Deck 14: Principles of Chemical Equilibrium
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/70
Play
Full screen (f)
Deck 14: Principles of Chemical Equilibrium
1
Explain the dynamic nature of equilibrium in terms of reversibility.
At equilibrium, the rates of the forward and reverse reactions are equal. An equilibrium expression relates the concentrations of reactants and products at equilibrium. Every elementary reaction that goes in the forward direction can also go in the reverse direction.
2
Understand some of the properties of equilibrium constants.
Concentrations or partial pressures can usually be used in place of activities in equilibrium constant expressions. The activity of a pure solid or liquid is equal to 1.
3
Relate the equilibrium position to thermodynamic quantities.
Reactions always move in the direction that minimizes the total free energy.
4
Predict the effects on the equilibrium position of changing concentrations or temperature.

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
5
Solve quantitative equilibrium problems.

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
6
Perform equilibrium calculations on reactions in aqueous solution.

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
7
A system in chemical equilibrium is characterized by one of the following:
A) molecules no longer undergo reactions of any kinds.
B) macroscopic changes are observed.
C) unaffected by changes in temperature.
D) unaffected by addition of catalyst.
E) concentrations are changing at a fixed constant rate.
A) molecules no longer undergo reactions of any kinds.
B) macroscopic changes are observed.
C) unaffected by changes in temperature.
D) unaffected by addition of catalyst.
E) concentrations are changing at a fixed constant rate.

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
8
A flask is filled with hydrogen, oxygen and several mL of water. This flask is then connected to a flask containing oxygen gas consisting of only the 17O isotope at the same pressure as the first flask. Which of the following will not occur?
A) Some water will evaporate.
B) The 17O2 will diffuse into the other flask.
C) Hydrogen and water vapour will diffuse into the second flask.
D) 17O will be incorporated into the water.
E) 16O2 will diffuse into the second flask.
A) Some water will evaporate.
B) The 17O2 will diffuse into the other flask.
C) Hydrogen and water vapour will diffuse into the second flask.
D) 17O will be incorporated into the water.
E) 16O2 will diffuse into the second flask.

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
9
The equilibrium constant
A) for an aqueous phase reaction is based on concentration of reactants and products.
B) for a gas phase reaction is based on pressures of the reactants and products.
C) is based on activities of all species.
D) is dependent on the initial concentrations of all species.
E) is dependent on the initial concentration of reactants only.
A) for an aqueous phase reaction is based on concentration of reactants and products.
B) for a gas phase reaction is based on pressures of the reactants and products.
C) is based on activities of all species.
D) is dependent on the initial concentrations of all species.
E) is dependent on the initial concentration of reactants only.

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
10
When is a reaction at equilibrium?
A) when K = 1
B) when all concentrations are equal
C) when Q = 1
D) when all concentrations are equal to 1 M or 1 atm
E) when Q = K
A) when K = 1
B) when all concentrations are equal
C) when Q = 1
D) when all concentrations are equal to 1 M or 1 atm
E) when Q = K

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
11
The copper (I) ion is a curious species. In aqueous solutions, there are a number of reactions that it can undergo; one is the reaction with other copper(I) ions:2 Cu+ (aq)  Cu2+(aq) + Cu(s)
Cu2+(aq) + Cu(s) 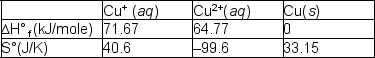 Using the tabulated data, calculate the equilibrium constant for this reaction of Cu+ (aq) at 298 and predict whether it will increase or decrease with increasing temperature. Choose from the following.
Using the tabulated data, calculate the equilibrium constant for this reaction of Cu+ (aq) at 298 and predict whether it will increase or decrease with increasing temperature. Choose from the following.
A) 3.5 x 104; increase
B) 1.2 x 106; decrease
C) 2.2 x 107 increase
D) 2.2 x 107 decrease
E) 3.5 x 104 decrease
 Cu2+(aq) + Cu(s)
Cu2+(aq) + Cu(s)  Using the tabulated data, calculate the equilibrium constant for this reaction of Cu+ (aq) at 298 and predict whether it will increase or decrease with increasing temperature. Choose from the following.
Using the tabulated data, calculate the equilibrium constant for this reaction of Cu+ (aq) at 298 and predict whether it will increase or decrease with increasing temperature. Choose from the following.A) 3.5 x 104; increase
B) 1.2 x 106; decrease
C) 2.2 x 107 increase
D) 2.2 x 107 decrease
E) 3.5 x 104 decrease

