Deck 15: Chemical Equilibrium
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/109
Play
Full screen (f)
Deck 15: Chemical Equilibrium
1
A chemical system is considered to have reached dynamic equilibrium when
A)the frequency of collisions between the reactant molecules is equal to the frequency of collisions between the product molecules.
B)the sum of the concentrations of each of the reactant species is equal to the sum of the concentrations of each of the product species.
C)the activation energy of the forward reaction is equal to the activation energy of the reverse reaction.
D)the rate of production of each of the products is equal to the rate of their consumption by the reverse reaction.
E)the rate of production of each of the product species is equal to the rate of consumption of each of the reactant species.
A)the frequency of collisions between the reactant molecules is equal to the frequency of collisions between the product molecules.
B)the sum of the concentrations of each of the reactant species is equal to the sum of the concentrations of each of the product species.
C)the activation energy of the forward reaction is equal to the activation energy of the reverse reaction.
D)the rate of production of each of the products is equal to the rate of their consumption by the reverse reaction.
E)the rate of production of each of the product species is equal to the rate of consumption of each of the reactant species.
the rate of production of each of the products is equal to the rate of their consumption by the reverse reaction.
2
A chemical system is considered to have reached equilibrium when
A)the rate of consumption of each of the product species by the reverse reaction is equal to the rate of production of each of the reactant species by the reverse reaction.
B)the sum of the concentrations of each of the reactant species is equal to the sum of the concentrations of each of the product species.
C)the rate of production of each of the product species is equal to the rate of consumption of each of the product species by the reverse reaction.
D)the rate of production of each of the product species is equal to the rate of consumption of each of the reactant species by the reverse reaction.
E)the rate of production of each of the product species by the forward reaction is equal to the rate of production of each of the reactant species by the reverse reaction.
A)the rate of consumption of each of the product species by the reverse reaction is equal to the rate of production of each of the reactant species by the reverse reaction.
B)the sum of the concentrations of each of the reactant species is equal to the sum of the concentrations of each of the product species.
C)the rate of production of each of the product species is equal to the rate of consumption of each of the product species by the reverse reaction.
D)the rate of production of each of the product species is equal to the rate of consumption of each of the reactant species by the reverse reaction.
E)the rate of production of each of the product species by the forward reaction is equal to the rate of production of each of the reactant species by the reverse reaction.
the rate of production of each of the product species is equal to the rate of consumption of each of the product species by the reverse reaction.
3
Which statement about chemical equilibrium is not true?
A)At equilibrium, the reactant and the product concentrations show no further change with time.
B)Chemical equilibrium can only be attained by starting with reagents from the reactant side of the equation.
C)At equilibrium, the forward reaction rate equals the reverse reaction rate.
D)The same equilibrium state can be attained starting either from the reactant or product side of the equation.
E)At equilibrium, the reactant and product concentrations are constant.
A)At equilibrium, the reactant and the product concentrations show no further change with time.
B)Chemical equilibrium can only be attained by starting with reagents from the reactant side of the equation.
C)At equilibrium, the forward reaction rate equals the reverse reaction rate.
D)The same equilibrium state can be attained starting either from the reactant or product side of the equation.
E)At equilibrium, the reactant and product concentrations are constant.
Chemical equilibrium can only be attained by starting with reagents from the reactant side of the equation.
4
Which of these statements are true about chemical equilibrium in general?
A)At equilibrium, the rate constant of the forward reaction is equal to the rate of the reverse reaction.
B)At equilibrium, the rate of the forward reaction is equal to the rate of the reverse reaction.
C)Equilibrium is the result of the cessation of all chemical change.
D)There is only one set of equilibrium concentrations that equals the Kc value.
E)At equilibrium the sum of the concentration of products equals the total concentration of the reactants.
A)At equilibrium, the rate constant of the forward reaction is equal to the rate of the reverse reaction.
B)At equilibrium, the rate of the forward reaction is equal to the rate of the reverse reaction.
C)Equilibrium is the result of the cessation of all chemical change.
D)There is only one set of equilibrium concentrations that equals the Kc value.
E)At equilibrium the sum of the concentration of products equals the total concentration of the reactants.

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
5
In a study of the system, H2(g)+ I2(g)  2 HI(g), at 699 K, several different reaction mixtures, described below, were prepared, placed in 5.00 liter containers, and allowed to attain equilibrium at 699 K. Despite having the different starting compositions shown, four of the five mixtures had the identical composition at equilibrium. Which one of the systems attained a different equilibrium composition than the others?
2 HI(g), at 699 K, several different reaction mixtures, described below, were prepared, placed in 5.00 liter containers, and allowed to attain equilibrium at 699 K. Despite having the different starting compositions shown, four of the five mixtures had the identical composition at equilibrium. Which one of the systems attained a different equilibrium composition than the others?
A)System 1: 0.100 moles of H2, 0.000 moles of I2 and 0.600 moles of HI
B)System 2: 0.175 moles of H2, 0.075 moles of I2 and 0.450 moles of HI
C)System 3: 0.400 moles of H2, 0.300 moles of I2 and 0.000 moles of HI
D)System 4: 0.150 moles of H2, 0.100 moles of I2 and 0.500 moles of HI
E)System 5: 0.300 moles of H2, 0.200 moles of I2 and 0.200 moles of HI
 2 HI(g), at 699 K, several different reaction mixtures, described below, were prepared, placed in 5.00 liter containers, and allowed to attain equilibrium at 699 K. Despite having the different starting compositions shown, four of the five mixtures had the identical composition at equilibrium. Which one of the systems attained a different equilibrium composition than the others?
2 HI(g), at 699 K, several different reaction mixtures, described below, were prepared, placed in 5.00 liter containers, and allowed to attain equilibrium at 699 K. Despite having the different starting compositions shown, four of the five mixtures had the identical composition at equilibrium. Which one of the systems attained a different equilibrium composition than the others?A)System 1: 0.100 moles of H2, 0.000 moles of I2 and 0.600 moles of HI
B)System 2: 0.175 moles of H2, 0.075 moles of I2 and 0.450 moles of HI
C)System 3: 0.400 moles of H2, 0.300 moles of I2 and 0.000 moles of HI
D)System 4: 0.150 moles of H2, 0.100 moles of I2 and 0.500 moles of HI
E)System 5: 0.300 moles of H2, 0.200 moles of I2 and 0.200 moles of HI

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
6
Given the two reactions shown with their equilibrium constants, 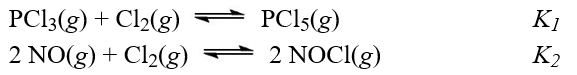 What is the equilibrium constant for the reaction,PCl5(g)+ 2 NO(g)
What is the equilibrium constant for the reaction,PCl5(g)+ 2 NO(g)  PCl3(g)+ 2 NOCl(g)
PCl3(g)+ 2 NOCl(g)
A)K1K2
B)K2/K1
C)K1/K2
D)(K1K2)?1
E)K2 ? K1
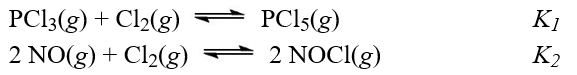 What is the equilibrium constant for the reaction,PCl5(g)+ 2 NO(g)
What is the equilibrium constant for the reaction,PCl5(g)+ 2 NO(g)  PCl3(g)+ 2 NOCl(g)
PCl3(g)+ 2 NOCl(g)A)K1K2
B)K2/K1
C)K1/K2
D)(K1K2)?1
E)K2 ? K1

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
7
In a study of the system, Cl2(g)+ Br2(g)  2 BrCl(g), at 350 K, several different reaction mixtures, described below, were prepared, placed in 5.00 liter containers, and allowed to attain equilibrium at 350 K. Despite having the different starting compositions shown, four of the five mixtures had the identical composition at equilibrium. Which one of the systems attained a different equilibrium composition than the others?
2 BrCl(g), at 350 K, several different reaction mixtures, described below, were prepared, placed in 5.00 liter containers, and allowed to attain equilibrium at 350 K. Despite having the different starting compositions shown, four of the five mixtures had the identical composition at equilibrium. Which one of the systems attained a different equilibrium composition than the others?
A)System 1: 0.100 moles of Cl2, 0.000 moles of Br2 and 0.600 moles of BrCl
B)System 2: 0.250 moles of Cl2, 0.150 moles of Br2 and 0.300 moles of BrCl
C)System 3: 0.400 moles of Cl2, 0.300 moles of Br2 and 0.000 moles of BrCl
D)System 4: 0.300 moles of Cl2, 0.250 moles of Br2 and 0.250 moles of BrCl
E)System 5: 0.350 moles of Cl2, 0.250 moles of Br2 and 0.100 moles of BrCl
 2 BrCl(g), at 350 K, several different reaction mixtures, described below, were prepared, placed in 5.00 liter containers, and allowed to attain equilibrium at 350 K. Despite having the different starting compositions shown, four of the five mixtures had the identical composition at equilibrium. Which one of the systems attained a different equilibrium composition than the others?
2 BrCl(g), at 350 K, several different reaction mixtures, described below, were prepared, placed in 5.00 liter containers, and allowed to attain equilibrium at 350 K. Despite having the different starting compositions shown, four of the five mixtures had the identical composition at equilibrium. Which one of the systems attained a different equilibrium composition than the others?A)System 1: 0.100 moles of Cl2, 0.000 moles of Br2 and 0.600 moles of BrCl
B)System 2: 0.250 moles of Cl2, 0.150 moles of Br2 and 0.300 moles of BrCl
C)System 3: 0.400 moles of Cl2, 0.300 moles of Br2 and 0.000 moles of BrCl
D)System 4: 0.300 moles of Cl2, 0.250 moles of Br2 and 0.250 moles of BrCl
E)System 5: 0.350 moles of Cl2, 0.250 moles of Br2 and 0.100 moles of BrCl

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
8
In a study of the system, N2O4(g)  2 NO2(g), at 585 K, several different reaction mixtures, described below, were prepared, placed in 5.00 liter containers, and allowed to attain equilibrium at 480 K. Despite having different starting compositions shown, four of the five mixtures had the identical composition at equilibrium. Which one of the systems attained a different equilibrium composition than the others?
2 NO2(g), at 585 K, several different reaction mixtures, described below, were prepared, placed in 5.00 liter containers, and allowed to attain equilibrium at 480 K. Despite having different starting compositions shown, four of the five mixtures had the identical composition at equilibrium. Which one of the systems attained a different equilibrium composition than the others?
A)System 1: 0.400 moles of N2O4 and 0.800 moles of NO2
B)System 2: 0.300 moles of N2O4 and 1.000 moles of NO2
C)System 3: 0.800 moles of N2O4 and 0.000 moles of NO2
D)System 4: 0.600 moles of N2O4 and 0.400 moles of NO2
E)System 5: 0.400 moles of N2O4 and 0.700 moles of NO2
 2 NO2(g), at 585 K, several different reaction mixtures, described below, were prepared, placed in 5.00 liter containers, and allowed to attain equilibrium at 480 K. Despite having different starting compositions shown, four of the five mixtures had the identical composition at equilibrium. Which one of the systems attained a different equilibrium composition than the others?
2 NO2(g), at 585 K, several different reaction mixtures, described below, were prepared, placed in 5.00 liter containers, and allowed to attain equilibrium at 480 K. Despite having different starting compositions shown, four of the five mixtures had the identical composition at equilibrium. Which one of the systems attained a different equilibrium composition than the others?A)System 1: 0.400 moles of N2O4 and 0.800 moles of NO2
B)System 2: 0.300 moles of N2O4 and 1.000 moles of NO2
C)System 3: 0.800 moles of N2O4 and 0.000 moles of NO2
D)System 4: 0.600 moles of N2O4 and 0.400 moles of NO2
E)System 5: 0.400 moles of N2O4 and 0.700 moles of NO2

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
9
In a study of the system, Cl2(g)+ 2 NO(g)  2 NOCl(g), at 425 K, several different reaction mixtures, described below, were prepared, placed in 5.00 liter containers, and allowed to attain equilibrium at 425 K. Despite having different starting compositions shown, four of the five mixtures had the identical composition at equilibrium. Which one of the systems attained a different equilibrium composition than the others?
2 NOCl(g), at 425 K, several different reaction mixtures, described below, were prepared, placed in 5.00 liter containers, and allowed to attain equilibrium at 425 K. Despite having different starting compositions shown, four of the five mixtures had the identical composition at equilibrium. Which one of the systems attained a different equilibrium composition than the others?
A)System 1: 0.100 moles of Cl2, 0.000 moles of NO and 0.600 moles of NOCl
B)System 2: 0.250 moles of Cl2, 0.300 moles of NO and 0.300 moles of NOCl
C)System 3: 0.400 moles of Cl2, 0.600 moles of NO and 0.000 moles of NOCl
D)System 4: 0.300 moles of Cl2, 0.250 moles of NO and 0.250 moles of NOCl
E)System 5: 0.350 moles of Cl2, 0.500 moles of NO and 0.100 moles of NOCl
 2 NOCl(g), at 425 K, several different reaction mixtures, described below, were prepared, placed in 5.00 liter containers, and allowed to attain equilibrium at 425 K. Despite having different starting compositions shown, four of the five mixtures had the identical composition at equilibrium. Which one of the systems attained a different equilibrium composition than the others?
2 NOCl(g), at 425 K, several different reaction mixtures, described below, were prepared, placed in 5.00 liter containers, and allowed to attain equilibrium at 425 K. Despite having different starting compositions shown, four of the five mixtures had the identical composition at equilibrium. Which one of the systems attained a different equilibrium composition than the others?A)System 1: 0.100 moles of Cl2, 0.000 moles of NO and 0.600 moles of NOCl
B)System 2: 0.250 moles of Cl2, 0.300 moles of NO and 0.300 moles of NOCl
C)System 3: 0.400 moles of Cl2, 0.600 moles of NO and 0.000 moles of NOCl
D)System 4: 0.300 moles of Cl2, 0.250 moles of NO and 0.250 moles of NOCl
E)System 5: 0.350 moles of Cl2, 0.500 moles of NO and 0.100 moles of NOCl

