Deck 28: Pericyclic Reactions, and Synthetic Polymers
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/23
Play
Full screen (f)
Deck 28: Pericyclic Reactions, and Synthetic Polymers
1
Bakelite is formed by the acid-catalyzed polymerization of phenol with formaldehyde. What is (are) the product(s) of the first step in this polymerization, shown below? (Note: in the answers below the hydroxymethyl group is -CH2OH.) 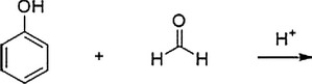
A) Ortho andpara-hydroxybenzaldehyde
B) Meta-(hydroxymethyl)phenol
C) Ortho andpara-(hydroxymethyl)phenol
D) Meta-hydroxybenzaldehyde

A) Ortho andpara-hydroxybenzaldehyde
B) Meta-(hydroxymethyl)phenol
C) Ortho andpara-(hydroxymethyl)phenol
D) Meta-hydroxybenzaldehyde
Ortho andpara-(hydroxymethyl)phenol
2
Which of the following polymers can be recycled into floor mats?
A) LDPE
B) PVC
C) HDPE
D) PET
A) LDPE
B) PVC
C) HDPE
D) PET
PVC
3
Which of the following polymers can be made at least in part from glucose derived from a renewable plant source?
A) Sorona
B) Polypropylene
C) Polyethylene
D) Teflon
A) Sorona
B) Polypropylene
C) Polyethylene
D) Teflon
Sorona
4
What type of sigmatropic rearrangement is illustrated below? ![<strong>What type of sigmatropic rearrangement is illustrated below? </strong> A) [1,3] B) [1,4] C) [1,5] D) [3,3]](https://d2lvgg3v3hfg70.cloudfront.net/TBMG1035/11ee4665_5cf6_e6c9_862d_bb87ea604df2_TBMG1035_00.jpg)
A) [1,3]
B) [1,4]
C) [1,5]
D) [3,3]
![<strong>What type of sigmatropic rearrangement is illustrated below? </strong> A) [1,3] B) [1,4] C) [1,5] D) [3,3]](https://d2lvgg3v3hfg70.cloudfront.net/TBMG1035/11ee4665_5cf6_e6c9_862d_bb87ea604df2_TBMG1035_00.jpg)
A) [1,3]
B) [1,4]
C) [1,5]
D) [3,3]

Unlock Deck
Unlock for access to all 23 flashcards in this deck.
Unlock Deck
k this deck
5
Which of the following statements about an electrocyclic ring-opening reaction isnot true?
A) The product of an electrocyclic ring-opening reaction contains one fewerp bond than the reactant.
B) The product of an electrocyclic ring-opening reaction contains one morep bond than the reactant.
C) An electrocyclic ring-opening reaction requires a source of energy (heat or light).
D) An electrocyclic ring-opening reaction is an intramolecular reaction.
A) The product of an electrocyclic ring-opening reaction contains one fewerp bond than the reactant.
B) The product of an electrocyclic ring-opening reaction contains one morep bond than the reactant.
C) An electrocyclic ring-opening reaction requires a source of energy (heat or light).
D) An electrocyclic ring-opening reaction is an intramolecular reaction.

Unlock Deck
Unlock for access to all 23 flashcards in this deck.
Unlock Deck
k this deck
6
Which of the following statements about orbital symmetry and cycloaddition reactions is true?
A) Thermal cycloadditions involving an odd number ofp bonds proceed by an antarafacial pathway.
B) Thermal cycloadditions involving an even number ofp bonds proceed by a suprafacial pathway.
C) Photochemical cycloadditions involving an even number ofp bonds proceed by an antarafacial pathway.
D) Photochemical cycloadditions involving an even number ofp bonds proceed by a suprafacial pathway.
A) Thermal cycloadditions involving an odd number ofp bonds proceed by an antarafacial pathway.
B) Thermal cycloadditions involving an even number ofp bonds proceed by a suprafacial pathway.
C) Photochemical cycloadditions involving an even number ofp bonds proceed by an antarafacial pathway.
D) Photochemical cycloadditions involving an even number ofp bonds proceed by a suprafacial pathway.

