Deck 20: Designer Proteins and Protein Folding
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/59
Play
Full screen (f)
Deck 20: Designer Proteins and Protein Folding
1
If you would synthesize a 15-amino acid long peptide, and each cycle of the solid-phase synthesis had a 99% yield, what would be your overall yield?
A) 99%
B) 75%
C) 80%
D) 86%
E) 95%
A) 99%
B) 75%
C) 80%
D) 86%
E) 95%
86%
2
The conserved folds found in proteins prove that these structures are selected by ________.
A) evolution
B) scientists
C) random sampling
D) mutation
E) default
A) evolution
B) scientists
C) random sampling
D) mutation
E) default
evolution
3
Protein engineering is possible because of advances in biochemistry and molecular biology.
Various _______ in a protein sequence are _____ in order to determine the role they played in the protein.
A) side chains; activated
B) amino acids; mutated
C) amino acids; activated
D) side chains; truncated
E) functional groups; replaced
Various _______ in a protein sequence are _____ in order to determine the role they played in the protein.
A) side chains; activated
B) amino acids; mutated
C) amino acids; activated
D) side chains; truncated
E) functional groups; replaced
amino acids; mutated
4
An _______ is a _________ monomer used in peptide synthesis. A peptide is a ____ polymer and contains _____ bonds.
A) acid; single; homogeneous; covalent
B) amino acid; heterogeneous; condensation; hydrogen
C) amino acid; bifunctional; condensation; amide
D) ?-hydroxy acid; bifunctional; homogeneous; amide
E) none of the above
A) acid; single; homogeneous; covalent
B) amino acid; heterogeneous; condensation; hydrogen
C) amino acid; bifunctional; condensation; amide
D) ?-hydroxy acid; bifunctional; homogeneous; amide
E) none of the above

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
5
Using a resin in solid-phase protein synthesis allows for _______.
I. efficient washing steps to remove the side products
II. keeping the growing peptide in solid phase
III. obtaining higher product yields
IV. using lower reaction temperatures
A) I and IV
B) II and III
C) I, II and III
D) II and IV
E) II, III and IV
I. efficient washing steps to remove the side products
II. keeping the growing peptide in solid phase
III. obtaining higher product yields
IV. using lower reaction temperatures
A) I and IV
B) II and III
C) I, II and III
D) II and IV
E) II, III and IV

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
6
A solid-phase protein synthesis reaction cycle includes_______________.
A) washing steps
B) deprotection of the peptide amino terminus using piperidine
C) activation of the carboxy group on the amino acid monomer to be added to the growing peptide
D) coupling of the activated amino acid with the amino group of the growing peptide polymer
E) all of the above
A) washing steps
B) deprotection of the peptide amino terminus using piperidine
C) activation of the carboxy group on the amino acid monomer to be added to the growing peptide
D) coupling of the activated amino acid with the amino group of the growing peptide polymer
E) all of the above

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
7
During solid-phase protein synthesis (SPPS) it is common to link the first amino acid to the resin by a(n) ___________.
A) hydrogen bond
B) ionic bond
C) ester bond
D) amide bond
E) none of the above
A) hydrogen bond
B) ionic bond
C) ester bond
D) amide bond
E) none of the above

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
8
The amine group of the amino acid monomers used in SPPS can be protected by a ____________.
I. phosphate group
II. tert-butoxycarbonyl group (t-Boc)
III. fluoprenylmethyloxycarbonyl group (Fmoc)
IV. sulfonamide group
A) I
B) II
C) III
D) II and III
E) III and IV
I. phosphate group
II. tert-butoxycarbonyl group (t-Boc)
III. fluoprenylmethyloxycarbonyl group (Fmoc)
IV. sulfonamide group
A) I
B) II
C) III
D) II and III
E) III and IV

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
9
Which amino acid side chains would need to be protected in SPPS?
I. hydroxyl groups of tyrosine and serine
II. carboxyl groups of glutamic acid and aspartic acid
III. amine group of lysine
IV. methyl group of alanine
V. phenyl group of phenylalanine
A) I
B) II
C) III and V
D) I, II and III
E) II, III and IV
I. hydroxyl groups of tyrosine and serine
II. carboxyl groups of glutamic acid and aspartic acid
III. amine group of lysine
IV. methyl group of alanine
V. phenyl group of phenylalanine
A) I
B) II
C) III and V
D) I, II and III
E) II, III and IV