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
10
Which of the following is the correct equilibrium law for the following reaction?N2H4(g)+ 6H2O2(g) ![<strong>Which of the following is the correct equilibrium law for the following reaction?N<sub>2</sub>H<sub>4</sub>(g)+ 6H<sub>2</sub>O<sub>2</sub>(g) 2(g)+ 8H<sub>2</sub>O(g)</strong> A)K<sub>c</sub> = B)K<sub>c</sub> = [NO<sub>2</sub>]<sup>2</sup>[H<sub>2</sub>O]<sup>8</sup>[N<sub>2</sub>H<sub>4</sub>] [H<sub>2</sub>O<sub>2</sub>]<sup>6</sup> C)K<sub>c</sub> = [NO<sub>2</sub>]<sup> </sup>[H<sub>2</sub>O]<sup> </sup>[N<sub>2</sub>H<sub>4</sub>] [H<sub>2</sub>O<sub>2</sub>] D)K<sub>c</sub> = E)K<sub>c</sub> =](https://d2lvgg3v3hfg70.cloudfront.net/TBW1039/11ee6e7b_ba80_cd51_bf7f_d325b7708052_TBW1039_11.jpg) 2(g)+ 8H2O(g)
2(g)+ 8H2O(g)
A)Kc =
![<strong>Which of the following is the correct equilibrium law for the following reaction?N<sub>2</sub>H<sub>4</sub>(g)+ 6H<sub>2</sub>O<sub>2</sub>(g) 2(g)+ 8H<sub>2</sub>O(g)</strong> A)K<sub>c</sub> = B)K<sub>c</sub> = [NO<sub>2</sub>]<sup>2</sup>[H<sub>2</sub>O]<sup>8</sup>[N<sub>2</sub>H<sub>4</sub>] [H<sub>2</sub>O<sub>2</sub>]<sup>6</sup> C)K<sub>c</sub> = [NO<sub>2</sub>]<sup> </sup>[H<sub>2</sub>O]<sup> </sup>[N<sub>2</sub>H<sub>4</sub>] [H<sub>2</sub>O<sub>2</sub>] D)K<sub>c</sub> = E)K<sub>c</sub> =](https://d2lvgg3v3hfg70.cloudfront.net/TBW1039/11ee6e7b_ba80_cd52_bf7f_11a7e1a582bf_TBW1039_11.jpg)
B)Kc = [NO2]2[H2O]8[N2H4] [H2O2]6
C)Kc = [NO2] [H2O] [N2H4] [H2O2]
D)Kc =
![<strong>Which of the following is the correct equilibrium law for the following reaction?N<sub>2</sub>H<sub>4</sub>(g)+ 6H<sub>2</sub>O<sub>2</sub>(g) 2(g)+ 8H<sub>2</sub>O(g)</strong> A)K<sub>c</sub> = B)K<sub>c</sub> = [NO<sub>2</sub>]<sup>2</sup>[H<sub>2</sub>O]<sup>8</sup>[N<sub>2</sub>H<sub>4</sub>] [H<sub>2</sub>O<sub>2</sub>]<sup>6</sup> C)K<sub>c</sub> = [NO<sub>2</sub>]<sup> </sup>[H<sub>2</sub>O]<sup> </sup>[N<sub>2</sub>H<sub>4</sub>] [H<sub>2</sub>O<sub>2</sub>] D)K<sub>c</sub> = E)K<sub>c</sub> =](https://d2lvgg3v3hfg70.cloudfront.net/TBW1039/11ee6e7b_ba80_cd53_bf7f_2b8fea4d056c_TBW1039_11.jpg)
E)Kc =
![<strong>Which of the following is the correct equilibrium law for the following reaction?N<sub>2</sub>H<sub>4</sub>(g)+ 6H<sub>2</sub>O<sub>2</sub>(g) 2(g)+ 8H<sub>2</sub>O(g)</strong> A)K<sub>c</sub> = B)K<sub>c</sub> = [NO<sub>2</sub>]<sup>2</sup>[H<sub>2</sub>O]<sup>8</sup>[N<sub>2</sub>H<sub>4</sub>] [H<sub>2</sub>O<sub>2</sub>]<sup>6</sup> C)K<sub>c</sub> = [NO<sub>2</sub>]<sup> </sup>[H<sub>2</sub>O]<sup> </sup>[N<sub>2</sub>H<sub>4</sub>] [H<sub>2</sub>O<sub>2</sub>] D)K<sub>c</sub> = E)K<sub>c</sub> =](https://d2lvgg3v3hfg70.cloudfront.net/TBW1039/11ee6e7b_ba80_cd54_bf7f_dd3e0cb79a27_TBW1039_11.jpg)
![<strong>Which of the following is the correct equilibrium law for the following reaction?N<sub>2</sub>H<sub>4</sub>(g)+ 6H<sub>2</sub>O<sub>2</sub>(g) 2(g)+ 8H<sub>2</sub>O(g)</strong> A)K<sub>c</sub> = B)K<sub>c</sub> = [NO<sub>2</sub>]<sup>2</sup>[H<sub>2</sub>O]<sup>8</sup>[N<sub>2</sub>H<sub>4</sub>] [H<sub>2</sub>O<sub>2</sub>]<sup>6</sup> C)K<sub>c</sub> = [NO<sub>2</sub>]<sup> </sup>[H<sub>2</sub>O]<sup> </sup>[N<sub>2</sub>H<sub>4</sub>] [H<sub>2</sub>O<sub>2</sub>] D)K<sub>c</sub> = E)K<sub>c</sub> =](https://d2lvgg3v3hfg70.cloudfront.net/TBW1039/11ee6e7b_ba80_cd51_bf7f_d325b7708052_TBW1039_11.jpg) 2(g)+ 8H2O(g)
2(g)+ 8H2O(g)A)Kc =
![<strong>Which of the following is the correct equilibrium law for the following reaction?N<sub>2</sub>H<sub>4</sub>(g)+ 6H<sub>2</sub>O<sub>2</sub>(g) 2(g)+ 8H<sub>2</sub>O(g)</strong> A)K<sub>c</sub> = B)K<sub>c</sub> = [NO<sub>2</sub>]<sup>2</sup>[H<sub>2</sub>O]<sup>8</sup>[N<sub>2</sub>H<sub>4</sub>] [H<sub>2</sub>O<sub>2</sub>]<sup>6</sup> C)K<sub>c</sub> = [NO<sub>2</sub>]<sup> </sup>[H<sub>2</sub>O]<sup> </sup>[N<sub>2</sub>H<sub>4</sub>] [H<sub>2</sub>O<sub>2</sub>] D)K<sub>c</sub> = E)K<sub>c</sub> =](https://d2lvgg3v3hfg70.cloudfront.net/TBW1039/11ee6e7b_ba80_cd52_bf7f_11a7e1a582bf_TBW1039_11.jpg)
B)Kc = [NO2]2[H2O]8[N2H4] [H2O2]6
C)Kc = [NO2] [H2O] [N2H4] [H2O2]
D)Kc =
![<strong>Which of the following is the correct equilibrium law for the following reaction?N<sub>2</sub>H<sub>4</sub>(g)+ 6H<sub>2</sub>O<sub>2</sub>(g) 2(g)+ 8H<sub>2</sub>O(g)</strong> A)K<sub>c</sub> = B)K<sub>c</sub> = [NO<sub>2</sub>]<sup>2</sup>[H<sub>2</sub>O]<sup>8</sup>[N<sub>2</sub>H<sub>4</sub>] [H<sub>2</sub>O<sub>2</sub>]<sup>6</sup> C)K<sub>c</sub> = [NO<sub>2</sub>]<sup> </sup>[H<sub>2</sub>O]<sup> </sup>[N<sub>2</sub>H<sub>4</sub>] [H<sub>2</sub>O<sub>2</sub>] D)K<sub>c</sub> = E)K<sub>c</sub> =](https://d2lvgg3v3hfg70.cloudfront.net/TBW1039/11ee6e7b_ba80_cd53_bf7f_2b8fea4d056c_TBW1039_11.jpg)
E)Kc =
![<strong>Which of the following is the correct equilibrium law for the following reaction?N<sub>2</sub>H<sub>4</sub>(g)+ 6H<sub>2</sub>O<sub>2</sub>(g) 2(g)+ 8H<sub>2</sub>O(g)</strong> A)K<sub>c</sub> = B)K<sub>c</sub> = [NO<sub>2</sub>]<sup>2</sup>[H<sub>2</sub>O]<sup>8</sup>[N<sub>2</sub>H<sub>4</sub>] [H<sub>2</sub>O<sub>2</sub>]<sup>6</sup> C)K<sub>c</sub> = [NO<sub>2</sub>]<sup> </sup>[H<sub>2</sub>O]<sup> </sup>[N<sub>2</sub>H<sub>4</sub>] [H<sub>2</sub>O<sub>2</sub>] D)K<sub>c</sub> = E)K<sub>c</sub> =](https://d2lvgg3v3hfg70.cloudfront.net/TBW1039/11ee6e7b_ba80_cd54_bf7f_dd3e0cb79a27_TBW1039_11.jpg)

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
11
Which of the following is the correct equilibrium law for the following reaction?N2(g)+ 3H2(g) ![<strong>Which of the following is the correct equilibrium law for the following reaction?N<sub>2</sub>(g)+ 3H<sub>2</sub>(g) 2NH<sub>3</sub>(g)</strong> A)K<sub>c</sub> = B)K<sub>c</sub> = [NH<sub>3</sub>]<sup>2</sup>[N<sub>2</sub>] [H<sub>2</sub>]<sup>3</sup> C)K<sub>c</sub> = [NH<sub>3</sub>]<sup> </sup>[N<sub>2</sub>] [H<sub>2</sub>] D)K<sub>c</sub> = E)K<sub>c</sub> =](https://d2lvgg3v3hfg70.cloudfront.net/TBW1039/11ee6e7b_ba80_cd55_bf7f_5526e561c018_TBW1039_11.jpg) 2NH3(g)
2NH3(g)
A)Kc =
![<strong>Which of the following is the correct equilibrium law for the following reaction?N<sub>2</sub>(g)+ 3H<sub>2</sub>(g) 2NH<sub>3</sub>(g)</strong> A)K<sub>c</sub> = B)K<sub>c</sub> = [NH<sub>3</sub>]<sup>2</sup>[N<sub>2</sub>] [H<sub>2</sub>]<sup>3</sup> C)K<sub>c</sub> = [NH<sub>3</sub>]<sup> </sup>[N<sub>2</sub>] [H<sub>2</sub>] D)K<sub>c</sub> = E)K<sub>c</sub> =](https://d2lvgg3v3hfg70.cloudfront.net/TBW1039/11ee6e7b_ba80_cd56_bf7f_9dd45e6d8680_TBW1039_11.jpg)
B)Kc = [NH3]2[N2] [H2]3
C)Kc = [NH3] [N2] [H2]
D)Kc =
![<strong>Which of the following is the correct equilibrium law for the following reaction?N<sub>2</sub>(g)+ 3H<sub>2</sub>(g) 2NH<sub>3</sub>(g)</strong> A)K<sub>c</sub> = B)K<sub>c</sub> = [NH<sub>3</sub>]<sup>2</sup>[N<sub>2</sub>] [H<sub>2</sub>]<sup>3</sup> C)K<sub>c</sub> = [NH<sub>3</sub>]<sup> </sup>[N<sub>2</sub>] [H<sub>2</sub>] D)K<sub>c</sub> = E)K<sub>c</sub> =](https://d2lvgg3v3hfg70.cloudfront.net/TBW1039/11ee6e7b_ba80_f467_bf7f_691079e40f3f_TBW1039_11.jpg)
E)Kc =
![<strong>Which of the following is the correct equilibrium law for the following reaction?N<sub>2</sub>(g)+ 3H<sub>2</sub>(g) 2NH<sub>3</sub>(g)</strong> A)K<sub>c</sub> = B)K<sub>c</sub> = [NH<sub>3</sub>]<sup>2</sup>[N<sub>2</sub>] [H<sub>2</sub>]<sup>3</sup> C)K<sub>c</sub> = [NH<sub>3</sub>]<sup> </sup>[N<sub>2</sub>] [H<sub>2</sub>] D)K<sub>c</sub> = E)K<sub>c</sub> =](https://d2lvgg3v3hfg70.cloudfront.net/TBW1039/11ee6e7b_ba80_f468_bf7f_97b1cdc9efdf_TBW1039_11.jpg)
![<strong>Which of the following is the correct equilibrium law for the following reaction?N<sub>2</sub>(g)+ 3H<sub>2</sub>(g) 2NH<sub>3</sub>(g)</strong> A)K<sub>c</sub> = B)K<sub>c</sub> = [NH<sub>3</sub>]<sup>2</sup>[N<sub>2</sub>] [H<sub>2</sub>]<sup>3</sup> C)K<sub>c</sub> = [NH<sub>3</sub>]<sup> </sup>[N<sub>2</sub>] [H<sub>2</sub>] D)K<sub>c</sub> = E)K<sub>c</sub> =](https://d2lvgg3v3hfg70.cloudfront.net/TBW1039/11ee6e7b_ba80_cd55_bf7f_5526e561c018_TBW1039_11.jpg) 2NH3(g)
2NH3(g)A)Kc =
![<strong>Which of the following is the correct equilibrium law for the following reaction?N<sub>2</sub>(g)+ 3H<sub>2</sub>(g) 2NH<sub>3</sub>(g)</strong> A)K<sub>c</sub> = B)K<sub>c</sub> = [NH<sub>3</sub>]<sup>2</sup>[N<sub>2</sub>] [H<sub>2</sub>]<sup>3</sup> C)K<sub>c</sub> = [NH<sub>3</sub>]<sup> </sup>[N<sub>2</sub>] [H<sub>2</sub>] D)K<sub>c</sub> = E)K<sub>c</sub> =](https://d2lvgg3v3hfg70.cloudfront.net/TBW1039/11ee6e7b_ba80_cd56_bf7f_9dd45e6d8680_TBW1039_11.jpg)
B)Kc = [NH3]2[N2] [H2]3
C)Kc = [NH3] [N2] [H2]
D)Kc =
![<strong>Which of the following is the correct equilibrium law for the following reaction?N<sub>2</sub>(g)+ 3H<sub>2</sub>(g) 2NH<sub>3</sub>(g)</strong> A)K<sub>c</sub> = B)K<sub>c</sub> = [NH<sub>3</sub>]<sup>2</sup>[N<sub>2</sub>] [H<sub>2</sub>]<sup>3</sup> C)K<sub>c</sub> = [NH<sub>3</sub>]<sup> </sup>[N<sub>2</sub>] [H<sub>2</sub>] D)K<sub>c</sub> = E)K<sub>c</sub> =](https://d2lvgg3v3hfg70.cloudfront.net/TBW1039/11ee6e7b_ba80_f467_bf7f_691079e40f3f_TBW1039_11.jpg)
E)Kc =
![<strong>Which of the following is the correct equilibrium law for the following reaction?N<sub>2</sub>(g)+ 3H<sub>2</sub>(g) 2NH<sub>3</sub>(g)</strong> A)K<sub>c</sub> = B)K<sub>c</sub> = [NH<sub>3</sub>]<sup>2</sup>[N<sub>2</sub>] [H<sub>2</sub>]<sup>3</sup> C)K<sub>c</sub> = [NH<sub>3</sub>]<sup> </sup>[N<sub>2</sub>] [H<sub>2</sub>] D)K<sub>c</sub> = E)K<sub>c</sub> =](https://d2lvgg3v3hfg70.cloudfront.net/TBW1039/11ee6e7b_ba80_f468_bf7f_97b1cdc9efdf_TBW1039_11.jpg)

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
12
Given the two reactions shown with their equilibrium constants, 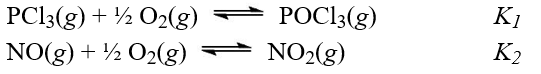 What is the equilibrium constant for the reaction,PCl3(g)+ NO2(g)
What is the equilibrium constant for the reaction,PCl3(g)+ NO2(g)  POCl3(g)+ NO(g)
POCl3(g)+ NO(g)
A)K1K2
B)K2/K1
C)K1/K2
D)(K1K2)-1
E)K2 - K1
 What is the equilibrium constant for the reaction,PCl3(g)+ NO2(g)
What is the equilibrium constant for the reaction,PCl3(g)+ NO2(g)  POCl3(g)+ NO(g)
POCl3(g)+ NO(g)A)K1K2
B)K2/K1
C)K1/K2
D)(K1K2)-1
E)K2 - K1

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
13
Consider the following reactions and their associated equilibrium constants:  For the reaction A + 2B
For the reaction A + 2B  D + E, having equilibrium constant Kc,
D + E, having equilibrium constant Kc,
A)Kc = (K1)(K2)
B)Kc = K2/K1
C)Kc = K1/K2
D)Kc = K1 -K2
E)Kc = K1 + K2
 For the reaction A + 2B
For the reaction A + 2B  D + E, having equilibrium constant Kc,
D + E, having equilibrium constant Kc,A)Kc = (K1)(K2)
B)Kc = K2/K1
C)Kc = K1/K2
D)Kc = K1 -K2
E)Kc = K1 + K2

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
14
The equilibrium constant for the reaction, H2(g)+ I2(g)  2 HI(g)is 54.9 at 699.0 K. What is the equilibrium constant for 4 HI(g)
2 HI(g)is 54.9 at 699.0 K. What is the equilibrium constant for 4 HI(g)  2 H2(g)+ 2 I2(g)under the same conditions?
2 H2(g)+ 2 I2(g)under the same conditions?
A)109.8
B)0.00911
C)0.000332
D)?109.8
E)0.0182
 2 HI(g)is 54.9 at 699.0 K. What is the equilibrium constant for 4 HI(g)
2 HI(g)is 54.9 at 699.0 K. What is the equilibrium constant for 4 HI(g)  2 H2(g)+ 2 I2(g)under the same conditions?
2 H2(g)+ 2 I2(g)under the same conditions?A)109.8
B)0.00911
C)0.000332
D)?109.8
E)0.0182

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
15
For a specific reaction, which statement can be made about the equilibrium constant?
A)It can change with temperature.
B)It may be changed by addition of a catalyst.
C)It increases if the concentration of one of the products is increased.
D)It increases if the concentration of one of the products is decreased.
E)It always remains the same.
A)It can change with temperature.
B)It may be changed by addition of a catalyst.
C)It increases if the concentration of one of the products is increased.
D)It increases if the concentration of one of the products is decreased.
E)It always remains the same.