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
12
Adding water to the reaction vessel in which the following reaction is occurring will result inBa(OH)2.8H2O(s) + NH4NO3(s) Ba(NO3)2(s) + H2O(l) + NH3(aq)
A) no change.
B) reaction will shift right as the concentration of NH3 is decreased.
C) reaction will shift to the left as more product is added.
D) reaction will shift to the right as more product is added.
E) reaction will shift left as the concentration of NH3 is increased.
A) no change.
B) reaction will shift right as the concentration of NH3 is decreased.
C) reaction will shift to the left as more product is added.
D) reaction will shift to the right as more product is added.
E) reaction will shift left as the concentration of NH3 is increased.

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
13
If the dissolution of CaCl2 is endothermic, will any of the following increase the amount of CaCl2 that will dissolve in water?
A) addition of NaCl
B) addition of Ca(NO3)2
C) addition of HCl
D) increase in temperature
E) decrease in temperature
A) addition of NaCl
B) addition of Ca(NO3)2
C) addition of HCl
D) increase in temperature
E) decrease in temperature

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
14
Which of the following will decrease the amount of [NH4][NO3] that will dissolve in water?
A) addition of NaCl
B) addition of Ca(NO3)2
C) addition of HCl
D) addition of NaOH
E) increase the pressure
A) addition of NaCl
B) addition of Ca(NO3)2
C) addition of HCl
D) addition of NaOH
E) increase the pressure

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
15
A system containing nitrogen, ammonia, and hydrogen is at equilibrium. The exothermic reaction will shift to greater production of ammonia if
A) H2 is added.
B) NH3 is added.
C) Ar is added.
D) a catalyst is added.
E) the temperature is increased.
A) H2 is added.
B) NH3 is added.
C) Ar is added.
D) a catalyst is added.
E) the temperature is increased.

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
16
Which way will the Haber process shift if a container at equilibrium has its volume decreased?
N2 + 3 H2 2 NH3
2 NH3
A) Shift to the reactants.
B) Shift to the products.
C) There will be no change.
D) The temperature will increase.
E) Initially will shift to the products, then readjust to original pressures.
N2 + 3 H2
 2 NH3
2 NH3A) Shift to the reactants.
B) Shift to the products.
C) There will be no change.
D) The temperature will increase.
E) Initially will shift to the products, then readjust to original pressures.

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
17
Which way will the Haber process (a spontaneous reaction) shift if the temperature of a container at equilibrium is raised?
N2 + 3 H2 2 NH3
2 NH3
A) Shift to the reactants.
B) Shift to the products.
C) There will be no change.
D) The rate of the reaction will increase.
E) Initially will shift to the products, then readjust to original pressures.
N2 + 3 H2
 2 NH3
2 NH3A) Shift to the reactants.
B) Shift to the products.
C) There will be no change.
D) The rate of the reaction will increase.
E) Initially will shift to the products, then readjust to original pressures.

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
18
When solving equilibrium problems the best way to simplify the problem is to
A) apply approximations.
B) use the quadratic equation.
C) approach equilibrium from the side from which the change is smallest.
D) divide by zero.
E) find the rate-determining step.
A) apply approximations.
B) use the quadratic equation.
C) approach equilibrium from the side from which the change is smallest.
D) divide by zero.
E) find the rate-determining step.

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
19
When 0.1 mole of methylamine is dissolved in 500 mL of water, the following hydrolysis reaction occurs:
H2O(l) + CH3NH2(aq) CH3NH3+(aq) + OH-(aq)
CH3NH3+(aq) + OH-(aq)
The hydroxide concentration is found to be 8.6 x10-3 M when equilibrium is reached. What is the value of the equilibrium constant for this reaction?
A) 4.3 x 10-2
B) 7.4 x10-4
C) 7.4 x 10-5
D) 3.9 x 10-4
E) 7.4 x 10-3
H2O(l) + CH3NH2(aq)
 CH3NH3+(aq) + OH-(aq)
CH3NH3+(aq) + OH-(aq)The hydroxide concentration is found to be 8.6 x10-3 M when equilibrium is reached. What is the value of the equilibrium constant for this reaction?
A) 4.3 x 10-2
B) 7.4 x10-4
C) 7.4 x 10-5
D) 3.9 x 10-4
E) 7.4 x 10-3