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
10
Protecting groups used for side chains of amino acids are different than the protecting group used on the terminal amine because ___________.
A) they can ionize.
B) of chemical interference.
C) of more activity.
D) they should not be deprotected during the deprotection reaction of the terminal amine
E) none of the above
A) they can ionize.
B) of chemical interference.
C) of more activity.
D) they should not be deprotected during the deprotection reaction of the terminal amine
E) none of the above

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
11
The purity of a peptide synthesized by SPPS is analyzed by ____________.
A) UV-VIS spectrophotometry
B) reverse phase HPLC
C) circular dichroism
D) GC-MS
E) IR
A) UV-VIS spectrophotometry
B) reverse phase HPLC
C) circular dichroism
D) GC-MS
E) IR

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
12
What are the disadvantages of studying proteins in their purified form?
A) The natural regulators and molecular interactors are missing from the assay, which can lead to missing out on the actual function of the protein.
B) The protein may not be soluble.
C) The protein may not be stable.
D) The protein may aggregate.
E) none of the above
A) The natural regulators and molecular interactors are missing from the assay, which can lead to missing out on the actual function of the protein.
B) The protein may not be soluble.
C) The protein may not be stable.
D) The protein may aggregate.
E) none of the above

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
13
Proteins can be synthesized in the lab by using ____________.
I. solid-phase peptide synthesis (SPPS)
II. protein overexpression in biological systems (cells)
III. esterification reactions
IV. polymerization reactions
V. hydrolysis reactions
A) I
B) II
C) III
D) I and II
E) IV and V
I. solid-phase peptide synthesis (SPPS)
II. protein overexpression in biological systems (cells)
III. esterification reactions
IV. polymerization reactions
V. hydrolysis reactions
A) I
B) II
C) III
D) I and II
E) IV and V

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
14
Which statement is true about SPPS:
A) In SPPS the carboxy terminus of the peptide is covalently linked to the resin.
B) The next amino acid is coupled to the N- terminus of the growing peptide.
C) The carboxylic group of the amino acid to be added to the growing peptide is activated by using dicyclohexylcarbodiimide (DCC)
D) SPPS is used to synthesize up to 100-amino acid long peptides.
E) all of the above
A) In SPPS the carboxy terminus of the peptide is covalently linked to the resin.
B) The next amino acid is coupled to the N- terminus of the growing peptide.
C) The carboxylic group of the amino acid to be added to the growing peptide is activated by using dicyclohexylcarbodiimide (DCC)
D) SPPS is used to synthesize up to 100-amino acid long peptides.
E) all of the above

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
15
Engineered proteins can be studied in their natural environment in the cell. Natural proteins are engineered to contain a tag or a fusion protein in order to ____________.
I. improve protein stability at high temperatures
II. detect the localization of the natural protein in the cell
III. analyze the level of expression in the cell
IV. detect interactions with potential protein ligands in the cell
V. improve stability of the protein in the cell
A) I and II
B) II, III and IV
C) I, III and V
D) II, III and V
E) I and IV
I. improve protein stability at high temperatures
II. detect the localization of the natural protein in the cell
III. analyze the level of expression in the cell
IV. detect interactions with potential protein ligands in the cell
V. improve stability of the protein in the cell
A) I and II
B) II, III and IV
C) I, III and V
D) II, III and V
E) I and IV

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
16
Expression vectors are engineered fragments of DNA that contain the necessary information, such as ______, for the cell to be able to express the protein at a high level.
I. cloning sites tailored to various restriction enzymes
II. promoters designed to work in the individual cell lines
III. an origin of replication
IV. visualization sites
V. antibiotics resistance marker gene
A) I
B) II
C) III
D) II, III, and V
E) I, II, and III
I. cloning sites tailored to various restriction enzymes
II. promoters designed to work in the individual cell lines
III. an origin of replication
IV. visualization sites
V. antibiotics resistance marker gene
A) I
B) II
C) III
D) II, III, and V
E) I, II, and III

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
17
The origin of replication enables the plasmid to ______________.
A) fuse with the call wall
B) replicate within the cell
C) fold properly
D) react to antibiotics
E) all of the above
A) fuse with the call wall
B) replicate within the cell
C) fold properly
D) react to antibiotics
E) all of the above