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
16
Using this data,  calculate a value for Kc for the reaction,NOCl(g)+ ½ O2(g)
calculate a value for Kc for the reaction,NOCl(g)+ ½ O2(g)  NO2(g)+ ½ Cl2(g)
NO2(g)+ ½ Cl2(g)
A)2.06 × 10-4
B)4.84 × 10-3
C)0.223
D)4.49
E)20.2
 calculate a value for Kc for the reaction,NOCl(g)+ ½ O2(g)
calculate a value for Kc for the reaction,NOCl(g)+ ½ O2(g)  NO2(g)+ ½ Cl2(g)
NO2(g)+ ½ Cl2(g)A)2.06 × 10-4
B)4.84 × 10-3
C)0.223
D)4.49
E)20.2

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
17
For the reaction, 2SO2(g)+ O2(g)  2SO3(g), at 900.0 K the equilibrium constant, Kc, has a value of 13.0. Calculate the value of Kp at the same temperature.
2SO3(g), at 900.0 K the equilibrium constant, Kc, has a value of 13.0. Calculate the value of Kp at the same temperature.
A)97.3 × 103
B)0.176
C)960
D)0.00174
E)0.077
 2SO3(g), at 900.0 K the equilibrium constant, Kc, has a value of 13.0. Calculate the value of Kp at the same temperature.
2SO3(g), at 900.0 K the equilibrium constant, Kc, has a value of 13.0. Calculate the value of Kp at the same temperature.A)97.3 × 103
B)0.176
C)960
D)0.00174
E)0.077

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
18
A student is preparing a study of the reaction, 2CO2(g)  2CO(g)+ O2(g), for which
2CO(g)+ O2(g), for which 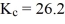 at 827 °C. What is the value of Kp at that same temperature?
at 827 °C. What is the value of Kp at that same temperature?
A)2.90 × 10-1
B)3.86 × 10-1
C)1.78 × 103
D)2.37 × 103
E)2.40 × 105
 2CO(g)+ O2(g), for which
2CO(g)+ O2(g), for which 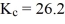 at 827 °C. What is the value of Kp at that same temperature?
at 827 °C. What is the value of Kp at that same temperature?A)2.90 × 10-1
B)3.86 × 10-1
C)1.78 × 103
D)2.37 × 103
E)2.40 × 105

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
19
For which one of the following reactions is Kp equal to Kc?
A)4NH3(g)+ 5O2(g) 4NO(g)+ 6H2O(g)
4NO(g)+ 6H2O(g)
B)C(s)+ CO2(g) 2CO(g)
2CO(g)
C)6CO2(g)+ 6H2O(l) C6H12O6(s)+ 6O2(g)
C6H12O6(s)+ 6O2(g)
D)CaCO3(s) CaO(s)+ CO2(g)
CaO(s)+ CO2(g)
E)H2O(g)+ C(s) H2(g)+ CO(g)
H2(g)+ CO(g)
A)4NH3(g)+ 5O2(g)
 4NO(g)+ 6H2O(g)
4NO(g)+ 6H2O(g)B)C(s)+ CO2(g)
 2CO(g)
2CO(g)C)6CO2(g)+ 6H2O(l)
 C6H12O6(s)+ 6O2(g)
C6H12O6(s)+ 6O2(g)D)CaCO3(s)
 CaO(s)+ CO2(g)
CaO(s)+ CO2(g)E)H2O(g)+ C(s)
 H2(g)+ CO(g)
H2(g)+ CO(g)
Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
20
For which one of the following reactions is Kp equal to Kc?
A)3O2(g) 2O3(g)
2O3(g)
B)2NH3(g) 3H2(g)+ N2(g)
3H2(g)+ N2(g)
C)CaCO3(s) CaO(s)+ CO2(g)
CaO(s)+ CO2(g)
D)NH4I(s) NH3(g)+ HI(g)
NH3(g)+ HI(g)
E)SnO2(s)+ 2H2(g) Sn(s)+ 2H2O(g)
Sn(s)+ 2H2O(g)
A)3O2(g)
 2O3(g)
2O3(g)B)2NH3(g)
 3H2(g)+ N2(g)
3H2(g)+ N2(g)C)CaCO3(s)
 CaO(s)+ CO2(g)
CaO(s)+ CO2(g)D)NH4I(s)
 NH3(g)+ HI(g)
NH3(g)+ HI(g)E)SnO2(s)+ 2H2(g)
 Sn(s)+ 2H2O(g)
Sn(s)+ 2H2O(g)
Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
21
The concentration of a pure solid or a pure liquid is left out of the expression for the equilibrium constant because
A)solids and liquids drive the reaction in an undetermined fashion.
B)solids and liquids do not react.
C)their concentrations cannot be determined.
D)solids and liquids react too slowly.
E)their activity is constant and independent of the amount of solid or liquid present.
A)solids and liquids drive the reaction in an undetermined fashion.
B)solids and liquids do not react.
C)their concentrations cannot be determined.
D)solids and liquids react too slowly.
E)their activity is constant and independent of the amount of solid or liquid present.

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
22
Write the mass action expression for the following reaction:4Cr(s)+ 3CCl4(g) ![<strong>Write the mass action expression for the following reaction:4Cr(s)+ 3CCl<sub>4</sub>(g) 4CrCl<sub>3</sub>(g)+ 4C(s)</strong> A)K<sub>c</sub> = B)K<sub>c</sub> = C)K<sub>c</sub> = D)K<sub>c</sub> = E)K<sub>c</sub> = [CrCl<sub>3</sub>]<sup>4</sup> + [CCl<sub>4</sub>]<sup>3</sup>](https://d2lvgg3v3hfg70.cloudfront.net/TBW1039/11ee6e7b_ba81_69ae_bf7f_ad24def5c9ca_TBW1039_11.jpg) 4CrCl3(g)+ 4C(s)
4CrCl3(g)+ 4C(s)
A)Kc =
![<strong>Write the mass action expression for the following reaction:4Cr(s)+ 3CCl<sub>4</sub>(g) 4CrCl<sub>3</sub>(g)+ 4C(s)</strong> A)K<sub>c</sub> = B)K<sub>c</sub> = C)K<sub>c</sub> = D)K<sub>c</sub> = E)K<sub>c</sub> = [CrCl<sub>3</sub>]<sup>4</sup> + [CCl<sub>4</sub>]<sup>3</sup>](https://d2lvgg3v3hfg70.cloudfront.net/TBW1039/11ee6e7b_ba81_69af_bf7f_d9e2ed7da608_TBW1039_11.jpg)
B)Kc =
![<strong>Write the mass action expression for the following reaction:4Cr(s)+ 3CCl<sub>4</sub>(g) 4CrCl<sub>3</sub>(g)+ 4C(s)</strong> A)K<sub>c</sub> = B)K<sub>c</sub> = C)K<sub>c</sub> = D)K<sub>c</sub> = E)K<sub>c</sub> = [CrCl<sub>3</sub>]<sup>4</sup> + [CCl<sub>4</sub>]<sup>3</sup>](https://d2lvgg3v3hfg70.cloudfront.net/TBW1039/11ee6e7b_ba81_69b0_bf7f_fb8e65339f13_TBW1039_11.jpg)
C)Kc =
![<strong>Write the mass action expression for the following reaction:4Cr(s)+ 3CCl<sub>4</sub>(g) 4CrCl<sub>3</sub>(g)+ 4C(s)</strong> A)K<sub>c</sub> = B)K<sub>c</sub> = C)K<sub>c</sub> = D)K<sub>c</sub> = E)K<sub>c</sub> = [CrCl<sub>3</sub>]<sup>4</sup> + [CCl<sub>4</sub>]<sup>3</sup>](https://d2lvgg3v3hfg70.cloudfront.net/TBW1039/11ee6e7b_ba81_69b1_bf7f_adf100cff37b_TBW1039_11.jpg)
D)Kc =
![<strong>Write the mass action expression for the following reaction:4Cr(s)+ 3CCl<sub>4</sub>(g) 4CrCl<sub>3</sub>(g)+ 4C(s)</strong> A)K<sub>c</sub> = B)K<sub>c</sub> = C)K<sub>c</sub> = D)K<sub>c</sub> = E)K<sub>c</sub> = [CrCl<sub>3</sub>]<sup>4</sup> + [CCl<sub>4</sub>]<sup>3</sup>](https://d2lvgg3v3hfg70.cloudfront.net/TBW1039/11ee6e7b_ba81_69b2_bf7f_67eee919a9e0_TBW1039_11.jpg)
E)Kc = [CrCl3]4 + [CCl4]3
![<strong>Write the mass action expression for the following reaction:4Cr(s)+ 3CCl<sub>4</sub>(g) 4CrCl<sub>3</sub>(g)+ 4C(s)</strong> A)K<sub>c</sub> = B)K<sub>c</sub> = C)K<sub>c</sub> = D)K<sub>c</sub> = E)K<sub>c</sub> = [CrCl<sub>3</sub>]<sup>4</sup> + [CCl<sub>4</sub>]<sup>3</sup>](https://d2lvgg3v3hfg70.cloudfront.net/TBW1039/11ee6e7b_ba81_69ae_bf7f_ad24def5c9ca_TBW1039_11.jpg) 4CrCl3(g)+ 4C(s)
4CrCl3(g)+ 4C(s)A)Kc =
![<strong>Write the mass action expression for the following reaction:4Cr(s)+ 3CCl<sub>4</sub>(g) 4CrCl<sub>3</sub>(g)+ 4C(s)</strong> A)K<sub>c</sub> = B)K<sub>c</sub> = C)K<sub>c</sub> = D)K<sub>c</sub> = E)K<sub>c</sub> = [CrCl<sub>3</sub>]<sup>4</sup> + [CCl<sub>4</sub>]<sup>3</sup>](https://d2lvgg3v3hfg70.cloudfront.net/TBW1039/11ee6e7b_ba81_69af_bf7f_d9e2ed7da608_TBW1039_11.jpg)
B)Kc =
![<strong>Write the mass action expression for the following reaction:4Cr(s)+ 3CCl<sub>4</sub>(g) 4CrCl<sub>3</sub>(g)+ 4C(s)</strong> A)K<sub>c</sub> = B)K<sub>c</sub> = C)K<sub>c</sub> = D)K<sub>c</sub> = E)K<sub>c</sub> = [CrCl<sub>3</sub>]<sup>4</sup> + [CCl<sub>4</sub>]<sup>3</sup>](https://d2lvgg3v3hfg70.cloudfront.net/TBW1039/11ee6e7b_ba81_69b0_bf7f_fb8e65339f13_TBW1039_11.jpg)
C)Kc =
![<strong>Write the mass action expression for the following reaction:4Cr(s)+ 3CCl<sub>4</sub>(g) 4CrCl<sub>3</sub>(g)+ 4C(s)</strong> A)K<sub>c</sub> = B)K<sub>c</sub> = C)K<sub>c</sub> = D)K<sub>c</sub> = E)K<sub>c</sub> = [CrCl<sub>3</sub>]<sup>4</sup> + [CCl<sub>4</sub>]<sup>3</sup>](https://d2lvgg3v3hfg70.cloudfront.net/TBW1039/11ee6e7b_ba81_69b1_bf7f_adf100cff37b_TBW1039_11.jpg)
D)Kc =
![<strong>Write the mass action expression for the following reaction:4Cr(s)+ 3CCl<sub>4</sub>(g) 4CrCl<sub>3</sub>(g)+ 4C(s)</strong> A)K<sub>c</sub> = B)K<sub>c</sub> = C)K<sub>c</sub> = D)K<sub>c</sub> = E)K<sub>c</sub> = [CrCl<sub>3</sub>]<sup>4</sup> + [CCl<sub>4</sub>]<sup>3</sup>](https://d2lvgg3v3hfg70.cloudfront.net/TBW1039/11ee6e7b_ba81_69b2_bf7f_67eee919a9e0_TBW1039_11.jpg)
E)Kc = [CrCl3]4 + [CCl4]3

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
23
The equilibrium law for the system, CaO(s)+ CO2(g) ![<strong>The equilibrium law for the system, CaO(s)+ CO<sub>2</sub>(g) CaCO<sub>3</sub>(s), is</strong> A)K<sub>c</sub> = [CO<sub>2</sub>]. B)K<sub>c</sub> = [CaCO<sub>3</sub>]/([CaO] [CO<sub>2</sub>]). C)K<sub>c</sub> = 1/[CO<sub>2</sub>]. D)K<sub>c</sub> = [CaCO<sub>3</sub>]/[CaO]. E)K<sub>c</sub> = ([CaO] [CO<sub>2</sub>])/[CaCO<sub>3</sub>].](https://d2lvgg3v3hfg70.cloudfront.net/TBW1039/11ee6e7b_ba81_69b3_bf7f_c1bd90ca84c4_TBW1039_11.jpg) CaCO3(s), is
CaCO3(s), is
A)Kc = [CO2].
B)Kc = [CaCO3]/([CaO] [CO2]).
C)Kc = 1/[CO2].
D)Kc = [CaCO3]/[CaO].
E)Kc = ([CaO] [CO2])/[CaCO3].
![<strong>The equilibrium law for the system, CaO(s)+ CO<sub>2</sub>(g) CaCO<sub>3</sub>(s), is</strong> A)K<sub>c</sub> = [CO<sub>2</sub>]. B)K<sub>c</sub> = [CaCO<sub>3</sub>]/([CaO] [CO<sub>2</sub>]). C)K<sub>c</sub> = 1/[CO<sub>2</sub>]. D)K<sub>c</sub> = [CaCO<sub>3</sub>]/[CaO]. E)K<sub>c</sub> = ([CaO] [CO<sub>2</sub>])/[CaCO<sub>3</sub>].](https://d2lvgg3v3hfg70.cloudfront.net/TBW1039/11ee6e7b_ba81_69b3_bf7f_c1bd90ca84c4_TBW1039_11.jpg) CaCO3(s), is
CaCO3(s), isA)Kc = [CO2].
B)Kc = [CaCO3]/([CaO] [CO2]).
C)Kc = 1/[CO2].
D)Kc = [CaCO3]/[CaO].
E)Kc = ([CaO] [CO2])/[CaCO3].