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
20
When ammonia dissolves in water the new major species present are
A) H2O; NH2-; H+
B) H2O; NH3
C) H2O; NH4+; OH-
D) H2O; NH2-; H+
E) H2O; N3-; H+
A) H2O; NH2-; H+
B) H2O; NH3
C) H2O; NH4+; OH-
D) H2O; NH2-; H+
E) H2O; N3-; H+

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
21
Addition of sodium mono-hydrogencarbonate to water gives what minor species?
A) H2O; Na+; H+; CO32-
B) H2O; Na+; OH-; H2CO3
C) H2O; NaHCO3
D) H2O; Na+; H3CO3+; OH-
E) H2O; Na+; HCO3-
A) H2O; Na+; H+; CO32-
B) H2O; Na+; OH-; H2CO3
C) H2O; NaHCO3
D) H2O; Na+; H3CO3+; OH-
E) H2O; Na+; HCO3-

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
22
Addition of sodium mono-hydrogencarbonate to water gives what major species?
A) H2O; H+; CO32-
B) H+, CO32-, OH-; H2CO3
C) NaHCO3
D) H2O; Na+; H2CO3, OH-
E) H2O; Na+; HCO3-
A) H2O; H+; CO32-
B) H+, CO32-, OH-; H2CO3
C) NaHCO3
D) H2O; Na+; H2CO3, OH-
E) H2O; Na+; HCO3-

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
23
What are the minor species present upon adding the slightly soluble PbCl2(s) to water?
A) H2O; Pb+2, Cl-, Cl22-
B) Pb+2, Cl-, Cl22-
C) Pb+2, Cl-
D) H2O; Pb+2, Cl-
E) PbCl2(s)
A) H2O; Pb+2, Cl-, Cl22-
B) Pb+2, Cl-, Cl22-
C) Pb+2, Cl-
D) H2O; Pb+2, Cl-
E) PbCl2(s)

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
24
Ammonia and aqueous HCl are combined in an Erlenmeyer flask. Which of the following reactions occurs?
A) precipitation reaction
B) donation of a proton to water
C) dissociation of a complex
D) donation of a proton to a base
E) dissolution of a salt in water
A) precipitation reaction
B) donation of a proton to water
C) dissociation of a complex
D) donation of a proton to a base
E) dissolution of a salt in water

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
25
Classify the equilibrium constant for the following reaction as:
Ag+(aq) + 2 NH3(aq) Ag(NH3)2+(aq)
Ag(NH3)2+(aq)
A) Ksp
B) Kion
C) Kf
D) Ka
E) Kb
Ag+(aq) + 2 NH3(aq)
 Ag(NH3)2+(aq)
Ag(NH3)2+(aq)A) Ksp
B) Kion
C) Kf
D) Ka
E) Kb

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
26
Classify the equilibrium constant for the following reaction as:
CH3NH2(aq) + H2O(l) CH3NH3+(aq) + OH-(aq)
CH3NH3+(aq) + OH-(aq)
A) Ksp
B) Kion
C) Kf
D) Ka
E) Kb
CH3NH2(aq) + H2O(l)
 CH3NH3+(aq) + OH-(aq)
CH3NH3+(aq) + OH-(aq)A) Ksp
B) Kion
C) Kf
D) Ka
E) Kb

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
27
Classify the equilibrium constant for the following reaction as:
Fe(OH)3(s) Fe+3(aq) + 3 OH-(aq)
Fe+3(aq) + 3 OH-(aq)
A) Ksp
B) Kion
C) Kf
D) Ka
E) Kb
Fe(OH)3(s)
 Fe+3(aq) + 3 OH-(aq)
Fe+3(aq) + 3 OH-(aq)A) Ksp
B) Kion
C) Kf
D) Ka
E) Kb

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
28
In the manufacture of ammonia, nitrogen molecules dissociate to give nitrogen atoms on the surface of the catalyst. Draw a molecular picture showing the reverse of this process.

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
29
Draw a graph of concentration vs. time for a system containing cis- and trans-butene in equilibrium (assume the equilibrium constant is 3.0) to which is added an amount of trans-butene equal to that present in the original mixture and monitored until equilibrium is reached again.

