Deck 29: Spectroscopy : Mass Spectrometry, Infrared Spectroscopy, and Nuclear Magnetic Resonance Spectroscopy
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/12
Play
Full screen (f)
Deck 29: Spectroscopy : Mass Spectrometry, Infrared Spectroscopy, and Nuclear Magnetic Resonance Spectroscopy
1
Which of the following statements about13C NMR isnot true?
A) In13C proton-decoupled NMR spectra, all peaks are singlets.
B) 13C NMR easily differentiates between the different hybridized carbons (sp3,sp2, andsp hybridized carbons).
C) 13C NMR chemical shifts occur over a greater range than1H NMR chemical shifts.
D) 13C NMR spectra display peaks for only carbons that bear hydrogen atoms.
A) In13C proton-decoupled NMR spectra, all peaks are singlets.
B) 13C NMR easily differentiates between the different hybridized carbons (sp3,sp2, andsp hybridized carbons).
C) 13C NMR chemical shifts occur over a greater range than1H NMR chemical shifts.
D) 13C NMR spectra display peaks for only carbons that bear hydrogen atoms.
13C NMR spectra display peaks for only carbons that bear hydrogen atoms.
2
How could spectroscopy be used to distinguish between the following compounds? 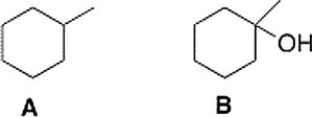
A) Compound A has a triplet in its 1H NMR spectrum at 1.0 ppm and compound B has a peak at 3200-3500 cm-1 in its IR spectrum.
B) Compound B has a peak at 3200-3500 cm-1 in its IR spectrum.
C) Compound A has a triplet in its1H NMR spectrum at 1.0 ppm.
D) Compound A has a peak in its IR spectrum at 2900 cm-1.

A) Compound A has a triplet in its 1H NMR spectrum at 1.0 ppm and compound B has a peak at 3200-3500 cm-1 in its IR spectrum.
B) Compound B has a peak at 3200-3500 cm-1 in its IR spectrum.
C) Compound A has a triplet in its1H NMR spectrum at 1.0 ppm.
D) Compound A has a peak in its IR spectrum at 2900 cm-1.
Compound B has a peak at 3200-3500 cm-1 in its IR spectrum.
3
Which of the following statements is (are) true about a compound that has a molecular ion peak in its mass spectrum at mass 94 and shows prominent peaks in its IR spectrum at 3600-3200 and 1600 cm-1?
A) Both (The compound has a molecular mass of 94) and (The compound contains an OH group and a benzene ring) are true statements.
B) Both (The compound has a molecular mass of 94) and (The compound contains a C=O group and Csp3-H hybridized bonds) are true statements.
C) The compound has a molecular mass of 94.
D) The compound contains an OH group and a benzene ring.
E) The compound contains a C=O group and Csp3-H hybridized bonds.
A) Both (The compound has a molecular mass of 94) and (The compound contains an OH group and a benzene ring) are true statements.
B) Both (The compound has a molecular mass of 94) and (The compound contains a C=O group and Csp3-H hybridized bonds) are true statements.
C) The compound has a molecular mass of 94.
D) The compound contains an OH group and a benzene ring.
E) The compound contains a C=O group and Csp3-H hybridized bonds.
Both (The compound has a molecular mass of 94) and (The compound contains an OH group and a benzene ring) are true statements.
4
Examine the IR below and classify the compound. 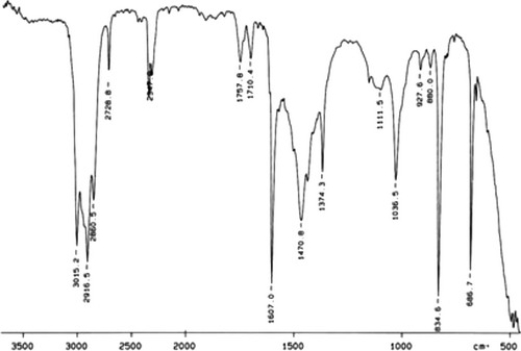
A) Amine
B) Carbocylic acid
C) Ketone
D) Alcohol
E) Arene