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
24
For the chemical reaction, N2(g)+ 3H2(g) ![<strong>For the chemical reaction, N<sub>2</sub>(g)+ 3H<sub>2</sub>(g) 2NH<sub>3</sub>(g),K<sub>c</sub> = 4.2 × 10<sup>2</sup> at some temperature that we call T<sub>1</sub>. 4.00 moles of NH<sub>3</sub> were placed in a 50.0-liter container at T<sub>1</sub> and allowed to come to equilibrium. Which situation below is true at equilibrium?</strong> A)[NH<sub>3</sub>] = 3 × [H<sub>2</sub>] B)[NH<sub>3</sub>] > [H<sub>2</sub>] C)[H<sub>2</sub>] > [NH<sub>3</sub>] D)[NH<sub>3</sub>] = [H<sub>2</sub>] E)[N<sub>2</sub>] = [NH<sub>3</sub>]](https://d2lvgg3v3hfg70.cloudfront.net/TBW1039/11ee6e7b_ba81_90c4_bf7f_7f60032b9736_TBW1039_11.jpg) 2NH3(g),Kc = 4.2 × 102 at some temperature that we call T1. 4.00 moles of NH3 were placed in a 50.0-liter container at T1 and allowed to come to equilibrium. Which situation below is true at equilibrium?
2NH3(g),Kc = 4.2 × 102 at some temperature that we call T1. 4.00 moles of NH3 were placed in a 50.0-liter container at T1 and allowed to come to equilibrium. Which situation below is true at equilibrium?
A)[NH3] = 3 × [H2]
B)[NH3] > [H2]
C)[H2] > [NH3]
D)[NH3] = [H2]
E)[N2] = [NH3]
![<strong>For the chemical reaction, N<sub>2</sub>(g)+ 3H<sub>2</sub>(g) 2NH<sub>3</sub>(g),K<sub>c</sub> = 4.2 × 10<sup>2</sup> at some temperature that we call T<sub>1</sub>. 4.00 moles of NH<sub>3</sub> were placed in a 50.0-liter container at T<sub>1</sub> and allowed to come to equilibrium. Which situation below is true at equilibrium?</strong> A)[NH<sub>3</sub>] = 3 × [H<sub>2</sub>] B)[NH<sub>3</sub>] > [H<sub>2</sub>] C)[H<sub>2</sub>] > [NH<sub>3</sub>] D)[NH<sub>3</sub>] = [H<sub>2</sub>] E)[N<sub>2</sub>] = [NH<sub>3</sub>]](https://d2lvgg3v3hfg70.cloudfront.net/TBW1039/11ee6e7b_ba81_90c4_bf7f_7f60032b9736_TBW1039_11.jpg) 2NH3(g),Kc = 4.2 × 102 at some temperature that we call T1. 4.00 moles of NH3 were placed in a 50.0-liter container at T1 and allowed to come to equilibrium. Which situation below is true at equilibrium?
2NH3(g),Kc = 4.2 × 102 at some temperature that we call T1. 4.00 moles of NH3 were placed in a 50.0-liter container at T1 and allowed to come to equilibrium. Which situation below is true at equilibrium?A)[NH3] = 3 × [H2]
B)[NH3] > [H2]
C)[H2] > [NH3]
D)[NH3] = [H2]
E)[N2] = [NH3]

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
25
The equilibrium constant for the reaction, R2  D2 is 6.8 × 10?10. Which one of the following statements is true?
D2 is 6.8 × 10?10. Which one of the following statements is true?
A)The equilibrium concentration of D2 is always greater than that of R2.
B)The equilibrium concentration of R2 is always greater than that of D2.
C)Adding more R2 will increase the value of the equilibrium constant.
D)Adding a catalyst will increase the equilibrium concentration of D2.
E)Adding a catalyst will increase the value of the equilibrium constant.
 D2 is 6.8 × 10?10. Which one of the following statements is true?
D2 is 6.8 × 10?10. Which one of the following statements is true?A)The equilibrium concentration of D2 is always greater than that of R2.
B)The equilibrium concentration of R2 is always greater than that of D2.
C)Adding more R2 will increase the value of the equilibrium constant.
D)Adding a catalyst will increase the equilibrium concentration of D2.
E)Adding a catalyst will increase the value of the equilibrium constant.

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
26
The following reactions occur at 298 K. Arrange them in order of increasing tendency to proceed to completion (least completion ? greatest completion) 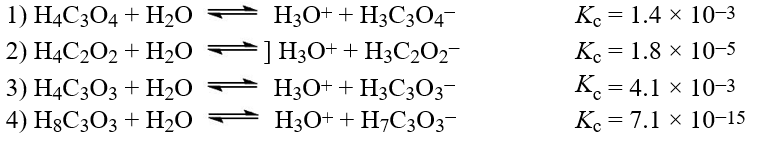
A)3 < 1 < 2 < 4
B)4 < 2 < 1 < 3
C)4 < 3 < 2 < 1
D)4 < 2 < 3 < 1
E)3 < 2 < 4 < 1
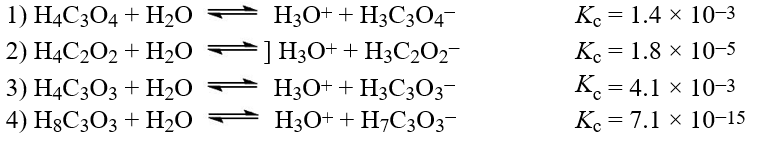
A)3 < 1 < 2 < 4
B)4 < 2 < 1 < 3
C)4 < 3 < 2 < 1
D)4 < 2 < 3 < 1
E)3 < 2 < 4 < 1

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
27
The following reactions occur at 298 K. Arrange them in order of increasing tendency to proceed to completion (least completion ? greatest completion). 
A)1 < 2 < 4 < 3
B)3 < 1 < 2 < 4
C)3 < 4 < 2 < 1
D)1 < 3 < 2 < 4
E)4 < 1 < 2 < 3

A)1 < 2 < 4 < 3
B)3 < 1 < 2 < 4
C)3 < 4 < 2 < 1
D)1 < 3 < 2 < 4
E)4 < 1 < 2 < 3

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
28
For a system, H2(g)+ I2(g) ![<strong>For a system, H<sub>2</sub>(g)+ I<sub>2</sub>(g) 2 HI(g), K<sub>c</sub> = 62.9 at 750 K. 2.80 moles of HI were placed in a 10.0-liter container, it was brought up to 750 K, and allowed to come to equilibrium. Which situation described below is true, at equilibrium?</strong> A)[HI] = 2 × [H<sub>2</sub>] B)[HI] = [H<sub>2</sub>] C)[HI] < [H<sub>2</sub>] D)[HI] > [H<sub>2</sub>] E)[H<sub>2</sub>] > [I<sub>2</sub>]](https://d2lvgg3v3hfg70.cloudfront.net/TBW1039/11ee6e7b_ba81_b7d8_bf7f_710c2233b6a8_TBW1039_11.jpg) 2 HI(g), Kc = 62.9 at 750 K. 2.80 moles of HI were placed in a 10.0-liter container, it was brought up to 750 K, and allowed to come to equilibrium. Which situation described below is true, at equilibrium?
2 HI(g), Kc = 62.9 at 750 K. 2.80 moles of HI were placed in a 10.0-liter container, it was brought up to 750 K, and allowed to come to equilibrium. Which situation described below is true, at equilibrium?
A)[HI] = 2 × [H2]
B)[HI] = [H2]
C)[HI] < [H2]
D)[HI] > [H2]
E)[H2] > [I2]
![<strong>For a system, H<sub>2</sub>(g)+ I<sub>2</sub>(g) 2 HI(g), K<sub>c</sub> = 62.9 at 750 K. 2.80 moles of HI were placed in a 10.0-liter container, it was brought up to 750 K, and allowed to come to equilibrium. Which situation described below is true, at equilibrium?</strong> A)[HI] = 2 × [H<sub>2</sub>] B)[HI] = [H<sub>2</sub>] C)[HI] < [H<sub>2</sub>] D)[HI] > [H<sub>2</sub>] E)[H<sub>2</sub>] > [I<sub>2</sub>]](https://d2lvgg3v3hfg70.cloudfront.net/TBW1039/11ee6e7b_ba81_b7d8_bf7f_710c2233b6a8_TBW1039_11.jpg) 2 HI(g), Kc = 62.9 at 750 K. 2.80 moles of HI were placed in a 10.0-liter container, it was brought up to 750 K, and allowed to come to equilibrium. Which situation described below is true, at equilibrium?
2 HI(g), Kc = 62.9 at 750 K. 2.80 moles of HI were placed in a 10.0-liter container, it was brought up to 750 K, and allowed to come to equilibrium. Which situation described below is true, at equilibrium?A)[HI] = 2 × [H2]
B)[HI] = [H2]
C)[HI] < [H2]
D)[HI] > [H2]
E)[H2] > [I2]

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
29
The following reactions have equilibrium values all measured at 500 K. Arrange them in order of increasing tendency to proceed to completion (least completion ? greatest completion). 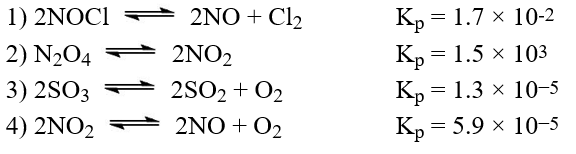
A)2 < 1 < 3 < 4
B)4 < 3 < 1 < 2
C)3 < 1 < 4 < 2
D)3 < 4 < 1 < 2
E)4 < 3 < 2 < 1
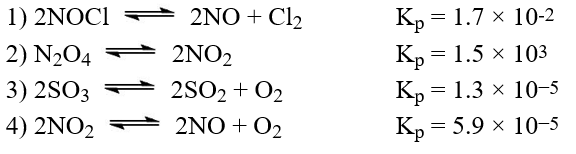
A)2 < 1 < 3 < 4
B)4 < 3 < 1 < 2
C)3 < 1 < 4 < 2
D)3 < 4 < 1 < 2
E)4 < 3 < 2 < 1

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
30
For the reaction, 2SO2(g)+ O2(g) ![<strong>For the reaction, 2SO<sub>2</sub>(g)+ O<sub>2</sub>(g) 2SO<sub>3</sub>(g), at 450.0 K the equilibrium constant, K<sub>c</sub>, has a value of 4.62. A system was charged to give these initial concentrations, [SO<sub>3</sub>] = 0.254 M, [O<sub>2</sub>] = 0.00855 M, [SO<sub>2</sub>] = 0.500 M. In which direction will it go? Hint: Find the value for Q and then compare that value to the given value for K<sub>c</sub>.</strong> A)to the right or the left depending on the pressure B)to the left C)it will remain at the same concentrations D)to the right E)to the right or the left depending on the volume](https://d2lvgg3v3hfg70.cloudfront.net/TBW1039/11ee6e7b_ba81_deea_bf7f_8555db019309_TBW1039_11.jpg) 2SO3(g), at 450.0 K the equilibrium constant, Kc, has a value of 4.62. A system was charged to give these initial concentrations, [SO3] = 0.254 M, [O2] = 0.00855 M, [SO2] = 0.500 M. In which direction will it go? Hint: Find the value for Q and then compare that value to the given value for Kc.
2SO3(g), at 450.0 K the equilibrium constant, Kc, has a value of 4.62. A system was charged to give these initial concentrations, [SO3] = 0.254 M, [O2] = 0.00855 M, [SO2] = 0.500 M. In which direction will it go? Hint: Find the value for Q and then compare that value to the given value for Kc.
A)to the right or the left depending on the pressure
B)to the left
C)it will remain at the same concentrations
D)to the right
E)to the right or the left depending on the volume
![<strong>For the reaction, 2SO<sub>2</sub>(g)+ O<sub>2</sub>(g) 2SO<sub>3</sub>(g), at 450.0 K the equilibrium constant, K<sub>c</sub>, has a value of 4.62. A system was charged to give these initial concentrations, [SO<sub>3</sub>] = 0.254 M, [O<sub>2</sub>] = 0.00855 M, [SO<sub>2</sub>] = 0.500 M. In which direction will it go? Hint: Find the value for Q and then compare that value to the given value for K<sub>c</sub>.</strong> A)to the right or the left depending on the pressure B)to the left C)it will remain at the same concentrations D)to the right E)to the right or the left depending on the volume](https://d2lvgg3v3hfg70.cloudfront.net/TBW1039/11ee6e7b_ba81_deea_bf7f_8555db019309_TBW1039_11.jpg) 2SO3(g), at 450.0 K the equilibrium constant, Kc, has a value of 4.62. A system was charged to give these initial concentrations, [SO3] = 0.254 M, [O2] = 0.00855 M, [SO2] = 0.500 M. In which direction will it go? Hint: Find the value for Q and then compare that value to the given value for Kc.
2SO3(g), at 450.0 K the equilibrium constant, Kc, has a value of 4.62. A system was charged to give these initial concentrations, [SO3] = 0.254 M, [O2] = 0.00855 M, [SO2] = 0.500 M. In which direction will it go? Hint: Find the value for Q and then compare that value to the given value for Kc.A)to the right or the left depending on the pressure
B)to the left
C)it will remain at the same concentrations
D)to the right
E)to the right or the left depending on the volume

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
31
For the reaction, 2SO2(g)+ O2(g) ![<strong>For the reaction, 2SO<sub>2</sub>(g)+ O<sub>2</sub>(g) 2SO<sub>3</sub>(g), at 450.0 K the equilibrium constant, K<sub>c</sub>, has a value of 4.62. A system was charged to give these initial concentrations: [SO<sub>3</sub>] = 0.500 M, [O<sub>2</sub>] = 0.00855 M, [SO<sub>2</sub>] = 0.254 M. In which direction will it go?Hint: Find the value for Q and then compare that value to the given value for K<sub>c</sub></strong> A)to the right B)it will remain at the same concentrations C)to the left D)to the right or the left depending on the pressure E)to the right or the left depending on the volume](https://d2lvgg3v3hfg70.cloudfront.net/TBW1039/11ee6e7b_ba81_deeb_bf7f_d35115c95bc2_TBW1039_11.jpg) 2SO3(g), at 450.0 K the equilibrium constant, Kc, has a value of 4.62. A system was charged to give these initial concentrations: [SO3] = 0.500 M, [O2] = 0.00855 M, [SO2] = 0.254 M. In which direction will it go?Hint: Find the value for Q and then compare that value to the given value for Kc
2SO3(g), at 450.0 K the equilibrium constant, Kc, has a value of 4.62. A system was charged to give these initial concentrations: [SO3] = 0.500 M, [O2] = 0.00855 M, [SO2] = 0.254 M. In which direction will it go?Hint: Find the value for Q and then compare that value to the given value for Kc
A)to the right
B)it will remain at the same concentrations
C)to the left
D)to the right or the left depending on the pressure
E)to the right or the left depending on the volume
![<strong>For the reaction, 2SO<sub>2</sub>(g)+ O<sub>2</sub>(g) 2SO<sub>3</sub>(g), at 450.0 K the equilibrium constant, K<sub>c</sub>, has a value of 4.62. A system was charged to give these initial concentrations: [SO<sub>3</sub>] = 0.500 M, [O<sub>2</sub>] = 0.00855 M, [SO<sub>2</sub>] = 0.254 M. In which direction will it go?Hint: Find the value for Q and then compare that value to the given value for K<sub>c</sub></strong> A)to the right B)it will remain at the same concentrations C)to the left D)to the right or the left depending on the pressure E)to the right or the left depending on the volume](https://d2lvgg3v3hfg70.cloudfront.net/TBW1039/11ee6e7b_ba81_deeb_bf7f_d35115c95bc2_TBW1039_11.jpg) 2SO3(g), at 450.0 K the equilibrium constant, Kc, has a value of 4.62. A system was charged to give these initial concentrations: [SO3] = 0.500 M, [O2] = 0.00855 M, [SO2] = 0.254 M. In which direction will it go?Hint: Find the value for Q and then compare that value to the given value for Kc
2SO3(g), at 450.0 K the equilibrium constant, Kc, has a value of 4.62. A system was charged to give these initial concentrations: [SO3] = 0.500 M, [O2] = 0.00855 M, [SO2] = 0.254 M. In which direction will it go?Hint: Find the value for Q and then compare that value to the given value for KcA)to the right
B)it will remain at the same concentrations
C)to the left
D)to the right or the left depending on the pressure
E)to the right or the left depending on the volume