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
18
Plasmids are _________. They can be delivered into the cell by ______ or _______.
A) circular pieces of DNA; transfection; electroporation
B) pieces of DNA; translocation; transfusion
C) pieces of RNA; translocation; transfusion
D) pieces of RNA; transfection; elimination
E) none of the above
A) circular pieces of DNA; transfection; electroporation
B) pieces of DNA; translocation; transfusion
C) pieces of RNA; translocation; transfusion
D) pieces of RNA; transfection; elimination
E) none of the above

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
19
Bacterial expression systems are used __________________.
A) when single chain proteins are expressed
B) to produce large quantities of protein
C) with a variety of expression vectors
D) with a variety of promoters to control the expression level of the protein
E) all of the above
A) when single chain proteins are expressed
B) to produce large quantities of protein
C) with a variety of expression vectors
D) with a variety of promoters to control the expression level of the protein
E) all of the above

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
20
Mammalian protein expression systems __________.
I. sometimes use the strong viral promoter CMV
II. are used when the expressed proteins require post-translational modifications
III. require expensive cell culture reagents
IV. are easy to handle
V. are used for producing insulin analogs
A) I
B) II
C) III
D) I, II and III
E) II, IV and V
I. sometimes use the strong viral promoter CMV
II. are used when the expressed proteins require post-translational modifications
III. require expensive cell culture reagents
IV. are easy to handle
V. are used for producing insulin analogs
A) I
B) II
C) III
D) I, II and III
E) II, IV and V

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
21
An insect expression system uses a baculovirus that is engineered to encode the protein of interest. The virus is used to infect ovarian cells from Spodoptera frugiperda, the fall armyworm. The advantages of using this system to produce engineered proteins are _________.
A) correct post-translational modifications on the protein of interest
B) cells grow in suspension in a simple broth
C) cells lyse at the end of the viral life cycle releasing the protein of interest
D) high expression level of the engineered protein
E) all of the above
A) correct post-translational modifications on the protein of interest
B) cells grow in suspension in a simple broth
C) cells lyse at the end of the viral life cycle releasing the protein of interest
D) high expression level of the engineered protein
E) all of the above

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
22
The advantages of using a yeast expression system are due to _______.
A) a fast grow rate of yeast
B) the proteins being secreted into the growth media
C) the ability of producing large quantities of engineered protein
D) the lower cost than the mammalian expression systems
E) all of the above
A) a fast grow rate of yeast
B) the proteins being secreted into the growth media
C) the ability of producing large quantities of engineered protein
D) the lower cost than the mammalian expression systems
E) all of the above

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
23
Proteins expressed in bacterial cells ________.
I. fold properly if they contain several domains
II. have to be released from the cells during a cell lysis step
III. do not go through post-translational modifications
IV. are secreted into the growth media
A) I
B) II
C) III
D) II and III
E) III and IV
I. fold properly if they contain several domains
II. have to be released from the cells during a cell lysis step
III. do not go through post-translational modifications
IV. are secreted into the growth media
A) I
B) II
C) III
D) II and III
E) III and IV

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
24
Engineered proteins can be expressed in a variety of host cells (mammalian, bacterial, etc). Choosing the host cell depends on ___________.
A) how fast the cells grow
B) the post-translational modifications that are required for the expressed protein to be biologically active
C) the type of single-domain or multi-domain protein that is expressed
D) amount of protein that needs to be produced
E) all of the above
A) how fast the cells grow
B) the post-translational modifications that are required for the expressed protein to be biologically active
C) the type of single-domain or multi-domain protein that is expressed
D) amount of protein that needs to be produced
E) all of the above

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
25
Using site-directed mutagenesis, the mutation of a serine residue to an alanine residue might be used to _________.
I. decrease the solubility of a protein
II. eliminate the possibility of phosphorylation at that location
III. observe the impact of a non-polar side chain at that location
IV. prove that serine had a catalytic function in the native protein
V. promote proper folding of the protein
A) I and II
B) I and III
C) II, III and IV
D) II, IV and V
E) I, II and V
I. decrease the solubility of a protein
II. eliminate the possibility of phosphorylation at that location
III. observe the impact of a non-polar side chain at that location
IV. prove that serine had a catalytic function in the native protein
V. promote proper folding of the protein
A) I and II
B) I and III
C) II, III and IV
D) II, IV and V
E) I, II and V

