Deck
k this deck