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
32
For the reaction, 2SO2(g)+ O2(g) ![<strong>For the reaction, 2SO<sub>2</sub>(g)+ O<sub>2</sub>(g) 2SO<sub>3</sub>(g), at 450.0 K the equilibrium constant, K<sub>c</sub>, has a value of 4.62. A system was charged to give these initial concentrations: [SO<sub>3</sub>] = 0.0254 M, [O<sub>2</sub>] = 0.00855 M, [SO<sub>2</sub>] = 0.500 M. In which direction will it go? Hint: Find the value for Q and then compare that value to the given value for K<sub>c</sub></strong> A)to the right B)to the left C)to the right or the left depending on the pressure D)it will remain at the same concentrations E)to the right or the left depending on the volume](https://d2lvgg3v3hfg70.cloudfront.net/TBW1039/11ee6e7b_ba81_deec_bf7f_7b6ac6fe0fbe_TBW1039_11.jpg) 2SO3(g), at 450.0 K the equilibrium constant, Kc, has a value of 4.62. A system was charged to give these initial concentrations: [SO3] = 0.0254 M, [O2] = 0.00855 M, [SO2] = 0.500 M. In which direction will it go? Hint: Find the value for Q and then compare that value to the given value for Kc
2SO3(g), at 450.0 K the equilibrium constant, Kc, has a value of 4.62. A system was charged to give these initial concentrations: [SO3] = 0.0254 M, [O2] = 0.00855 M, [SO2] = 0.500 M. In which direction will it go? Hint: Find the value for Q and then compare that value to the given value for Kc
A)to the right
B)to the left
C)to the right or the left depending on the pressure
D)it will remain at the same concentrations
E)to the right or the left depending on the volume
![<strong>For the reaction, 2SO<sub>2</sub>(g)+ O<sub>2</sub>(g) 2SO<sub>3</sub>(g), at 450.0 K the equilibrium constant, K<sub>c</sub>, has a value of 4.62. A system was charged to give these initial concentrations: [SO<sub>3</sub>] = 0.0254 M, [O<sub>2</sub>] = 0.00855 M, [SO<sub>2</sub>] = 0.500 M. In which direction will it go? Hint: Find the value for Q and then compare that value to the given value for K<sub>c</sub></strong> A)to the right B)to the left C)to the right or the left depending on the pressure D)it will remain at the same concentrations E)to the right or the left depending on the volume](https://d2lvgg3v3hfg70.cloudfront.net/TBW1039/11ee6e7b_ba81_deec_bf7f_7b6ac6fe0fbe_TBW1039_11.jpg) 2SO3(g), at 450.0 K the equilibrium constant, Kc, has a value of 4.62. A system was charged to give these initial concentrations: [SO3] = 0.0254 M, [O2] = 0.00855 M, [SO2] = 0.500 M. In which direction will it go? Hint: Find the value for Q and then compare that value to the given value for Kc
2SO3(g), at 450.0 K the equilibrium constant, Kc, has a value of 4.62. A system was charged to give these initial concentrations: [SO3] = 0.0254 M, [O2] = 0.00855 M, [SO2] = 0.500 M. In which direction will it go? Hint: Find the value for Q and then compare that value to the given value for KcA)to the right
B)to the left
C)to the right or the left depending on the pressure
D)it will remain at the same concentrations
E)to the right or the left depending on the volume

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
33
Given the reaction, 2NO(g)+ O2(g)  2NO2(g), for which the enthalpy of reaction is -118.9 kJ .Which one of the following actions will cause an increase in the equilibrium concentration of NO(g)in a closed reaction chamber?
2NO2(g), for which the enthalpy of reaction is -118.9 kJ .Which one of the following actions will cause an increase in the equilibrium concentration of NO(g)in a closed reaction chamber?
A)adding more O2(g)through an injection nozzle
B)increasing the temperature of the system
C)removing the NO2(g)from the system
D)increasing the pressure of the system while temperature is kept constant
E)adding a catalyst
 2NO2(g), for which the enthalpy of reaction is -118.9 kJ .Which one of the following actions will cause an increase in the equilibrium concentration of NO(g)in a closed reaction chamber?
2NO2(g), for which the enthalpy of reaction is -118.9 kJ .Which one of the following actions will cause an increase in the equilibrium concentration of NO(g)in a closed reaction chamber?A)adding more O2(g)through an injection nozzle
B)increasing the temperature of the system
C)removing the NO2(g)from the system
D)increasing the pressure of the system while temperature is kept constant
E)adding a catalyst

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
34
The reaction, 2 SO3(g)  2 SO2(g)+ O2(g)is endothermic. Predict what will happen if the temperature is increased.
2 SO2(g)+ O2(g)is endothermic. Predict what will happen if the temperature is increased.
A)Kc remains the same.
B)Kc decreases.
C)The pressure decreases.
D)More SO3(g)is produced.
E)Kc increases.
 2 SO2(g)+ O2(g)is endothermic. Predict what will happen if the temperature is increased.
2 SO2(g)+ O2(g)is endothermic. Predict what will happen if the temperature is increased.A)Kc remains the same.
B)Kc decreases.
C)The pressure decreases.
D)More SO3(g)is produced.
E)Kc increases.

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
35
Consider the following system, which is at equilibrium,CO(g)+ 3H2(g)  CH4(g)+ H2O(g).The result of removing some CH4(g)and H2O(g)from the system is that
CH4(g)+ H2O(g).The result of removing some CH4(g)and H2O(g)from the system is that
A)more CH4(g)and H2O(g)are produced to replace that which is removed.
B)Kc decreases.
C)more CO(g)is produced.
D)more H2O(g)is consumed to restore the equilibrium.
E)more CH4(g)is consumed to restore the equilibrium.
 CH4(g)+ H2O(g).The result of removing some CH4(g)and H2O(g)from the system is that
CH4(g)+ H2O(g).The result of removing some CH4(g)and H2O(g)from the system is thatA)more CH4(g)and H2O(g)are produced to replace that which is removed.
B)Kc decreases.
C)more CO(g)is produced.
D)more H2O(g)is consumed to restore the equilibrium.
E)more CH4(g)is consumed to restore the equilibrium.

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
36
Consider the following system, which is at equilibrium, 3C(s)+ 3H2(g)  CH4(g)+ C2H2(g)The result of removing some CH4(g)and C2H2(g)from the system is that
CH4(g)+ C2H2(g)The result of removing some CH4(g)and C2H2(g)from the system is that
A)no further change occurs.
B)Kc increases.
C)more C(s)is produced.
D)more C2H2(g)is consumed to restore the equilibrium.
E)more CH4(g)and C2H2(g)are produced to replace that which is removed.
 CH4(g)+ C2H2(g)The result of removing some CH4(g)and C2H2(g)from the system is that
CH4(g)+ C2H2(g)The result of removing some CH4(g)and C2H2(g)from the system is thatA)no further change occurs.
B)Kc increases.
C)more C(s)is produced.
D)more C2H2(g)is consumed to restore the equilibrium.
E)more CH4(g)and C2H2(g)are produced to replace that which is removed.

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
37
Consider the following system, which is at equilibrium, 3C(s)+ 3H2(g)  CH4(g)+ C2H2(g)The result of removing some C(s)from the system will be:
CH4(g)+ C2H2(g)The result of removing some C(s)from the system will be:
A)Kc increases.
B)more C(s)is produced.
C)no further change occurs.
D)more CH4(g)and C2H2(g)are produced to restore the equilibrium.
E)more C2H2(g)is consumed to restore the equilibrium.
 CH4(g)+ C2H2(g)The result of removing some C(s)from the system will be:
CH4(g)+ C2H2(g)The result of removing some C(s)from the system will be:A)Kc increases.
B)more C(s)is produced.
C)no further change occurs.
D)more CH4(g)and C2H2(g)are produced to restore the equilibrium.
E)more C2H2(g)is consumed to restore the equilibrium.

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
38
The system, 2H2O(g)+ 2Cl2(g)  4HCl(g)+ O2(g), has a value of 8.00 for Kp. Initially, the partial pressures of H2O(g)and Cl2(g)are set at 0.100 atm, while those of HCl(g)and O2(g)are set at 0.250 atm. Which statement below is true?
4HCl(g)+ O2(g), has a value of 8.00 for Kp. Initially, the partial pressures of H2O(g)and Cl2(g)are set at 0.100 atm, while those of HCl(g)and O2(g)are set at 0.250 atm. Which statement below is true?
A)Qp > Kp and the reaction proceeds to the right to reach equilibrium.
B)Qp < Kp and the reaction proceeds to the left to reach equilibrium.
C)The reaction system is already at equilibrium.
D)Qp > Kp and the reaction proceeds to the left to reach equilibrium.
E)Qp < Kp and the reaction proceeds to the right to reach equilibrium.
 4HCl(g)+ O2(g), has a value of 8.00 for Kp. Initially, the partial pressures of H2O(g)and Cl2(g)are set at 0.100 atm, while those of HCl(g)and O2(g)are set at 0.250 atm. Which statement below is true?
4HCl(g)+ O2(g), has a value of 8.00 for Kp. Initially, the partial pressures of H2O(g)and Cl2(g)are set at 0.100 atm, while those of HCl(g)and O2(g)are set at 0.250 atm. Which statement below is true?A)Qp > Kp and the reaction proceeds to the right to reach equilibrium.
B)Qp < Kp and the reaction proceeds to the left to reach equilibrium.
C)The reaction system is already at equilibrium.
D)Qp > Kp and the reaction proceeds to the left to reach equilibrium.
E)Qp < Kp and the reaction proceeds to the right to reach equilibrium.

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
39
The system, H2(g)+ X2(g)  2HX(g)has a value of 24.4 for Kc. A system being studied in a 3.00 liter reactor was charged with 0.150 moles of H2, 0.150 moles of X2, and 0.600 moles of HX. A catalyst was introduced using a remote unit. Which statement below describes the situation? Hint: Be sure to find molarities of all reactants and products before calculating Q.
2HX(g)has a value of 24.4 for Kc. A system being studied in a 3.00 liter reactor was charged with 0.150 moles of H2, 0.150 moles of X2, and 0.600 moles of HX. A catalyst was introduced using a remote unit. Which statement below describes the situation? Hint: Be sure to find molarities of all reactants and products before calculating Q.
A)The reaction goes to the right, Q < K.
B)The reaction goes to the left, Q < K.
C)The reaction goes to the right, Q > K.
D)The reaction goes to the left, Q > K.
E)It is not possible to predict in which direction the system will travel as it reacts.
 2HX(g)has a value of 24.4 for Kc. A system being studied in a 3.00 liter reactor was charged with 0.150 moles of H2, 0.150 moles of X2, and 0.600 moles of HX. A catalyst was introduced using a remote unit. Which statement below describes the situation? Hint: Be sure to find molarities of all reactants and products before calculating Q.
2HX(g)has a value of 24.4 for Kc. A system being studied in a 3.00 liter reactor was charged with 0.150 moles of H2, 0.150 moles of X2, and 0.600 moles of HX. A catalyst was introduced using a remote unit. Which statement below describes the situation? Hint: Be sure to find molarities of all reactants and products before calculating Q.A)The reaction goes to the right, Q < K.
B)The reaction goes to the left, Q < K.
C)The reaction goes to the right, Q > K.
D)The reaction goes to the left, Q > K.
E)It is not possible to predict in which direction the system will travel as it reacts.

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
40
A study of the system, 4NH3(g)+ 7O2(g) ![<strong>A study of the system, 4NH<sub>3</sub>(g)+ 7O<sub>2</sub>(g) 2N<sub>2</sub>O<sub>4</sub>(g)+ 6H<sub>2</sub>O(g), was carried out. A system was prepared with [NH<sub>3</sub>] = [O<sub>2</sub>] = 3.60 M as the only components initially. At equilibrium, [N<sub>2</sub>O<sub>4</sub>] is 0.60 M. Calculate the equilibrium concentration of O<sub>2</sub>. Hint: Organize your information using an ICE table, then use the equilibrium N<sub>2</sub>O<sub>4</sub> concentration to find the concentration of O<sub>2</sub>.</strong> A)3.00 M B)2.40 M C)1.50 M D)2.10 M E)3.30 M](https://d2lvgg3v3hfg70.cloudfront.net/TBW1039/11ee6e7b_ba82_5424_bf7f_a1142ca41f1d_TBW1039_11.jpg) 2N2O4(g)+ 6H2O(g), was carried out. A system was prepared with [NH3] = [O2] = 3.60 M as the only components initially. At equilibrium, [N2O4] is 0.60 M. Calculate the equilibrium concentration of O2. Hint: Organize your information using an ICE table, then use the equilibrium N2O4 concentration to find the concentration of O2.
2N2O4(g)+ 6H2O(g), was carried out. A system was prepared with [NH3] = [O2] = 3.60 M as the only components initially. At equilibrium, [N2O4] is 0.60 M. Calculate the equilibrium concentration of O2. Hint: Organize your information using an ICE table, then use the equilibrium N2O4 concentration to find the concentration of O2.
A)3.00 M
B)2.40 M
C)1.50 M
D)2.10 M
E)3.30 M
![<strong>A study of the system, 4NH<sub>3</sub>(g)+ 7O<sub>2</sub>(g) 2N<sub>2</sub>O<sub>4</sub>(g)+ 6H<sub>2</sub>O(g), was carried out. A system was prepared with [NH<sub>3</sub>] = [O<sub>2</sub>] = 3.60 M as the only components initially. At equilibrium, [N<sub>2</sub>O<sub>4</sub>] is 0.60 M. Calculate the equilibrium concentration of O<sub>2</sub>. Hint: Organize your information using an ICE table, then use the equilibrium N<sub>2</sub>O<sub>4</sub> concentration to find the concentration of O<sub>2</sub>.</strong> A)3.00 M B)2.40 M C)1.50 M D)2.10 M E)3.30 M](https://d2lvgg3v3hfg70.cloudfront.net/TBW1039/11ee6e7b_ba82_5424_bf7f_a1142ca41f1d_TBW1039_11.jpg) 2N2O4(g)+ 6H2O(g), was carried out. A system was prepared with [NH3] = [O2] = 3.60 M as the only components initially. At equilibrium, [N2O4] is 0.60 M. Calculate the equilibrium concentration of O2. Hint: Organize your information using an ICE table, then use the equilibrium N2O4 concentration to find the concentration of O2.
2N2O4(g)+ 6H2O(g), was carried out. A system was prepared with [NH3] = [O2] = 3.60 M as the only components initially. At equilibrium, [N2O4] is 0.60 M. Calculate the equilibrium concentration of O2. Hint: Organize your information using an ICE table, then use the equilibrium N2O4 concentration to find the concentration of O2.A)3.00 M
B)2.40 M
C)1.50 M
D)2.10 M
E)3.30 M

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
41
A study of the system, 4NH3(g)+ 5O2(g) ![<strong>A study of the system, 4NH<sub>3</sub>(g)+ 5O<sub>2</sub>(g) 4NO(g)+ 6H<sub>2</sub>O(g), was carried out. A system was prepared with [NH<sub>3</sub>] = [O<sub>2</sub>] = 5.60 M as the only components initially. At equilibrium, [NO] is 0.36 M. Calculate the equilibrium concentration of O<sub>2</sub>.Hint: Organize your information using an ICE table, then use the equilibrium NO concentration to find the concentration of O<sub>2</sub>.</strong> A)6.05 M B)5.15 M C)5.24 M D)0.60 M E)5.51 M](https://d2lvgg3v3hfg70.cloudfront.net/TBW1039/11ee6e7b_ba82_7b35_bf7f_f9fbfb24007c_TBW1039_11.jpg) 4NO(g)+ 6H2O(g), was carried out. A system was prepared with [NH3] = [O2] = 5.60 M as the only components initially. At equilibrium, [NO] is 0.36 M. Calculate the equilibrium concentration of O2.Hint: Organize your information using an ICE table, then use the equilibrium NO concentration to find the concentration of O2.
4NO(g)+ 6H2O(g), was carried out. A system was prepared with [NH3] = [O2] = 5.60 M as the only components initially. At equilibrium, [NO] is 0.36 M. Calculate the equilibrium concentration of O2.Hint: Organize your information using an ICE table, then use the equilibrium NO concentration to find the concentration of O2.
A)6.05 M
B)5.15 M
C)5.24 M
D)0.60 M
E)5.51 M
![<strong>A study of the system, 4NH<sub>3</sub>(g)+ 5O<sub>2</sub>(g) 4NO(g)+ 6H<sub>2</sub>O(g), was carried out. A system was prepared with [NH<sub>3</sub>] = [O<sub>2</sub>] = 5.60 M as the only components initially. At equilibrium, [NO] is 0.36 M. Calculate the equilibrium concentration of O<sub>2</sub>.Hint: Organize your information using an ICE table, then use the equilibrium NO concentration to find the concentration of O<sub>2</sub>.</strong> A)6.05 M B)5.15 M C)5.24 M D)0.60 M E)5.51 M](https://d2lvgg3v3hfg70.cloudfront.net/TBW1039/11ee6e7b_ba82_7b35_bf7f_f9fbfb24007c_TBW1039_11.jpg) 4NO(g)+ 6H2O(g), was carried out. A system was prepared with [NH3] = [O2] = 5.60 M as the only components initially. At equilibrium, [NO] is 0.36 M. Calculate the equilibrium concentration of O2.Hint: Organize your information using an ICE table, then use the equilibrium NO concentration to find the concentration of O2.
4NO(g)+ 6H2O(g), was carried out. A system was prepared with [NH3] = [O2] = 5.60 M as the only components initially. At equilibrium, [NO] is 0.36 M. Calculate the equilibrium concentration of O2.Hint: Organize your information using an ICE table, then use the equilibrium NO concentration to find the concentration of O2.A)6.05 M
B)5.15 M
C)5.24 M
D)0.60 M
E)5.51 M