Unlock Deck
Unlock for access to all 23 flashcards in this deck.
Unlock Deck
k this deck
7
Which of the following statements about a thermal reaction involving an even number of electron pairs is true?
A) A thermal reaction involving an even number of electron pairs is conrotatory or suprafacial.
B) A thermal reaction involving an even number of electron pairs is conrotatory or antarafacial.
C) A thermal reaction involving an even number of electron pairs is disrotatory or suprafacial.
D) A thermal reaction involving an even number of electron pairs is disrotatory or antarafacial.
A) A thermal reaction involving an even number of electron pairs is conrotatory or suprafacial.
B) A thermal reaction involving an even number of electron pairs is conrotatory or antarafacial.
C) A thermal reaction involving an even number of electron pairs is disrotatory or suprafacial.
D) A thermal reaction involving an even number of electron pairs is disrotatory or antarafacial.

Unlock Deck
Unlock for access to all 23 flashcards in this deck.
Unlock Deck
k this deck
8
Which of the following statements about molecular orbitals is true?
A) The number of molecular orbitals formed is equal to the number of atomic orbitals used.
B) The number of molecular orbitals formed is equal to twice the number of atomic orbitals used.
C) The number of molecular orbitals formed is different from the number of atomic orbitals used.
D) The number of molecular orbitals formed is equal to half the number of atomic orbitals used.
A) The number of molecular orbitals formed is equal to the number of atomic orbitals used.
B) The number of molecular orbitals formed is equal to twice the number of atomic orbitals used.
C) The number of molecular orbitals formed is different from the number of atomic orbitals used.
D) The number of molecular orbitals formed is equal to half the number of atomic orbitals used.

Unlock Deck
Unlock for access to all 23 flashcards in this deck.
Unlock Deck
k this deck
9
Which of the following statements about sigmatropic rearrangements and orbital symmetry isnot true?
A) In an antarafacial rearrangement, the news bond forms on the opposite side of thep system as the brokens bond.
B) Reactions involving six atoms or fewer must take place by suprafacial pathways.
C) In a suprafacial rearrangement, the news bond forms on the opposite side of thep system as the brokens bond.
D) In a suprafacial rearrangement, the news bond forms on the same side of thep system as the brokens bond.
A) In an antarafacial rearrangement, the news bond forms on the opposite side of thep system as the brokens bond.
B) Reactions involving six atoms or fewer must take place by suprafacial pathways.
C) In a suprafacial rearrangement, the news bond forms on the opposite side of thep system as the brokens bond.
D) In a suprafacial rearrangement, the news bond forms on the same side of thep system as the brokens bond.

Unlock Deck
Unlock for access to all 23 flashcards in this deck.
Unlock Deck
k this deck
10
Which of the following statements about the Claisen rearrangement is true?
A) The Claisen rearrangement occurs readily in a suprafacial pathway under photochemical conditions.
B) The Claisen rearrangement involves three electron pairs; two inp bonds and one in as bond.
C) The Claisen rearrangement occurs readily in an antarafacial pathway under thermal conditions.
D) The Claisen rearrangement involves the rearrangement of an unsaturated ether to a b,g-unsaturated carbonyl compound.
A) The Claisen rearrangement occurs readily in a suprafacial pathway under photochemical conditions.
B) The Claisen rearrangement involves three electron pairs; two inp bonds and one in as bond.
C) The Claisen rearrangement occurs readily in an antarafacial pathway under thermal conditions.
D) The Claisen rearrangement involves the rearrangement of an unsaturated ether to a b,g-unsaturated carbonyl compound.

Unlock Deck
Unlock for access to all 23 flashcards in this deck.
Unlock Deck
k this deck
11
Which of the following statements about sigmatropic reactions isnot true?
A) Thep bonds rearrange in a sigmatropic reaction.
B) A sigmatropic reaction is an intramolecular pericyclic reaction.
C) In a sigmatropic reaction,s bond is broken in one of the reactants.
D) The number ofp bonds in the reactants and product differs in a sigmatropic reaction.
A) Thep bonds rearrange in a sigmatropic reaction.
B) A sigmatropic reaction is an intramolecular pericyclic reaction.
C) In a sigmatropic reaction,s bond is broken in one of the reactants.
D) The number ofp bonds in the reactants and product differs in a sigmatropic reaction.