A) Amine
B) Carbocylic acid
C) Ketone
D) Alcohol
E) Arene

Unlock Deck
Unlock for access to all 12 flashcards in this deck.
Unlock Deck
k this deck
5
Which of the following statements is (are) true about a compound that has a molecular ion peak in its mass spectrum at mass 104 and shows prominent peaks in its IR spectrum at 3200-2850 cm-1?
A) The compound contains an OH group and Csp3-H hybridized bonds.
B) Both (The compound has a molecular mass of 104) and (The compound contains a C=O group and Csp3-H hybridized bonds) are true statements.
C) The compound contains a C=O group and Csp3-H hybridized bonds.
D) Both (The compound has a molecular mass of 104) and (The compound contains an OH group and Csp3-H hybridized bonds) are true statements.
E) The compound has a molecular mass of 104.
A) The compound contains an OH group and Csp3-H hybridized bonds.
B) Both (The compound has a molecular mass of 104) and (The compound contains a C=O group and Csp3-H hybridized bonds) are true statements.
C) The compound contains a C=O group and Csp3-H hybridized bonds.
D) Both (The compound has a molecular mass of 104) and (The compound contains an OH group and Csp3-H hybridized bonds) are true statements.
E) The compound has a molecular mass of 104.

Unlock Deck
Unlock for access to all 12 flashcards in this deck.
Unlock Deck
k this deck
6
You are given a bottle of an organic liquid and told it must be either cyclohexane or 1-hexene. Which of the following statements is (are) true about these two compounds? 
A) 1-Hexene will show an absorption at 1650 cm-1 but cyclohexane will not.
B) Statements (The two compounds can be differentiated by their mass spectra because they will have molecular ions at different masses), (1-Hexene will show an absorption at 1650 cm-1 but cyclohexane will not), and (Both cyclohexane and 1- hexene will show C-H absorptions at about 2950 cm-1) are all true.
C) Statements (1-Hexene will show an absorption at 1650 cm-1 but cyclohexane will not) and (Both cyclohexane and 1-hexene will show C-H absorptions at about 2950 cm-1) are both true.
D) The two compounds can be differentiated by their mass spectra because they will have molecular ion peaks at different m/z.
E) Both cyclohexane and 1-hexene will show C-H absorptions at about 2950 cm-1.

A) 1-Hexene will show an absorption at 1650 cm-1 but cyclohexane will not.
B) Statements (The two compounds can be differentiated by their mass spectra because they will have molecular ions at different masses), (1-Hexene will show an absorption at 1650 cm-1 but cyclohexane will not), and (Both cyclohexane and 1- hexene will show C-H absorptions at about 2950 cm-1) are all true.
C) Statements (1-Hexene will show an absorption at 1650 cm-1 but cyclohexane will not) and (Both cyclohexane and 1-hexene will show C-H absorptions at about 2950 cm-1) are both true.
D) The two compounds can be differentiated by their mass spectra because they will have molecular ion peaks at different m/z.
E) Both cyclohexane and 1-hexene will show C-H absorptions at about 2950 cm-1.

Unlock Deck
Unlock for access to all 12 flashcards in this deck.
Unlock Deck
k this deck
7
Which of the following statements is (are) accurate about the IR spectrum of compounds I, II, and III below? 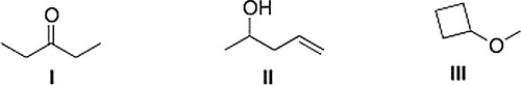
A) CompoundI shows absorptions at 2950 and 1700 cm-1.
B) Both (CompoundI shows absorptions at 2950 and 1700 cm-1) and (CompoundII shows absorptions at 3200-3600 and 1650 cm-1) are true.
C) CompoundIII shows absorptions at 3200-3600 and 2950 cm-1.
D) CompoundII shows absorptions at 3200-3600 and 1650 cm-1.

A) CompoundI shows absorptions at 2950 and 1700 cm-1.
B) Both (CompoundI shows absorptions at 2950 and 1700 cm-1) and (CompoundII shows absorptions at 3200-3600 and 1650 cm-1) are true.
C) CompoundIII shows absorptions at 3200-3600 and 2950 cm-1.
D) CompoundII shows absorptions at 3200-3600 and 1650 cm-1.

Unlock Deck
Unlock for access to all 12 flashcards in this deck.
Unlock Deck
k this deck
8
Consider the three organic compounds drawn below. Which of the following statements is (are) true about the IR spectra of I, II, and III? 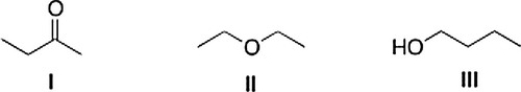
A) II shows strong absorptions at 2950 cm-1 and 2250 cm-1.
B) Statements (I shows strong absorptions at 2950 cm-1 and 1700 cm-1) and (III shows strong absorptions at 2950 cm-1 and 3200-3600 cm-1) are true.
C) III shows strong absorptions at 2950 cm-1 and 3200-3600 cm-1.
D) I shows strong absorptions at 2950 cm-1 and 1700 cm-1.

A) II shows strong absorptions at 2950 cm-1 and 2250 cm-1.
B) Statements (I shows strong absorptions at 2950 cm-1 and 1700 cm-1) and (III shows strong absorptions at 2950 cm-1 and 3200-3600 cm-1) are true.
C) III shows strong absorptions at 2950 cm-1 and 3200-3600 cm-1.
D) I shows strong absorptions at 2950 cm-1 and 1700 cm-1.

Unlock Deck
Unlock for access to all 12 flashcards in this deck.
Unlock Deck
k this deck
9
Which of the following statement(s) is (are) true about a compound that has a molecular ion peak in its mass spectrum at mass 69 and shows a prominent peak in its IR spectrum at 2250 cm-1?
A) Both (The compound has a molecular mass of 70) and (The compound contains a cyano or alkyne group) are true statements.
B) The compound contains a cyano or alkyne group.
C) The compound contains a C=O group.
D) Both (The compound has a molecular mass of 70) and (The compound contains a C=O group) are true statements.
E) The compound has a molecular mass of 70.
A) Both (The compound has a molecular mass of 70) and (The compound contains a cyano or alkyne group) are true statements.
B) The compound contains a cyano or alkyne group.
C) The compound contains a C=O group.
D) Both (The compound has a molecular mass of 70) and (The compound contains a C=O group) are true statements.
E) The compound has a molecular mass of 70.

Unlock Deck
Unlock for access to all 12 flashcards in this deck.
Unlock Deck
k this deck
10
Which of the following statements is (are) true about the IR spectrum of the compound drawn below? 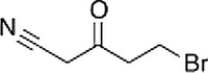
A) It shows absorptions at 2250 cm-1 and 1650 cm-1.
B) It shows absorptions at 3000-3150 cm-1 and 1720 cm-1.
C) It shows absorptions at 3000-2850 cm-1 and 2150 cm-1.
D) It shows absorptions at 2250 cm-1 and 1720 cm-1.
E) Both statements (It shows absorptions at 3000-2850 cm-1 and 2250 cm-1) and (It shows absorptions at 2250 cm-1 and 1720 cm-1) are true.

A) It shows absorptions at 2250 cm-1 and 1650 cm-1.
B) It shows absorptions at 3000-3150 cm-1 and 1720 cm-1.
C) It shows absorptions at 3000-2850 cm-1 and 2150 cm-1.
D) It shows absorptions at 2250 cm-1 and 1720 cm-1.
E) Both statements (It shows absorptions at 3000-2850 cm-1 and 2250 cm-1) and (It shows absorptions at 2250 cm-1 and 1720 cm-1) are true.

Unlock Deck
Unlock for access to all 12 flashcards in this deck.
Unlock Deck
k this deck
11
Which of the following statements is (are) true about a compound that has molecular ion peaks in its mass spectrum at mass 170 and 172 and shows prominent peaks in its IR spectrum at 3150-3000 and 1600 cm-1?
A) Both (The compound is not pure) and (The compound contains a halogen) are true statements.
B) Both (The compound is not pure) and (The compound contains an OH group and Csp3-H hybridized bonds) are true statements.
C) The compound contains an OH group and Csp3-H hybridized bonds.
D) The compound contains a halogen.
E) The compound is not pure.
A) Both (The compound is not pure) and (The compound contains a halogen) are true statements.
B) Both (The compound is not pure) and (The compound contains an OH group and Csp3-H hybridized bonds) are true statements.
C) The compound contains an OH group and Csp3-H hybridized bonds.
D) The compound contains a halogen.
E) The compound is not pure.

Unlock Deck
Unlock for access to all 12 flashcards in this deck.
Unlock Deck
k this deck
12
A compoundX has a molecular ion peak in its mass spectrum at m/z 136. What information does this tell us aboutX?
A) X has a molecular mass of 136.
B) Statements (X has a molecular mass of 136), (The molecular formula forX is C8H8O2), and (The empirical formula forX is C4H4O) are all true.
C) Both A (X has a molecular mass of 136) and B (The molecular formula forX is C8H8O2) are true.
D) The molecular formula forX is C8H8O2.
E) The empirical formula forX is C4H4O.
A) X has a molecular mass of 136.
B) Statements (X has a molecular mass of 136), (The molecular formula forX is C8H8O2), and (The empirical formula forX is C4H4O) are all true.
C) Both A (X has a molecular mass of 136) and B (The molecular formula forX is C8H8O2) are true.
D) The molecular formula forX is C8H8O2.
E) The empirical formula forX is C4H4O.

Unlock Deck
Unlock for access to all 12 flashcards in this deck.
Unlock Deck
k this deck