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
42
NH2CO2NH4(s)undergoes the following reaction at 450 K to produce a system thatreaches equilibrium:NH2CO2NH4(s)  2NH3(g)+ CO2(g)The total pressure in the closed container under these conditions is found to be 0.843 atm. Calculate a value for the equilibrium constant, Kp.Hint: Organize your information using an ICE table, then use Dalton's Law to find the equilibrium pressures of each product gas.
2NH3(g)+ CO2(g)The total pressure in the closed container under these conditions is found to be 0.843 atm. Calculate a value for the equilibrium constant, Kp.Hint: Organize your information using an ICE table, then use Dalton's Law to find the equilibrium pressures of each product gas.
A)0.00701
B)0.0888
C)0.222
D)0.599
 2NH3(g)+ CO2(g)The total pressure in the closed container under these conditions is found to be 0.843 atm. Calculate a value for the equilibrium constant, Kp.Hint: Organize your information using an ICE table, then use Dalton's Law to find the equilibrium pressures of each product gas.
2NH3(g)+ CO2(g)The total pressure in the closed container under these conditions is found to be 0.843 atm. Calculate a value for the equilibrium constant, Kp.Hint: Organize your information using an ICE table, then use Dalton's Law to find the equilibrium pressures of each product gas.A)0.00701
B)0.0888
C)0.222
D)0.599

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
43
For the reaction system, 2SO2(g)+ O2(g)  2SO3(g), the equilibrium concentrations are: SO3: 0.120 M SO2: 0.860 M O2: 0.330 M Calculate the value of Kc for this reaction.
2SO3(g), the equilibrium concentrations are: SO3: 0.120 M SO2: 0.860 M O2: 0.330 M Calculate the value of Kc for this reaction.
A)1.31
B)2.51
C)0.423
D)0.872
E)0.0590
 2SO3(g), the equilibrium concentrations are: SO3: 0.120 M SO2: 0.860 M O2: 0.330 M Calculate the value of Kc for this reaction.
2SO3(g), the equilibrium concentrations are: SO3: 0.120 M SO2: 0.860 M O2: 0.330 M Calculate the value of Kc for this reaction.A)1.31
B)2.51
C)0.423
D)0.872
E)0.0590

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
44
For the reaction system, H2(g)+ X2(g)  2HX(g), Kc = 24.4 at 300 K. A system made up from these components which is at equilibrium contains 0.150 moles of H2 and 0.600 moles of HX in a 3.00-liter container. Calculate the number of moles of X2(g)present at equilibrium. Hint: Be sure to find molarities of all reactants and products before finding the equilibrium amount of X2.
2HX(g), Kc = 24.4 at 300 K. A system made up from these components which is at equilibrium contains 0.150 moles of H2 and 0.600 moles of HX in a 3.00-liter container. Calculate the number of moles of X2(g)present at equilibrium. Hint: Be sure to find molarities of all reactants and products before finding the equilibrium amount of X2.
A)0.0984 mol
B)0.164 mol
C)0.0328 mol
D)0.131 mol
E)0.393 mol
 2HX(g), Kc = 24.4 at 300 K. A system made up from these components which is at equilibrium contains 0.150 moles of H2 and 0.600 moles of HX in a 3.00-liter container. Calculate the number of moles of X2(g)present at equilibrium. Hint: Be sure to find molarities of all reactants and products before finding the equilibrium amount of X2.
2HX(g), Kc = 24.4 at 300 K. A system made up from these components which is at equilibrium contains 0.150 moles of H2 and 0.600 moles of HX in a 3.00-liter container. Calculate the number of moles of X2(g)present at equilibrium. Hint: Be sure to find molarities of all reactants and products before finding the equilibrium amount of X2.A)0.0984 mol
B)0.164 mol
C)0.0328 mol
D)0.131 mol
E)0.393 mol

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
45
For the reaction system, H2(g)+ X2(g)  2HX(g), Kc = 24.4 at 300 K. A system made up from these components that is at equilibrium contains 0.200 moles of X2 and 0.600 moles of HX(g)in a 4.00 liter container. Calculate the number of moles of H2(g)present at equilibrium. Hint: Be sure to find molarities of all reactants and products before finding the equilibrium amount of H2.
2HX(g), Kc = 24.4 at 300 K. A system made up from these components that is at equilibrium contains 0.200 moles of X2 and 0.600 moles of HX(g)in a 4.00 liter container. Calculate the number of moles of H2(g)present at equilibrium. Hint: Be sure to find molarities of all reactants and products before finding the equilibrium amount of H2.
A)0.059 mol
B)0.074 mol
C)0.123 mol
D)0.148 mol
E)0.295 mol
 2HX(g), Kc = 24.4 at 300 K. A system made up from these components that is at equilibrium contains 0.200 moles of X2 and 0.600 moles of HX(g)in a 4.00 liter container. Calculate the number of moles of H2(g)present at equilibrium. Hint: Be sure to find molarities of all reactants and products before finding the equilibrium amount of H2.
2HX(g), Kc = 24.4 at 300 K. A system made up from these components that is at equilibrium contains 0.200 moles of X2 and 0.600 moles of HX(g)in a 4.00 liter container. Calculate the number of moles of H2(g)present at equilibrium. Hint: Be sure to find molarities of all reactants and products before finding the equilibrium amount of H2.A)0.059 mol
B)0.074 mol
C)0.123 mol
D)0.148 mol
E)0.295 mol

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
46
For the reaction system, 2SO2(g)+ O2(g)  2SO3(g), Kc has a value of 4.62 at 450.0 K. A system, at equilibrium, was found to contain 0.360 moles of SO2 and 0.900 moles of O2 in a 3.00 liter container. Calculate the number of moles of SO3 gas at equilibrium. Hint: Be sure to find molarities of all reactants and products before finding the equilibrium amount of SO3.
2SO3(g), Kc has a value of 4.62 at 450.0 K. A system, at equilibrium, was found to contain 0.360 moles of SO2 and 0.900 moles of O2 in a 3.00 liter container. Calculate the number of moles of SO3 gas at equilibrium. Hint: Be sure to find molarities of all reactants and products before finding the equilibrium amount of SO3.
A)0.166 M
B)0.734 M
C)0.539 M
D)0.424 M
E)0.141 M
 2SO3(g), Kc has a value of 4.62 at 450.0 K. A system, at equilibrium, was found to contain 0.360 moles of SO2 and 0.900 moles of O2 in a 3.00 liter container. Calculate the number of moles of SO3 gas at equilibrium. Hint: Be sure to find molarities of all reactants and products before finding the equilibrium amount of SO3.
2SO3(g), Kc has a value of 4.62 at 450.0 K. A system, at equilibrium, was found to contain 0.360 moles of SO2 and 0.900 moles of O2 in a 3.00 liter container. Calculate the number of moles of SO3 gas at equilibrium. Hint: Be sure to find molarities of all reactants and products before finding the equilibrium amount of SO3.A)0.166 M
B)0.734 M
C)0.539 M
D)0.424 M
E)0.141 M

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
47
For the reaction system, 2SO2(g)+ O2(g)  2SO3(g), Kc has a value of 4.62 at 450.0 K. A system, at equilibrium, was found to contain 0.440 moles of SO2 and 0.880 moles of O2 in a 4.00 liter container. Calculate the number of moles of SO3 gas at equilibrium. Hint: Find molarities of all reactants and products before finding the equilibrium amount of SO3.
2SO3(g), Kc has a value of 4.62 at 450.0 K. A system, at equilibrium, was found to contain 0.440 moles of SO2 and 0.880 moles of O2 in a 4.00 liter container. Calculate the number of moles of SO3 gas at equilibrium. Hint: Find molarities of all reactants and products before finding the equilibrium amount of SO3.
A)0.443 M
B)0.887 M
C)0.0492 M
D)0.111 M
E)0.0123 M
 2SO3(g), Kc has a value of 4.62 at 450.0 K. A system, at equilibrium, was found to contain 0.440 moles of SO2 and 0.880 moles of O2 in a 4.00 liter container. Calculate the number of moles of SO3 gas at equilibrium. Hint: Find molarities of all reactants and products before finding the equilibrium amount of SO3.
2SO3(g), Kc has a value of 4.62 at 450.0 K. A system, at equilibrium, was found to contain 0.440 moles of SO2 and 0.880 moles of O2 in a 4.00 liter container. Calculate the number of moles of SO3 gas at equilibrium. Hint: Find molarities of all reactants and products before finding the equilibrium amount of SO3.A)0.443 M
B)0.887 M
C)0.0492 M
D)0.111 M
E)0.0123 M

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
48
At 1500 °C the system, 2NO(g)  N2(g)+ O2(g), was allowed to come to equilibrium. The equilibrium concentrations were: NO(g)= 0.00035 M, N2(g)= 0.040 M, and O2(g)= 0.040 M. What is the value of Kc for the system at this temperature?
N2(g)+ O2(g), was allowed to come to equilibrium. The equilibrium concentrations were: NO(g)= 0.00035 M, N2(g)= 0.040 M, and O2(g)= 0.040 M. What is the value of Kc for the system at this temperature?
A)3.3 × 104
B)7.7 × 10-5
C)2.2 × 10-1
D)4.6
E)1.3 × 104
 N2(g)+ O2(g), was allowed to come to equilibrium. The equilibrium concentrations were: NO(g)= 0.00035 M, N2(g)= 0.040 M, and O2(g)= 0.040 M. What is the value of Kc for the system at this temperature?
N2(g)+ O2(g), was allowed to come to equilibrium. The equilibrium concentrations were: NO(g)= 0.00035 M, N2(g)= 0.040 M, and O2(g)= 0.040 M. What is the value of Kc for the system at this temperature?A)3.3 × 104
B)7.7 × 10-5
C)2.2 × 10-1
D)4.6
E)1.3 × 104

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
49
For the reaction system, 2SO2(g)+ O2(g) ![<strong>For the reaction system, 2SO<sub>2</sub>(g)+ O<sub>2</sub>(g) 2SO<sub>3</sub>(g), K<sub>c</sub> has a value of 4.62 at 450.0 K. A system, at equilibrium, has the following concentrations: [SO<sub>3</sub>] = 0.254 M, [O<sub>2</sub>] = 0.00855 M. What is the equilibrium concentration of SO<sub>2</sub>(g)?</strong> A)1.28 M B)0.216 M C)0.465 M D)6.43 M E)41.3 M](https://d2lvgg3v3hfg70.cloudfront.net/TBW1039/11ee6e7b_ba82_c95d_bf7f_c9f01b2b617a_TBW1039_11.jpg) 2SO3(g), Kc has a value of 4.62 at 450.0 K. A system, at equilibrium, has the following concentrations: [SO3] = 0.254 M, [O2] = 0.00855 M. What is the equilibrium concentration of SO2(g)?
2SO3(g), Kc has a value of 4.62 at 450.0 K. A system, at equilibrium, has the following concentrations: [SO3] = 0.254 M, [O2] = 0.00855 M. What is the equilibrium concentration of SO2(g)?
A)1.28 M
B)0.216 M
C)0.465 M
D)6.43 M
E)41.3 M
![<strong>For the reaction system, 2SO<sub>2</sub>(g)+ O<sub>2</sub>(g) 2SO<sub>3</sub>(g), K<sub>c</sub> has a value of 4.62 at 450.0 K. A system, at equilibrium, has the following concentrations: [SO<sub>3</sub>] = 0.254 M, [O<sub>2</sub>] = 0.00855 M. What is the equilibrium concentration of SO<sub>2</sub>(g)?</strong> A)1.28 M B)0.216 M C)0.465 M D)6.43 M E)41.3 M](https://d2lvgg3v3hfg70.cloudfront.net/TBW1039/11ee6e7b_ba82_c95d_bf7f_c9f01b2b617a_TBW1039_11.jpg) 2SO3(g), Kc has a value of 4.62 at 450.0 K. A system, at equilibrium, has the following concentrations: [SO3] = 0.254 M, [O2] = 0.00855 M. What is the equilibrium concentration of SO2(g)?
2SO3(g), Kc has a value of 4.62 at 450.0 K. A system, at equilibrium, has the following concentrations: [SO3] = 0.254 M, [O2] = 0.00855 M. What is the equilibrium concentration of SO2(g)?A)1.28 M
B)0.216 M
C)0.465 M
D)6.43 M
E)41.3 M

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
50
For the reaction, H2(g)+ I2(g)  2HI(g), Kc = 54.9 at 699.0 K. A system, at equilibrium, contains 2.50 moles of HI(g)and 2.12 moles of I2(g)in a 5.00 liter vessel. How many moles of H2(g)should there be in the container? Hint: Be sure to find molarities of all reactants and products before finding the equilibrium amount of H2.
2HI(g), Kc = 54.9 at 699.0 K. A system, at equilibrium, contains 2.50 moles of HI(g)and 2.12 moles of I2(g)in a 5.00 liter vessel. How many moles of H2(g)should there be in the container? Hint: Be sure to find molarities of all reactants and products before finding the equilibrium amount of H2.
A)0.0537 moles
B)0.380 moles
C)0.0215 moles
D)0.0107 moles
E)2.12 moles
 2HI(g), Kc = 54.9 at 699.0 K. A system, at equilibrium, contains 2.50 moles of HI(g)and 2.12 moles of I2(g)in a 5.00 liter vessel. How many moles of H2(g)should there be in the container? Hint: Be sure to find molarities of all reactants and products before finding the equilibrium amount of H2.
2HI(g), Kc = 54.9 at 699.0 K. A system, at equilibrium, contains 2.50 moles of HI(g)and 2.12 moles of I2(g)in a 5.00 liter vessel. How many moles of H2(g)should there be in the container? Hint: Be sure to find molarities of all reactants and products before finding the equilibrium amount of H2.A)0.0537 moles
B)0.380 moles
C)0.0215 moles
D)0.0107 moles
E)2.12 moles

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
51
The system, 2NO(g)  N2(g)+ O2(g)was allowed to come to equilibrium at 1500 °C. The equilibrium concentrations were: NO(g)= 0.00035 M, N2(g)= 0.040 M, and O2(g)= 0.040 M. What is the value of Kp for the system at this temperature?
N2(g)+ O2(g)was allowed to come to equilibrium at 1500 °C. The equilibrium concentrations were: NO(g)= 0.00035 M, N2(g)= 0.040 M, and O2(g)= 0.040 M. What is the value of Kp for the system at this temperature?
A)2.2 × 10-1
B)1.3 × 104
C)3.3 × 103
D)4.6
E)1.5
 N2(g)+ O2(g)was allowed to come to equilibrium at 1500 °C. The equilibrium concentrations were: NO(g)= 0.00035 M, N2(g)= 0.040 M, and O2(g)= 0.040 M. What is the value of Kp for the system at this temperature?
N2(g)+ O2(g)was allowed to come to equilibrium at 1500 °C. The equilibrium concentrations were: NO(g)= 0.00035 M, N2(g)= 0.040 M, and O2(g)= 0.040 M. What is the value of Kp for the system at this temperature?A)2.2 × 10-1
B)1.3 × 104
C)3.3 × 103
D)4.6
E)1.5