Unlock Deck
Unlock for access to all 23 flashcards in this deck.
Unlock Deck
k this deck
12
Which of the following statements about ap-bonding molecular orbital is true?
A) Ap-bonding molecular orbital is formed when twop orbitals of opposite phase overlap.
B) Ap-bonding molecular orbital is lower in energy than as-bonding molecular orbital.
C) Both the statements ap-bonding molecular orbital is formed when twop orbitals of similar phase overlap and ap-bonding molecular orbital is lower in energy than as-bonding molecular orbital are true.
D) Ap-bonding molecular orbital is formed when twop orbitals of similar phase overlap.
A) Ap-bonding molecular orbital is formed when twop orbitals of opposite phase overlap.
B) Ap-bonding molecular orbital is lower in energy than as-bonding molecular orbital.
C) Both the statements ap-bonding molecular orbital is formed when twop orbitals of similar phase overlap and ap-bonding molecular orbital is lower in energy than as-bonding molecular orbital are true.
D) Ap-bonding molecular orbital is formed when twop orbitals of similar phase overlap.

Unlock Deck
Unlock for access to all 23 flashcards in this deck.
Unlock Deck
k this deck
13
Which of the following statements about orbital symmetry and cycloaddition reactions is true?
A) Photochemical sycloadditions involving an even number ofp bonds proceed by an antarafacial pathway.
B) Photochemical cycloadditions involving an odd number ofp bonds proceed by a suprafacial pathway.
C) Thermal cycloadditions involving an even number ofp bonds proceed by a suprafacial pathway.
D) Thermal cycloadditions involving an odd number ofp bonds proceed by a suprafacial pathway.
A) Photochemical sycloadditions involving an even number ofp bonds proceed by an antarafacial pathway.
B) Photochemical cycloadditions involving an odd number ofp bonds proceed by a suprafacial pathway.
C) Thermal cycloadditions involving an even number ofp bonds proceed by a suprafacial pathway.
D) Thermal cycloadditions involving an odd number ofp bonds proceed by a suprafacial pathway.

Unlock Deck
Unlock for access to all 23 flashcards in this deck.
Unlock Deck
k this deck
14
Which of the following statements about ap* antibonding molecular orbital is true?
A) Ap* antibonding molecular orbital is formed when twop orbitals of similar phase overlap.
B) Both the statements ap* antibonding molecular orbital is formed when twop orbitals of opposite phase overlap and ap* antibonding molecular orbital is a higher-energy molecular orbital than ap bonding molecular orbital are true.
C) Ap* antibonding molecular orbital is formed when twop orbitals of opposite phase overlap.
D) Ap* antibonding molecular orbital is a higher-energy molecular orbital than ap bonding molecular orbital.
A) Ap* antibonding molecular orbital is formed when twop orbitals of similar phase overlap.
B) Both the statements ap* antibonding molecular orbital is formed when twop orbitals of opposite phase overlap and ap* antibonding molecular orbital is a higher-energy molecular orbital than ap bonding molecular orbital are true.
C) Ap* antibonding molecular orbital is formed when twop orbitals of opposite phase overlap.
D) Ap* antibonding molecular orbital is a higher-energy molecular orbital than ap bonding molecular orbital.