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
26
It is common, when making mutations in a cDNA sequence ___________________.
A) to remove a nucleotide
B) to use a codon with the smallest change in the nucleotides
C) to delete a large cDNA sequence
D) to exchange nucleic acids
E) to replace phosphate groups
A) to remove a nucleotide
B) to use a codon with the smallest change in the nucleotides
C) to delete a large cDNA sequence
D) to exchange nucleic acids
E) to replace phosphate groups

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
27
Adding restriction sites when constructing plasmids will help with _______.
A) extraction
B) purification
C) cloning
D) solubility
E) electrophoresis
A) extraction
B) purification
C) cloning
D) solubility
E) electrophoresis

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
28
When using cassette mutagenesis, in order to insert an engineered cDNA sequence into a plasmid, a __________ is used to cut out the sequence that is no longer needed, and a __________ is used to insert the new mutant cassette into the plasmid.
A) restriction enzyme; ligase
B) clone; enzyme
C) catalase; polymerase
D) reductase; polymerase
E) all of the above
A) restriction enzyme; ligase
B) clone; enzyme
C) catalase; polymerase
D) reductase; polymerase
E) all of the above

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
29
PCR-based mutagenesis is a widely used technique due to the mutation being introduced in _________.

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
30
Mutation of a serine residue to a(n) _____ residue is a good modeling tool for a phosphorylated serine.
A) alanine
B) methionine
C) lysine
D) aspartate
E) glutamate
A) alanine
B) methionine
C) lysine
D) aspartate
E) glutamate

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
31
__________ suppression is used to incorporate unnatural amino acids into a protein using cellular expression.

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
32
Subtilisin is a bacterial serine protease that is commonly used as an additive in laundry detergent. In its native state, it has a methionine that becomes oxidized, surprisingly leading to enzyme inactivation. To prevent inactivation, subtilisin could be engineered by mutating methionine residues to ___________.
I. isoleucine
II. alanine
III. cysteine
IV. phenylalanine
A) I
B) II
C) III
D) II, III and IV
E) I, II and IV
I. isoleucine
II. alanine
III. cysteine
IV. phenylalanine
A) I
B) II
C) III
D) II, III and IV
E) I, II and IV

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
33
The green fluorescent protein (GFP) ___________.
I. is a large protein, MW larger than 60 kDa
II. is used in chimeric protein constructs
III. is extremely useful in tagging proteins inside the cell, to visualize the localization of the expressed proteins
IV. was first isolated from the jellyfish Aequorea victoria
V. needs a substrate to exhibit fluorescence
A) II, III, and IV
B) II and V
C) III and IV
D) I and II
E) II and IV
I. is a large protein, MW larger than 60 kDa
II. is used in chimeric protein constructs
III. is extremely useful in tagging proteins inside the cell, to visualize the localization of the expressed proteins
IV. was first isolated from the jellyfish Aequorea victoria
V. needs a substrate to exhibit fluorescence
A) II, III, and IV
B) II and V
C) III and IV
D) I and II
E) II and IV

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
34
Proteins can be engineered to express a tag that is beneficial in __________ of the protein by _______ chromatography.

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
35
Choose fusion proteins from the list that could be used in biochemical or cellular assays:
I. GFP-protein constructs
II. extra cellular matrix proteins
III. anti-His6 tag-antibody conjugated to horseradish peroxidase
IV. maltose binding proteins
V. subtilisin
A) I
B) II
C) III
D) I, III and IV
E) II, III and V
I. GFP-protein constructs
II. extra cellular matrix proteins
III. anti-His6 tag-antibody conjugated to horseradish peroxidase
IV. maltose binding proteins
V. subtilisin
A) I
B) II
C) III
D) I, III and IV
E) II, III and V

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
36
His6-tagged proteins are often overexpressed in E. coli cells and purified by _______ chromatography.
A) ion exchange
B) size exclusion
C) nickel-affinity
D) high pressure
E) reverse phase
A) ion exchange
B) size exclusion
C) nickel-affinity
D) high pressure
E) reverse phase

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
37
In the cell, most proteins fold without assistance and within milliseconds. The information needed to fold a protein properly is found in the ______ sequence of the protein.
A) primary
B) secondary
C) tertiary
D) quaternary
E) complementary
A) primary
B) secondary
C) tertiary
D) quaternary
E) complementary

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
38
Protein folding is the process by which proteins ____________.
I. are stored
II. gain their secondary structure
III. gain their tertiary structure
IV. lose activity
V. burry hydrophobic side chains and expose hydrophilic ones
A) I
B) II
C) III and IV
D) II and V
E) II, III and V
I. are stored
II. gain their secondary structure
III. gain their tertiary structure
IV. lose activity
V. burry hydrophobic side chains and expose hydrophilic ones
A) I
B) II
C) III and IV
D) II and V
E) II, III and V

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
39
Levinthal's conclusion was that protein folding _______, because it would take way too long to complete the folding if all randomly available rotations around the main chain bonds would be sampled to achieve the most stable structure.