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
52
For the reaction, 2BrCl(g) ![<strong>For the reaction, 2BrCl(g) Cl<sub>2</sub>(g)+ Br<sub>2</sub>(g), K<sub>p</sub> has a value of 0.140 at 350 K. In a container housing this system, the [Cl<sub>2</sub>(g)] = [Br<sub>2</sub>(g)] = 0.0200 M at equilibrium. What is the value of the partial pressure of BrCl<sub>(</sub><sub>g</sub><sub>)</sub>? Hint: The ideal gas law is needed to convert molarity to pressure.</strong> A)2.86 × 10<sup>-3</sup> atm B)5.35 × 10<sup>-2</sup> atm C)8.21 × 10<sup>-2</sup> atm D)1.25 × 10<sup>-5 </sup>atm E)1.54 atm](https://d2lvgg3v3hfg70.cloudfront.net/TBW1039/11ee6e7b_ba82_f070_bf7f_bff0549aac44_TBW1039_11.jpg) Cl2(g)+ Br2(g), Kp has a value of 0.140 at 350 K. In a container housing this system, the [Cl2(g)] = [Br2(g)] = 0.0200 M at equilibrium. What is the value of the partial pressure of BrCl(g)? Hint: The ideal gas law is needed to convert molarity to pressure.
Cl2(g)+ Br2(g), Kp has a value of 0.140 at 350 K. In a container housing this system, the [Cl2(g)] = [Br2(g)] = 0.0200 M at equilibrium. What is the value of the partial pressure of BrCl(g)? Hint: The ideal gas law is needed to convert molarity to pressure.
A)2.86 × 10-3 atm
B)5.35 × 10-2 atm
C)8.21 × 10-2 atm
D)1.25 × 10-5 atm
E)1.54 atm
![<strong>For the reaction, 2BrCl(g) Cl<sub>2</sub>(g)+ Br<sub>2</sub>(g), K<sub>p</sub> has a value of 0.140 at 350 K. In a container housing this system, the [Cl<sub>2</sub>(g)] = [Br<sub>2</sub>(g)] = 0.0200 M at equilibrium. What is the value of the partial pressure of BrCl<sub>(</sub><sub>g</sub><sub>)</sub>? Hint: The ideal gas law is needed to convert molarity to pressure.</strong> A)2.86 × 10<sup>-3</sup> atm B)5.35 × 10<sup>-2</sup> atm C)8.21 × 10<sup>-2</sup> atm D)1.25 × 10<sup>-5 </sup>atm E)1.54 atm](https://d2lvgg3v3hfg70.cloudfront.net/TBW1039/11ee6e7b_ba82_f070_bf7f_bff0549aac44_TBW1039_11.jpg) Cl2(g)+ Br2(g), Kp has a value of 0.140 at 350 K. In a container housing this system, the [Cl2(g)] = [Br2(g)] = 0.0200 M at equilibrium. What is the value of the partial pressure of BrCl(g)? Hint: The ideal gas law is needed to convert molarity to pressure.
Cl2(g)+ Br2(g), Kp has a value of 0.140 at 350 K. In a container housing this system, the [Cl2(g)] = [Br2(g)] = 0.0200 M at equilibrium. What is the value of the partial pressure of BrCl(g)? Hint: The ideal gas law is needed to convert molarity to pressure.A)2.86 × 10-3 atm
B)5.35 × 10-2 atm
C)8.21 × 10-2 atm
D)1.25 × 10-5 atm
E)1.54 atm

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
53
A study of the system, 4NH3(g)+ 7O2(g) ![<strong>A study of the system, 4NH<sub>3</sub>(g)+ 7O<sub>2</sub>(g) 2N<sub>2</sub>O<sub>4</sub>(g)+ 6H<sub>2</sub>O(g), was carried out. A system was prepared with [NH<sub>3</sub>] = [O<sub>2</sub>] = 3.60 M as the only components initially. At equilibrium, [N<sub>2</sub>O<sub>4</sub>] is 0.600 M. Calculate the equilibrium concentration of NH<sub>3</sub>(g).Hint: Organize your information using an ICE table, then use the equilibrium N<sub>2</sub>O<sub>4</sub> concentration to find the concentration of NH<sub>3</sub>.</strong> A)3.00 M B)2.10 M C)3.30 M D)1.80 M E)2.40 M](https://d2lvgg3v3hfg70.cloudfront.net/TBW1039/11ee6e7b_ba82_f071_bf7f_1f0a350a681b_TBW1039_11.jpg) 2N2O4(g)+ 6H2O(g), was carried out. A system was prepared with [NH3] = [O2] = 3.60 M as the only components initially. At equilibrium, [N2O4] is 0.600 M. Calculate the equilibrium concentration of NH3(g).Hint: Organize your information using an ICE table, then use the equilibrium N2O4 concentration to find the concentration of NH3.
2N2O4(g)+ 6H2O(g), was carried out. A system was prepared with [NH3] = [O2] = 3.60 M as the only components initially. At equilibrium, [N2O4] is 0.600 M. Calculate the equilibrium concentration of NH3(g).Hint: Organize your information using an ICE table, then use the equilibrium N2O4 concentration to find the concentration of NH3.
A)3.00 M
B)2.10 M
C)3.30 M
D)1.80 M
E)2.40 M
![<strong>A study of the system, 4NH<sub>3</sub>(g)+ 7O<sub>2</sub>(g) 2N<sub>2</sub>O<sub>4</sub>(g)+ 6H<sub>2</sub>O(g), was carried out. A system was prepared with [NH<sub>3</sub>] = [O<sub>2</sub>] = 3.60 M as the only components initially. At equilibrium, [N<sub>2</sub>O<sub>4</sub>] is 0.600 M. Calculate the equilibrium concentration of NH<sub>3</sub>(g).Hint: Organize your information using an ICE table, then use the equilibrium N<sub>2</sub>O<sub>4</sub> concentration to find the concentration of NH<sub>3</sub>.</strong> A)3.00 M B)2.10 M C)3.30 M D)1.80 M E)2.40 M](https://d2lvgg3v3hfg70.cloudfront.net/TBW1039/11ee6e7b_ba82_f071_bf7f_1f0a350a681b_TBW1039_11.jpg) 2N2O4(g)+ 6H2O(g), was carried out. A system was prepared with [NH3] = [O2] = 3.60 M as the only components initially. At equilibrium, [N2O4] is 0.600 M. Calculate the equilibrium concentration of NH3(g).Hint: Organize your information using an ICE table, then use the equilibrium N2O4 concentration to find the concentration of NH3.
2N2O4(g)+ 6H2O(g), was carried out. A system was prepared with [NH3] = [O2] = 3.60 M as the only components initially. At equilibrium, [N2O4] is 0.600 M. Calculate the equilibrium concentration of NH3(g).Hint: Organize your information using an ICE table, then use the equilibrium N2O4 concentration to find the concentration of NH3.A)3.00 M
B)2.10 M
C)3.30 M
D)1.80 M
E)2.40 M

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
54
A study of the system, 4NH3(g)+ 7O2(g) ![<strong>A study of the system, 4NH<sub>3</sub>(g)+ 7O<sub>2</sub>(g) 2N<sub>2</sub>O<sub>4</sub>(g)+ 6H<sub>2</sub>O(g), was carried out. A system was prepared with [N<sub>2</sub>O<sub>4</sub>] = [H<sub>2</sub>O] = 3.60 M as the only components initially. At equilibrium, [H<sub>2</sub>O] is 0.600 M. Calculate the equilibrium concentration of NH<sub>3</sub>(g). Hint: Organize your information using an ICE table, then use the equilibrium H<sub>2</sub>O concentration to find the concentration of NH<sub>3</sub>.</strong> A)1.50 M B)0.90 M C)3.00 M D)2.40 M E)2.00 M](https://d2lvgg3v3hfg70.cloudfront.net/TBW1039/11ee6e7b_ba83_1782_bf7f_c99fdc1d388b_TBW1039_11.jpg) 2N2O4(g)+ 6H2O(g), was carried out. A system was prepared with [N2O4] = [H2O] = 3.60 M as the only components initially. At equilibrium, [H2O] is 0.600 M. Calculate the equilibrium concentration of NH3(g). Hint: Organize your information using an ICE table, then use the equilibrium H2O concentration to find the concentration of NH3.
2N2O4(g)+ 6H2O(g), was carried out. A system was prepared with [N2O4] = [H2O] = 3.60 M as the only components initially. At equilibrium, [H2O] is 0.600 M. Calculate the equilibrium concentration of NH3(g). Hint: Organize your information using an ICE table, then use the equilibrium H2O concentration to find the concentration of NH3.
A)1.50 M
B)0.90 M
C)3.00 M
D)2.40 M
E)2.00 M
![<strong>A study of the system, 4NH<sub>3</sub>(g)+ 7O<sub>2</sub>(g) 2N<sub>2</sub>O<sub>4</sub>(g)+ 6H<sub>2</sub>O(g), was carried out. A system was prepared with [N<sub>2</sub>O<sub>4</sub>] = [H<sub>2</sub>O] = 3.60 M as the only components initially. At equilibrium, [H<sub>2</sub>O] is 0.600 M. Calculate the equilibrium concentration of NH<sub>3</sub>(g). Hint: Organize your information using an ICE table, then use the equilibrium H<sub>2</sub>O concentration to find the concentration of NH<sub>3</sub>.</strong> A)1.50 M B)0.90 M C)3.00 M D)2.40 M E)2.00 M](https://d2lvgg3v3hfg70.cloudfront.net/TBW1039/11ee6e7b_ba83_1782_bf7f_c99fdc1d388b_TBW1039_11.jpg) 2N2O4(g)+ 6H2O(g), was carried out. A system was prepared with [N2O4] = [H2O] = 3.60 M as the only components initially. At equilibrium, [H2O] is 0.600 M. Calculate the equilibrium concentration of NH3(g). Hint: Organize your information using an ICE table, then use the equilibrium H2O concentration to find the concentration of NH3.
2N2O4(g)+ 6H2O(g), was carried out. A system was prepared with [N2O4] = [H2O] = 3.60 M as the only components initially. At equilibrium, [H2O] is 0.600 M. Calculate the equilibrium concentration of NH3(g). Hint: Organize your information using an ICE table, then use the equilibrium H2O concentration to find the concentration of NH3.A)1.50 M
B)0.90 M
C)3.00 M
D)2.40 M
E)2.00 M

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
55
A study of the following system, 4 NH3(g)+ 7 O2(g) ![<strong>A study of the following system, 4 NH<sub>3</sub>(g)+ 7 O<sub>2</sub>(g) 2 N<sub>2</sub>O<sub>4</sub>(g)+ 6 H<sub>2</sub>O(g), was carried out. A system was prepared with [N<sub>2</sub>O<sub>4</sub>] = [H<sub>2</sub>O] = 3.60 M as the only components initially. At equilibrium, [H<sub>2</sub>O] is 0.600 M. Calculate the equilibrium concentration of O<sub>2</sub>(g). Hint: Organize your information using an ICE table, then use the equilibrium H<sub>2</sub>O concentration to find the concentration of O<sub>2</sub>.</strong> A)0.700 M B)3.00 M C)1.00 M D)2.40 M E)3.50 M](https://d2lvgg3v3hfg70.cloudfront.net/TBW1039/11ee6e7b_ba83_1783_bf7f_db173944a9a9_TBW1039_11.jpg) 2 N2O4(g)+ 6 H2O(g), was carried out. A system was prepared with [N2O4] = [H2O] = 3.60 M as the only components initially. At equilibrium, [H2O] is 0.600 M. Calculate the equilibrium concentration of O2(g). Hint: Organize your information using an ICE table, then use the equilibrium H2O concentration to find the concentration of O2.
2 N2O4(g)+ 6 H2O(g), was carried out. A system was prepared with [N2O4] = [H2O] = 3.60 M as the only components initially. At equilibrium, [H2O] is 0.600 M. Calculate the equilibrium concentration of O2(g). Hint: Organize your information using an ICE table, then use the equilibrium H2O concentration to find the concentration of O2.
A)0.700 M
B)3.00 M
C)1.00 M
D)2.40 M
E)3.50 M
![<strong>A study of the following system, 4 NH<sub>3</sub>(g)+ 7 O<sub>2</sub>(g) 2 N<sub>2</sub>O<sub>4</sub>(g)+ 6 H<sub>2</sub>O(g), was carried out. A system was prepared with [N<sub>2</sub>O<sub>4</sub>] = [H<sub>2</sub>O] = 3.60 M as the only components initially. At equilibrium, [H<sub>2</sub>O] is 0.600 M. Calculate the equilibrium concentration of O<sub>2</sub>(g). Hint: Organize your information using an ICE table, then use the equilibrium H<sub>2</sub>O concentration to find the concentration of O<sub>2</sub>.</strong> A)0.700 M B)3.00 M C)1.00 M D)2.40 M E)3.50 M](https://d2lvgg3v3hfg70.cloudfront.net/TBW1039/11ee6e7b_ba83_1783_bf7f_db173944a9a9_TBW1039_11.jpg) 2 N2O4(g)+ 6 H2O(g), was carried out. A system was prepared with [N2O4] = [H2O] = 3.60 M as the only components initially. At equilibrium, [H2O] is 0.600 M. Calculate the equilibrium concentration of O2(g). Hint: Organize your information using an ICE table, then use the equilibrium H2O concentration to find the concentration of O2.
2 N2O4(g)+ 6 H2O(g), was carried out. A system was prepared with [N2O4] = [H2O] = 3.60 M as the only components initially. At equilibrium, [H2O] is 0.600 M. Calculate the equilibrium concentration of O2(g). Hint: Organize your information using an ICE table, then use the equilibrium H2O concentration to find the concentration of O2.A)0.700 M
B)3.00 M
C)1.00 M
D)2.40 M
E)3.50 M

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
56
A vessel was filled with NOCl gas until the concentration was 0.398 mol/liter. The temperature of the system was increased to 245 °C and the system was allowed to undergo the reaction, ![<strong>A vessel was filled with NOCl gas until the concentration was 0.398 mol/liter. The temperature of the system was increased to 245 °C and the system was allowed to undergo the reaction, , until equilibrium was attained. The physical chemistry students did a calculation and found the [Cl<sub>2</sub>] at that point was 0.0225 mol/liter. Calculate the value of K<sub>c</sub> at that temperature.</strong> A)2.87 × 10<sup>-3</sup> B)3.66 × 10<sup>-4</sup> C)8.13 × 10<sup>-3</sup> D)1.29 × 10<sup>-4</sup> E)9.14 × 10<sup>-5</sup>](https://d2lvgg3v3hfg70.cloudfront.net/TBW1039/11ee6e7b_ba83_3e94_bf7f_214a2ca03efa_TBW1039_11.jpg) , until equilibrium was attained. The physical chemistry students did a calculation and found the [Cl2] at that point was 0.0225 mol/liter. Calculate the value of Kc at that temperature.
, until equilibrium was attained. The physical chemistry students did a calculation and found the [Cl2] at that point was 0.0225 mol/liter. Calculate the value of Kc at that temperature.
A)2.87 × 10-3
B)3.66 × 10-4
C)8.13 × 10-3
D)1.29 × 10-4
E)9.14 × 10-5
![<strong>A vessel was filled with NOCl gas until the concentration was 0.398 mol/liter. The temperature of the system was increased to 245 °C and the system was allowed to undergo the reaction, , until equilibrium was attained. The physical chemistry students did a calculation and found the [Cl<sub>2</sub>] at that point was 0.0225 mol/liter. Calculate the value of K<sub>c</sub> at that temperature.</strong> A)2.87 × 10<sup>-3</sup> B)3.66 × 10<sup>-4</sup> C)8.13 × 10<sup>-3</sup> D)1.29 × 10<sup>-4</sup> E)9.14 × 10<sup>-5</sup>](https://d2lvgg3v3hfg70.cloudfront.net/TBW1039/11ee6e7b_ba83_3e94_bf7f_214a2ca03efa_TBW1039_11.jpg) , until equilibrium was attained. The physical chemistry students did a calculation and found the [Cl2] at that point was 0.0225 mol/liter. Calculate the value of Kc at that temperature.
, until equilibrium was attained. The physical chemistry students did a calculation and found the [Cl2] at that point was 0.0225 mol/liter. Calculate the value of Kc at that temperature.A)2.87 × 10-3
B)3.66 × 10-4
C)8.13 × 10-3
D)1.29 × 10-4
E)9.14 × 10-5