Unlock Deck
Unlock for access to all 23 flashcards in this deck.
Unlock Deck
k this deck
15
Which of the following isnot a correct designation for a sigmatropic rearrangement?
A) [3,3]
B) [1,3]
C) [3,1]
D) [1,5]
A) [3,3]
B) [1,3]
C) [3,1]
D) [1,5]

Unlock Deck
Unlock for access to all 23 flashcards in this deck.
Unlock Deck
k this deck
16
What type of sigmatropic rearrangement is illustrated below? ![<strong>What type of sigmatropic rearrangement is illustrated below? </strong> A) [3,3] B) [1,4] C) [1,5] D) [1,3]](https://d2lvgg3v3hfg70.cloudfront.net/TBMG1035/11ee4665_5cf7_5bfa_862d_db5d716529dd_TBMG1035_00.jpg)
A) [3,3]
B) [1,4]
C) [1,5]
D) [1,3]
![<strong>What type of sigmatropic rearrangement is illustrated below? </strong> A) [3,3] B) [1,4] C) [1,5] D) [1,3]](https://d2lvgg3v3hfg70.cloudfront.net/TBMG1035/11ee4665_5cf7_5bfa_862d_db5d716529dd_TBMG1035_00.jpg)
A) [3,3]
B) [1,4]
C) [1,5]
D) [1,3]

Unlock Deck
Unlock for access to all 23 flashcards in this deck.
Unlock Deck
k this deck
17
Why is the Diels-Alder reaction called a thermal [4+2] cycloaddition?
A) Because the reaction is initiated by light; the dienophile has fourp electrons and the diene has twop electrons.
B) Because the reaction is initiated by heat; the diene has fourp electrons and the dienophile has twop electrons.
C) Because the reaction is initiated by light; the diene has fourp electrons and the dienophile has twop electrons.
D) Because the reaction is initiated by heat; the dienophile has fourp electrons and the diene has twop electrons.
A) Because the reaction is initiated by light; the dienophile has fourp electrons and the diene has twop electrons.
B) Because the reaction is initiated by heat; the diene has fourp electrons and the dienophile has twop electrons.
C) Because the reaction is initiated by light; the diene has fourp electrons and the dienophile has twop electrons.
D) Because the reaction is initiated by heat; the dienophile has fourp electrons and the diene has twop electrons.

Unlock Deck
Unlock for access to all 23 flashcards in this deck.
Unlock Deck
k this deck
18
Which of the following statements about the Cope rearrangement isnot true?
A) The Cope rearrangement involves the rearrangement of a 1,5-diene to an isomeric 1,5-diene.
B) The Cope rearrangement takes place readily in a suprafacial pathway under photochemical conditions.
C) The Cope rearrangement involves three electron pairs; two inp bonds and one in as bond.
D) The Cope rearrangement takes place readily in a suprafacial pathway, when heated.
A) The Cope rearrangement involves the rearrangement of a 1,5-diene to an isomeric 1,5-diene.
B) The Cope rearrangement takes place readily in a suprafacial pathway under photochemical conditions.
C) The Cope rearrangement involves three electron pairs; two inp bonds and one in as bond.
D) The Cope rearrangement takes place readily in a suprafacial pathway, when heated.

Unlock Deck
Unlock for access to all 23 flashcards in this deck.
Unlock Deck
k this deck
19
What type of sigmatropic rearrangement is illustrated below? ![<strong>What type of sigmatropic rearrangement is illustrated below? </strong> A) [1,5] B) [1,3] C) [1,4] D) [3,3]](https://d2lvgg3v3hfg70.cloudfront.net/TBMG1035/11ee4665_5cf7_5bfb_862d_dde9c93157c1_TBMG1035_00.jpg)
A) [1,5]
B) [1,3]
C) [1,4]
D) [3,3]
![<strong>What type of sigmatropic rearrangement is illustrated below? </strong> A) [1,5] B) [1,3] C) [1,4] D) [3,3]](https://d2lvgg3v3hfg70.cloudfront.net/TBMG1035/11ee4665_5cf7_5bfb_862d_dde9c93157c1_TBMG1035_00.jpg)
A) [1,5]
B) [1,3]
C) [1,4]
D) [3,3]

Unlock Deck
Unlock for access to all 23 flashcards in this deck.
Unlock Deck
k this deck
20
Which of the following statements about photochemical electrocyclic reactions isnot true?
A) Photochemical electrocyclic reactions occur in a disrotatory fashion for a conjugated polyene with an even number ofp bonds.
B) Photochemical electrocyclic reactions occur in a conrotatory fashion for a conjugated polyene with an odd number ofp bonds.
C) In photochemical reactions, we consider the orbitals of the HOMO of the excited state to determine the course of the reaction.
D) In photochemical reactions, we consider the orbitals of the LUMO of the excited state to determine the course of the reaction.
A) Photochemical electrocyclic reactions occur in a disrotatory fashion for a conjugated polyene with an even number ofp bonds.
B) Photochemical electrocyclic reactions occur in a conrotatory fashion for a conjugated polyene with an odd number ofp bonds.
C) In photochemical reactions, we consider the orbitals of the HOMO of the excited state to determine the course of the reaction.
D) In photochemical reactions, we consider the orbitals of the LUMO of the excited state to determine the course of the reaction.

Unlock Deck
Unlock for access to all 23 flashcards in this deck.
Unlock Deck
k this deck
21
Which of the following statements about cycloaddition reactions isnot true?
A) Cycloaddition reactions are stereospecific.
B) Cycloaddition reactions are concerted.
C) The course of the reaction is determined by the symmetry of the molecular orbitals of the products.
D) Cycloaddition reactions form a cyclic product with two news bonds.
A) Cycloaddition reactions are stereospecific.
B) Cycloaddition reactions are concerted.
C) The course of the reaction is determined by the symmetry of the molecular orbitals of the products.
D) Cycloaddition reactions form a cyclic product with two news bonds.

Unlock Deck
Unlock for access to all 23 flashcards in this deck.
Unlock Deck
k this deck
22
Consider the following reaction sequence. What is the correct classification of the first reaction in the sequence? ![<strong>Consider the following reaction sequence. What is the correct classification of the first reaction in the sequence? </strong> A) [3,3] Sigmatropic rearrangement B) [1,3] Sigmatropic rearrangement C) [1,5] Sigmatropic rearrangement D) [5,5] Sigmatropic rearrangement](https://d2lvgg3v3hfg70.cloudfront.net/TBMG1035/11ee4665_5cf7_830c_862d_85158e53b564_TBMG1035_00.jpg)
A) [3,3] Sigmatropic rearrangement
B) [1,3] Sigmatropic rearrangement
C) [1,5] Sigmatropic rearrangement
D) [5,5] Sigmatropic rearrangement
![<strong>Consider the following reaction sequence. What is the correct classification of the first reaction in the sequence? </strong> A) [3,3] Sigmatropic rearrangement B) [1,3] Sigmatropic rearrangement C) [1,5] Sigmatropic rearrangement D) [5,5] Sigmatropic rearrangement](https://d2lvgg3v3hfg70.cloudfront.net/TBMG1035/11ee4665_5cf7_830c_862d_85158e53b564_TBMG1035_00.jpg)
A) [3,3] Sigmatropic rearrangement
B) [1,3] Sigmatropic rearrangement
C) [1,5] Sigmatropic rearrangement
D) [5,5] Sigmatropic rearrangement

Unlock Deck
Unlock for access to all 23 flashcards in this deck.
Unlock Deck
k this deck
23
Which of the following statements about thermal electrocyclic reactions isnot true?
A) The number ofs bonds in the conjugated polyene determines whether rotation is conrotatory or disrotatory.
B) In thermal reactions, we consider the orbitals of the HOMO of the ground state electronic configuration to determine the course of the reaction.
C) Thermal electrocyclic reactions occur in a conrotatory fashion for a conjugated polyene with an even number ofp bonds.
D) Thermal electrocyclic reactions occur in a disrotatory fashion for a conjugated polyene with an odd number ofp bonds.
A) The number ofs bonds in the conjugated polyene determines whether rotation is conrotatory or disrotatory.
B) In thermal reactions, we consider the orbitals of the HOMO of the ground state electronic configuration to determine the course of the reaction.
C) Thermal electrocyclic reactions occur in a conrotatory fashion for a conjugated polyene with an even number ofp bonds.
D) Thermal electrocyclic reactions occur in a disrotatory fashion for a conjugated polyene with an odd number ofp bonds.

Unlock Deck
Unlock for access to all 23 flashcards in this deck.
Unlock Deck
k this deck