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
40
During folding the entropy of the protein _______.
A) decreases
B) stays the same
C) converts
D) increases
E) none of the above
A) decreases
B) stays the same
C) converts
D) increases
E) none of the above

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
41
Proteins unfold (denature) under various experimental conditions, such as heat or presence of _______ in the buffer.
A) very low or very high pH
B) -mercaptoethanol
C) urea
D) guanidinium hydrochloride
E) all of the above
A) very low or very high pH
B) -mercaptoethanol
C) urea
D) guanidinium hydrochloride
E) all of the above

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
42
Unfolded (denatured) proteins can be re-folded by removing the chemicals in a process called _________.
A) chromatography
B) dialysis
C) precipitation
D) nucleation
E) condensation
A) chromatography
B) dialysis
C) precipitation
D) nucleation
E) condensation

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
43
Proteins can be denatured using -mercaptoethanol, since this reagent will _____________.
A) melt the protein
B) oxidize the amine groups
C) reduce the disulfide bonds
D) precipitate the protein
E) all of the above
A) melt the protein
B) oxidize the amine groups
C) reduce the disulfide bonds
D) precipitate the protein
E) all of the above

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
44
Proteins containing ________ residues may not re-fold properly.
A) few cysteine
B) multiple serine
C) few serine
D) numerous cysteine
E) multiple lysine
A) few cysteine
B) multiple serine
C) few serine
D) numerous cysteine
E) multiple lysine

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
45
Given a peptide: 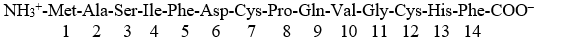
Salt bridges will form in this peptide in a buffer at pH 7 between:
I. the amino terminus -NH3+ and the carboxy terminus -COO-
II. the side chain of Asp6 and the amino terminus -NH3+
III. the side chains of Ser6 and Gln9
IV. the side chain of His13 and the carboxy terminus -COO-
V. the side chains of Gln9 and His13
A) I
B) II
C) III
D) I, II and IV
E) III, IV, and V
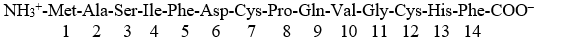
Salt bridges will form in this peptide in a buffer at pH 7 between:
I. the amino terminus -NH3+ and the carboxy terminus -COO-
II. the side chain of Asp6 and the amino terminus -NH3+
III. the side chains of Ser6 and Gln9
IV. the side chain of His13 and the carboxy terminus -COO-
V. the side chains of Gln9 and His13
A) I
B) II
C) III
D) I, II and IV
E) III, IV, and V

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
46
Given a peptide: 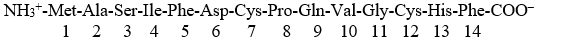 Disulfide bonds will form between the side chains of ______ in a buffer at pH 7 (under oxidizing conditions).
Disulfide bonds will form between the side chains of ______ in a buffer at pH 7 (under oxidizing conditions).
A) Met1 and Cys7
B) Met1 and Cys12
C) Cys 7 and Cys12
D) Ser3 and Cys12
E) none of the above
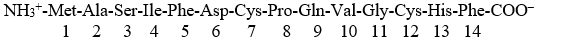 Disulfide bonds will form between the side chains of ______ in a buffer at pH 7 (under oxidizing conditions).
Disulfide bonds will form between the side chains of ______ in a buffer at pH 7 (under oxidizing conditions).A) Met1 and Cys7
B) Met1 and Cys12
C) Cys 7 and Cys12
D) Ser3 and Cys12
E) none of the above

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
47
Given a peptide: 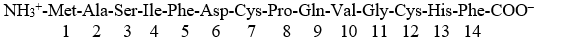
Hydrogen bonds will form in this peptide in a buffer at pH 7 between:
A) the amino terminus -NH3+ and Ser3
B) the side chain of Asp6 and Ser3
C) the side chains of Ser6 and His13
D) the side chains of Gln9 and His13
E) all of the above
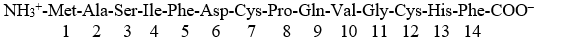
Hydrogen bonds will form in this peptide in a buffer at pH 7 between:
A) the amino terminus -NH3+ and Ser3
B) the side chain of Asp6 and Ser3
C) the side chains of Ser6 and His13
D) the side chains of Gln9 and His13
E) all of the above

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
48
Several neurodegenerative diseases (Alzheimer's, Parkinson's, etc.) present __________ structures at the molecular level.