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
57
A study of the system, 4 NH3(g)+ 7 O2(g) ![<strong>A study of the system, 4 NH<sub>3</sub>(g)+ 7 O<sub>2</sub>(g) 2 N<sub>2</sub>O<sub>4</sub>(g)+ 6 H<sub>2</sub>O(g), was carried out. A system was prepared with [NH<sub>3</sub>] = [O<sub>2</sub>] = 3.60 M as the only components initially. At equilibrium, [N<sub>2</sub>O<sub>4</sub>] is 0.60 M. Calculate the value of the equilibrium constant, K<sub>c</sub>, for the reaction.Hint: Organize your information using an ICE table, then use the equilibrium N<sub>2</sub>O<sub>4</sub> concentration to find the concentrations of all reactants and products to find K<sub>c</sub>.</strong> A)8.1 B)0.0000093 C)0.30 D)0.022 E)3.3](https://d2lvgg3v3hfg70.cloudfront.net/TBW1039/11ee6e7b_ba83_3e95_bf7f_9fc9c24d5be2_TBW1039_11.jpg) 2 N2O4(g)+ 6 H2O(g), was carried out. A system was prepared with [NH3] = [O2] = 3.60 M as the only components initially. At equilibrium, [N2O4] is 0.60 M. Calculate the value of the equilibrium constant, Kc, for the reaction.Hint: Organize your information using an ICE table, then use the equilibrium N2O4 concentration to find the concentrations of all reactants and products to find Kc.
2 N2O4(g)+ 6 H2O(g), was carried out. A system was prepared with [NH3] = [O2] = 3.60 M as the only components initially. At equilibrium, [N2O4] is 0.60 M. Calculate the value of the equilibrium constant, Kc, for the reaction.Hint: Organize your information using an ICE table, then use the equilibrium N2O4 concentration to find the concentrations of all reactants and products to find Kc.
A)8.1
B)0.0000093
C)0.30
D)0.022
E)3.3
![<strong>A study of the system, 4 NH<sub>3</sub>(g)+ 7 O<sub>2</sub>(g) 2 N<sub>2</sub>O<sub>4</sub>(g)+ 6 H<sub>2</sub>O(g), was carried out. A system was prepared with [NH<sub>3</sub>] = [O<sub>2</sub>] = 3.60 M as the only components initially. At equilibrium, [N<sub>2</sub>O<sub>4</sub>] is 0.60 M. Calculate the value of the equilibrium constant, K<sub>c</sub>, for the reaction.Hint: Organize your information using an ICE table, then use the equilibrium N<sub>2</sub>O<sub>4</sub> concentration to find the concentrations of all reactants and products to find K<sub>c</sub>.</strong> A)8.1 B)0.0000093 C)0.30 D)0.022 E)3.3](https://d2lvgg3v3hfg70.cloudfront.net/TBW1039/11ee6e7b_ba83_3e95_bf7f_9fc9c24d5be2_TBW1039_11.jpg) 2 N2O4(g)+ 6 H2O(g), was carried out. A system was prepared with [NH3] = [O2] = 3.60 M as the only components initially. At equilibrium, [N2O4] is 0.60 M. Calculate the value of the equilibrium constant, Kc, for the reaction.Hint: Organize your information using an ICE table, then use the equilibrium N2O4 concentration to find the concentrations of all reactants and products to find Kc.
2 N2O4(g)+ 6 H2O(g), was carried out. A system was prepared with [NH3] = [O2] = 3.60 M as the only components initially. At equilibrium, [N2O4] is 0.60 M. Calculate the value of the equilibrium constant, Kc, for the reaction.Hint: Organize your information using an ICE table, then use the equilibrium N2O4 concentration to find the concentrations of all reactants and products to find Kc.A)8.1
B)0.0000093
C)0.30
D)0.022
E)3.3

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
58
A study of the system, H2(g)+ I2(g)  2HI(g), was carried out. Kc = 54.9 at 699.0 K for this reaction. A system was charged with 2.50 moles of HI in a 5.00-liter vessel as the only component initially. The system attained equilibrium at 699.0 K. How many moles of H2(g)should there be in the container at that time?Hint: Be sure to find molarities of all reactants and products before plugging in to the equilibrium law and solving for the amount of H2.
2HI(g), was carried out. Kc = 54.9 at 699.0 K for this reaction. A system was charged with 2.50 moles of HI in a 5.00-liter vessel as the only component initially. The system attained equilibrium at 699.0 K. How many moles of H2(g)should there be in the container at that time?Hint: Be sure to find molarities of all reactants and products before plugging in to the equilibrium law and solving for the amount of H2.
A)0.337 moles
B)1.25 moles
C)0.297 moles
D)0.500 moles
E)0.266 moles
 2HI(g), was carried out. Kc = 54.9 at 699.0 K for this reaction. A system was charged with 2.50 moles of HI in a 5.00-liter vessel as the only component initially. The system attained equilibrium at 699.0 K. How many moles of H2(g)should there be in the container at that time?Hint: Be sure to find molarities of all reactants and products before plugging in to the equilibrium law and solving for the amount of H2.
2HI(g), was carried out. Kc = 54.9 at 699.0 K for this reaction. A system was charged with 2.50 moles of HI in a 5.00-liter vessel as the only component initially. The system attained equilibrium at 699.0 K. How many moles of H2(g)should there be in the container at that time?Hint: Be sure to find molarities of all reactants and products before plugging in to the equilibrium law and solving for the amount of H2.A)0.337 moles
B)1.25 moles
C)0.297 moles
D)0.500 moles
E)0.266 moles

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
59
A study of the system, H2(g)+ I2(g)  2HI(g), was carried out. Kc = 54.9 at 699.0 K for this reaction. A system was charged with 2.50 moles of H2(g)and 2.50 moles I2(g)in a 5.00 liter vessel as the only components initially. The system attained equilibrium at 699.0 K. How many moles of H2(g)should there be in the container at that time?Hint: Be sure to find molarities of all reactants and products before plugging in to the equilibrium law and solving for the amount of H2.
2HI(g), was carried out. Kc = 54.9 at 699.0 K for this reaction. A system was charged with 2.50 moles of H2(g)and 2.50 moles I2(g)in a 5.00 liter vessel as the only components initially. The system attained equilibrium at 699.0 K. How many moles of H2(g)should there be in the container at that time?Hint: Be sure to find molarities of all reactants and products before plugging in to the equilibrium law and solving for the amount of H2.
A)0.531 moles
B)0.674 moles
C)0.594 moles
D)1.00 moles
E)0.266 moles
 2HI(g), was carried out. Kc = 54.9 at 699.0 K for this reaction. A system was charged with 2.50 moles of H2(g)and 2.50 moles I2(g)in a 5.00 liter vessel as the only components initially. The system attained equilibrium at 699.0 K. How many moles of H2(g)should there be in the container at that time?Hint: Be sure to find molarities of all reactants and products before plugging in to the equilibrium law and solving for the amount of H2.
2HI(g), was carried out. Kc = 54.9 at 699.0 K for this reaction. A system was charged with 2.50 moles of H2(g)and 2.50 moles I2(g)in a 5.00 liter vessel as the only components initially. The system attained equilibrium at 699.0 K. How many moles of H2(g)should there be in the container at that time?Hint: Be sure to find molarities of all reactants and products before plugging in to the equilibrium law and solving for the amount of H2.A)0.531 moles
B)0.674 moles
C)0.594 moles
D)1.00 moles
E)0.266 moles

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
60
A student was assigned to carry out some calculations regarding the system described by the equation, I2(g)+ Br2(g)  2IBr(g). Kc = 250 for this system. The system was to be charged with 0.0500 M of I2 and of Br2, then allowed to attain equilibrium at the desired temperature. What value should the student obtain for the equilibrium concentration of IBr(g)?Hint: Organize your information using an ICE table, then use the equilibrium law to find the concentration of IBr.
2IBr(g). Kc = 250 for this system. The system was to be charged with 0.0500 M of I2 and of Br2, then allowed to attain equilibrium at the desired temperature. What value should the student obtain for the equilibrium concentration of IBr(g)?Hint: Organize your information using an ICE table, then use the equilibrium law to find the concentration of IBr.
A)0.0056 M
B)0.0444 M
C)0.0888 M
D)0.0950 M
E)0.100 M
 2IBr(g). Kc = 250 for this system. The system was to be charged with 0.0500 M of I2 and of Br2, then allowed to attain equilibrium at the desired temperature. What value should the student obtain for the equilibrium concentration of IBr(g)?Hint: Organize your information using an ICE table, then use the equilibrium law to find the concentration of IBr.
2IBr(g). Kc = 250 for this system. The system was to be charged with 0.0500 M of I2 and of Br2, then allowed to attain equilibrium at the desired temperature. What value should the student obtain for the equilibrium concentration of IBr(g)?Hint: Organize your information using an ICE table, then use the equilibrium law to find the concentration of IBr.A)0.0056 M
B)0.0444 M
C)0.0888 M
D)0.0950 M
E)0.100 M

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
61
For the system, H2(g)+ I2(g)  2HI(g), Kc = 24.4 at 300 K. A system was charged with 2.00 moles of HI in a 3.00-liter container. The catalyst was introduced using a remote unit, and the system was allowed to come to equilibrium. How many moles of H2(g)will be present when the system reaches equilibrium? Hint: Organize your information using an ICE table, then use the equilibrium expression to find the equilibrium amount of H2.
2HI(g), Kc = 24.4 at 300 K. A system was charged with 2.00 moles of HI in a 3.00-liter container. The catalyst was introduced using a remote unit, and the system was allowed to come to equilibrium. How many moles of H2(g)will be present when the system reaches equilibrium? Hint: Organize your information using an ICE table, then use the equilibrium expression to find the equilibrium amount of H2.
A)0.288 moles
B)0.337 moles
C)0.388 moles
D)0.404 moles
E)0.424 moles
 2HI(g), Kc = 24.4 at 300 K. A system was charged with 2.00 moles of HI in a 3.00-liter container. The catalyst was introduced using a remote unit, and the system was allowed to come to equilibrium. How many moles of H2(g)will be present when the system reaches equilibrium? Hint: Organize your information using an ICE table, then use the equilibrium expression to find the equilibrium amount of H2.
2HI(g), Kc = 24.4 at 300 K. A system was charged with 2.00 moles of HI in a 3.00-liter container. The catalyst was introduced using a remote unit, and the system was allowed to come to equilibrium. How many moles of H2(g)will be present when the system reaches equilibrium? Hint: Organize your information using an ICE table, then use the equilibrium expression to find the equilibrium amount of H2.A)0.288 moles
B)0.337 moles
C)0.388 moles
D)0.404 moles
E)0.424 moles

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
62
For the system, H2(g)+ I2(g)  2HI(g), Kc = 24.4 at 300 K. A system was charged with 1.00 moles of H2(g)and 1.00 moles of I2(g)in a 3.00 liter container. The catalyst was introduced using a remote unit, and the system was allowed to come to equilibrium. How many moles of H2(g)will be present when the system reaches equilibrium? Hint: Organize your information using an ICE table, then use the equilibrium expression to find the equilibrium amount of H2.
2HI(g), Kc = 24.4 at 300 K. A system was charged with 1.00 moles of H2(g)and 1.00 moles of I2(g)in a 3.00 liter container. The catalyst was introduced using a remote unit, and the system was allowed to come to equilibrium. How many moles of H2(g)will be present when the system reaches equilibrium? Hint: Organize your information using an ICE table, then use the equilibrium expression to find the equilibrium amount of H2.
A)0.288 moles
B)0.337 moles
C)0.388 moles
D)0.404 moles
E)0.442 moles
 2HI(g), Kc = 24.4 at 300 K. A system was charged with 1.00 moles of H2(g)and 1.00 moles of I2(g)in a 3.00 liter container. The catalyst was introduced using a remote unit, and the system was allowed to come to equilibrium. How many moles of H2(g)will be present when the system reaches equilibrium? Hint: Organize your information using an ICE table, then use the equilibrium expression to find the equilibrium amount of H2.
2HI(g), Kc = 24.4 at 300 K. A system was charged with 1.00 moles of H2(g)and 1.00 moles of I2(g)in a 3.00 liter container. The catalyst was introduced using a remote unit, and the system was allowed to come to equilibrium. How many moles of H2(g)will be present when the system reaches equilibrium? Hint: Organize your information using an ICE table, then use the equilibrium expression to find the equilibrium amount of H2.A)0.288 moles
B)0.337 moles
C)0.388 moles
D)0.404 moles
E)0.442 moles

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
63
By convention, the ________ are always written on the left side of an equilibrium expression.

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
64
When the direction of an equation is ________, the new equilibrium constant is the reciprocal of the original.

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
65
For a given equilibrium process A + B  C + D the value of Kc was found to be equal to 3.2 × 10−4. Therefore, the reaction favors ________.
C + D the value of Kc was found to be equal to 3.2 × 10−4. Therefore, the reaction favors ________.
 C + D the value of Kc was found to be equal to 3.2 × 10−4. Therefore, the reaction favors ________.
C + D the value of Kc was found to be equal to 3.2 × 10−4. Therefore, the reaction favors ________.
Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
66
Write the equilibrium law for the reaction,SO2Cl2(g)+ 2NO(g)  SO2(g)+ 2NOCl(g)
SO2(g)+ 2NOCl(g)
 SO2(g)+ 2NOCl(g)
SO2(g)+ 2NOCl(g)
Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
67
The equilibrium law for a reaction in which all the components are gases is: 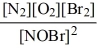 = KcWrite the balanced equation for the reaction described by this equilibrium constant.
= KcWrite the balanced equation for the reaction described by this equilibrium constant.
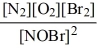 = KcWrite the balanced equation for the reaction described by this equilibrium constant.
= KcWrite the balanced equation for the reaction described by this equilibrium constant.
Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
68
Write the equilibrium law for the reaction,2AsF3(g)+ 3CCl4(g)  2AsCl3(g)+ 3CCl2F2(g)
2AsCl3(g)+ 3CCl2F2(g)
 2AsCl3(g)+ 3CCl2F2(g)
2AsCl3(g)+ 3CCl2F2(g)
Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
69
Write the equilibrium law, in terms of Kc, for the reaction,N2H4(g)+ 6H2O2(g)  2NO2(g)+ 8H2O(g)
2NO2(g)+ 8H2O(g)
 2NO2(g)+ 8H2O(g)
2NO2(g)+ 8H2O(g)
Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
70
Write the equilibrium law, in terms of Kc, for the following chemical reaction:Ni2+(aq)+ 6NH3(aq)→ [Ni(NH3)6]2+(aq)

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
71
Write the equilibrium law, in terms of Kp, for the reaction below.CH4(g)+ 2NO2(g)  CO2(g)+ 2H2O(g)+ N2(g)
CO2(g)+ 2H2O(g)+ N2(g)
 CO2(g)+ 2H2O(g)+ N2(g)
CO2(g)+ 2H2O(g)+ N2(g)
Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
72
Write the expression for Kp, for the reaction,N2(g)+ O2(g)  2NO(g)
2NO(g)
 2NO(g)
2NO(g)
Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
73
Write the expression for Kp, for the reaction,CO(g)+ 2H2(g)  CH3OH(g)
CH3OH(g)
 CH3OH(g)
CH3OH(g)
Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
74
Write the expression for Kp, for the reaction,4HCl(g)+ O2(g)  2H2O(g)+ 2Cl2(g)
2H2O(g)+ 2Cl2(g)
 2H2O(g)+ 2Cl2(g)
2H2O(g)+ 2Cl2(g)
Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
75
Write the expression for Kp, for the reaction,SnCl2(s)+ Cl2(g)  SnCl4(g)
SnCl4(g)
 SnCl4(g)
SnCl4(g)
Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
76
Write the expression for Kp, for the reaction,NH4Cl(s)  NH3(g)+ HCl(g)
NH3(g)+ HCl(g)
 NH3(g)+ HCl(g)
NH3(g)+ HCl(g)
Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
77
Write the expression for Kc, for the reaction,4Cr(s)+ 3CCl4(g)  4CrCl3(g)+ 4C(s)
4CrCl3(g)+ 4C(s)
 4CrCl3(g)+ 4C(s)
4CrCl3(g)+ 4C(s)
Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
78
Write the expression for Kc, for the reaction,C(s)+ H2O(g)  HCHO(g)
HCHO(g)
 HCHO(g)
HCHO(g)
Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
79
Write the equilibrium law for the following chemical reaction:TiO2(s)+ C(s)+ 2Cl2(g)→ TiCl4(g)+ CO2(g)

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
80
Write the equilibrium law for the following chemical reaction:Ni(H2O)62+(aq)+ 5NH3(aq)→ Ni(NH3)5(H2O)2+(aq)+ 5H2O(l)

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck



