Deck 2: Introduction to Organic Molecules and Functional Groups
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/10
Play
Full screen (f)
Deck 2: Introduction to Organic Molecules and Functional Groups
1
Which of the following compounds has the highest boiling point? 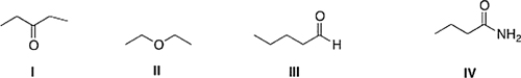
A) II
B) III
C) I
D) IV

A) II
B) III
C) I
D) IV
IV
2
Which of the following compounds has the highest boiling point? 
A) IV
B) II
C) III
D) I

A) IV
B) II
C) III
D) I
I
3
Which of the following is one of the families of biomolecules?
A) Simple sugars
B) Amino acids
C) All of these are correct.
D) Lipids
A) Simple sugars
B) Amino acids
C) All of these are correct.
D) Lipids
Amino acids
4
Which of the following compounds has the highest boiling point? 
A) II
B) III
C) IV
D) I

A) II
B) III
C) IV
D) I

Unlock Deck
Unlock for access to all 10 flashcards in this deck.
Unlock Deck
k this deck
5
Consider the molecule atenolol (a ? blocker used to treat hypertension). Which of the following lists the correct functional groups present in atenolol? 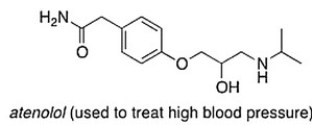
A) Secondary alcohol, amide, secondary amine, aromatic, ester
B) Secondary alcohol, amide, primary amine, aromatic, ether
C) Secondary alcohol, amide, secondary amine, aromatic, ether
D) Primary alcohol, amide, primary amine, aromatic, ether

A) Secondary alcohol, amide, secondary amine, aromatic, ester
B) Secondary alcohol, amide, primary amine, aromatic, ether
C) Secondary alcohol, amide, secondary amine, aromatic, ether
D) Primary alcohol, amide, primary amine, aromatic, ether

Unlock Deck
Unlock for access to all 10 flashcards in this deck.
Unlock Deck
k this deck
6
Which of the following statements best describes the relationship between the surface area of a molecule and the strength of the intermolecular forces?
A) The smaller the surface area, the stronger the intermolecular forces.
B) The larger the surface area, the stronger the intermolecular forces.
C) The larger the surface area, the weaker the intermolecular forces.
D) There is no relationship between surface area and intermolecular forces.
A) The smaller the surface area, the stronger the intermolecular forces.
B) The larger the surface area, the stronger the intermolecular forces.
C) The larger the surface area, the weaker the intermolecular forces.
D) There is no relationship between surface area and intermolecular forces.

Unlock Deck
Unlock for access to all 10 flashcards in this deck.
Unlock Deck
k this deck
7
Which of the following compounds has the highest boiling point? 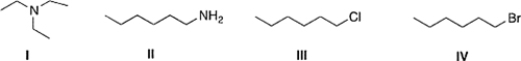
A) IV
B) I
C) III
D) II

A) IV
B) I
C) III
D) II

Unlock Deck
Unlock for access to all 10 flashcards in this deck.
Unlock Deck
k this deck
8
Which C=O functional group is present in the following molecule? 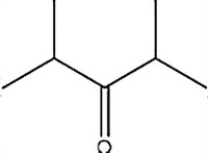
A) Aldehyde
B) Ketone
C) Ester
D) Carboxylic acid

A) Aldehyde
B) Ketone
C) Ester
D) Carboxylic acid

Unlock Deck
Unlock for access to all 10 flashcards in this deck.
Unlock Deck
k this deck
9
List the intermolecular forces present in the following molecule: 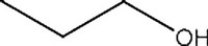
A) Dipole-dipole interactions
B) More than one of these answer choices is correct.
C) Van der Waals
D) Hydrogen bonding

A) Dipole-dipole interactions
B) More than one of these answer choices is correct.
C) Van der Waals
D) Hydrogen bonding

Unlock Deck
Unlock for access to all 10 flashcards in this deck.
Unlock Deck
k this deck
10
Which of the following statements about the solubility of organic compounds in H2O is true?
A) For an organic compound with one functional group that contains an O or N atom, the compound is water soluble only if it has 35 carbons.
B) The non-polar part of a molecule that is not attracted to water is said to be hydrophilic.
C) The polar part of a molecule that can that can bond hydrogen to water is said to be hydrophobic.
D) The non-polar part of a molecule that is not attracted to water is said to be hydrophobic.
A) For an organic compound with one functional group that contains an O or N atom, the compound is water soluble only if it has 35 carbons.
B) The non-polar part of a molecule that is not attracted to water is said to be hydrophilic.
C) The polar part of a molecule that can that can bond hydrogen to water is said to be hydrophobic.
D) The non-polar part of a molecule that is not attracted to water is said to be hydrophobic.

Unlock Deck
Unlock for access to all 10 flashcards in this deck.
Unlock Deck
k this deck