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
49
The 3D solution structure of a 20mer peptide could be determined by_________.
A) electron microscopy
B) x-ray crystallography
C) multidimensional NMR
D) circular dichroism
E) none of the above
A) electron microscopy
B) x-ray crystallography
C) multidimensional NMR
D) circular dichroism
E) none of the above

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
50
The thermodynamic stabilization of the protein in folded state is partly due to the hydrophobic effect that accompanies protein folding. The hydrophobic effect in proteins is manifested in ________ and _________.
A) burial of the hydrophilic side chains; release of the water molecules
B) burial of the exposed hydrophobic side chains; release of the solvating water molecules
C) release of the water molecules; solvation of cations
D) release of the water molecules; formation of salt bridges
E) none of the above
A) burial of the hydrophilic side chains; release of the water molecules
B) burial of the exposed hydrophobic side chains; release of the solvating water molecules
C) release of the water molecules; solvation of cations
D) release of the water molecules; formation of salt bridges
E) none of the above

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
51
Forming bonds _______ energy. Thus, formation of numerous _______ during protein folding contributes to the stability of the folded structure.
A) increases; ionic bonds
B) increases; Van der Waals interactions
C) releases; metallic bonds
D) releases; hydrogen bonds
E) none of the above
A) increases; ionic bonds
B) increases; Van der Waals interactions
C) releases; metallic bonds
D) releases; hydrogen bonds
E) none of the above

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
52
During protein folding the entropy of the solvent molecules _______.
A) does not change
B) decreases
C) increases
D) is zero
E) none of the above
A) does not change
B) decreases
C) increases
D) is zero
E) none of the above

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
53
The most stable 3D protein structure has the ______ free energy, and is therefore assumed to be the native conformation of the protein.
A) highest
B) lowest
C) widest
D) most defined
E) least defined
A) highest
B) lowest
C) widest
D) most defined
E) least defined

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
54
The ___________ sequence is needed to predict the 3D structure of a relatively small protein using de novo structure prediction software.
A) base
B) amino acid
C) side chain
D) ionization
E) propagation
A) base
B) amino acid
C) side chain
D) ionization
E) propagation

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
55
_____ of protein structures is a useful tool in building 3D structures for proteins that do not have a solved x-ray structure. It uses the x-ray structure of a related protein as a template and the amino acid sequence of the protein of interest.
A) Solvation
B) Structural dynamics
C) Molecular dynamics
D) Homology modeling
E) none of the above
A) Solvation
B) Structural dynamics
C) Molecular dynamics
D) Homology modeling
E) none of the above

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
56
Molecular dynamics simulations can be used to model the sequence in which the _____ structure of a protein forms during protein folding.

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
57
Protein folding can be described in terms of energy, and visualized by an energy funnel diagram .The width of the funnel is given by the________.
A) size of the funnel
B) number of conformational states of the protein
C) shape of the molecule
D) motion of the molecule
E) all of the above
A) size of the funnel
B) number of conformational states of the protein
C) shape of the molecule
D) motion of the molecule
E) all of the above

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
58
Depositing falsified protein structure data into the Protein Data Bank, PDB, is a huge disservice to the scientific community because it leads to ___________.
A) misleading protein-drug interactions when used in drug discovery
B) wasted financial resources
C) wasted time using erroneous structures
D) retracted publications
E) all of the above
A) misleading protein-drug interactions when used in drug discovery
B) wasted financial resources
C) wasted time using erroneous structures
D) retracted publications
E) all of the above

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
59
Whistleblowers are in general protected from being fired from the job by __________.
A) federal and state laws
B) regulations
C) internal guidelines
D) company policy
E) all of the above
A) federal and state laws
B) regulations
C) internal guidelines
D) company policy
E) all of the above

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck