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
30
Acetic acid dissolves in water and undergoes the following reaction to increase the concentration of hydronium ions:
CH3COOH (aq) H3O+ (aq) + CH3COO- (aq)
k1Express the equilibrium constant in terms of the rate constants of this elementary process and its reverse.
H3O+ (aq) + CH3COO- (aq) CH3COOH (aq) k-1
CH3COOH (aq) H3O+ (aq) + CH3COO- (aq)
k1Express the equilibrium constant in terms of the rate constants of this elementary process and its reverse.
H3O+ (aq) + CH3COO- (aq) CH3COOH (aq) k-1

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
31
What is the concentration based equilibrium constant for
4NO2(g) + O2(g) 2N2O5(g)?
4NO2(g) + O2(g) 2N2O5(g)?

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
32
Write the equilibrium constant expression for the following reaction:
2 N2 (g) + 2O2 (g) 4 NO (g)
4 NO (g)
2 N2 (g) + 2O2 (g)
 4 NO (g)
4 NO (g)
Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
33
Write the equilibrium constant expression for the following reaction:
C2H4 (g) + 2HCl (g) + O2 (g) CH2ClCH2Cl (g) + H2O (g)
CH2ClCH2Cl (g) + H2O (g)
C2H4 (g) + 2HCl (g) + O2 (g)
 CH2ClCH2Cl (g) + H2O (g)
CH2ClCH2Cl (g) + H2O (g) 
Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
34
Write the equilibrium constant expressions for the following reaction:
N2(g) + 3 H2(g) 2 NH3(g)
2 NH3(g)
N2(g) + 3 H2(g)
 2 NH3(g)
2 NH3(g)
Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
35
Write the equilibrium constant expression and state the reference concentrations for the reaction:
CaCO3(s) CaO(s) + CO2(g)
CaO(s) + CO2(g)
CaCO3(s)
 CaO(s) + CO2(g)
CaO(s) + CO2(g)
Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
36
Write the equilibrium constant expressions and state the reference concentrations for the reactants and products:
Cl2 (g) + 2 H2O (l ) HOCl (aq) + Cl- (aq) + H3O+ (aq)
HOCl (aq) + Cl- (aq) + H3O+ (aq)
Cl2 (g) + 2 H2O (l )
 HOCl (aq) + Cl- (aq) + H3O+ (aq)
HOCl (aq) + Cl- (aq) + H3O+ (aq)
Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
37
Write the equilibrium constant expression for the following reaction:
HCOOH(aq) + H2O(l) H3O+(aq) + HCOO-1(aq)
H3O+(aq) + HCOO-1(aq)
HCOOH(aq) + H2O(l)
 H3O+(aq) + HCOO-1(aq)
H3O+(aq) + HCOO-1(aq)
Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
38
Write the equilibrium constant expression for the following reaction in terms of concentrations:
2 PbS(s) + 3 O2(g) 2 PbO(s) + 2 SO2(g)
2 PbO(s) + 2 SO2(g)
2 PbS(s) + 3 O2(g)
 2 PbO(s) + 2 SO2(g)
2 PbO(s) + 2 SO2(g)
Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
39
Write the combined equilibrium constant expression for the following proposed reaction mechanism of the reaction between NO2 and CO:NO2(g) + NO2(g)  NO3(g) + NO(g)
NO3(g) + NO(g)
NO3(g) + CO(g) CO2(g) + NO2(g)
CO2(g) + NO2(g)
 NO3(g) + NO(g)
NO3(g) + NO(g)NO3(g) + CO(g)
 CO2(g) + NO2(g)
CO2(g) + NO2(g)
Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
40
The equilibrium constant for the following reaction is given:
4 NO2(g) 2 N2O(g) + 3 O2(g) K1 = 690What is the value for the equilibrium constant of the reverse reaction?
2 N2O(g) + 3 O2(g) K1 = 690What is the value for the equilibrium constant of the reverse reaction?
2 N2O(g) + 3 O2(g) 4 NO2(g) K2 =?
4 NO2(g) K2 =?
4 NO2(g)
 2 N2O(g) + 3 O2(g) K1 = 690What is the value for the equilibrium constant of the reverse reaction?
2 N2O(g) + 3 O2(g) K1 = 690What is the value for the equilibrium constant of the reverse reaction?2 N2O(g) + 3 O2(g)
 4 NO2(g) K2 =?
4 NO2(g) K2 =?
Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
41
Based on the following two reactions and equilibrium constants, determine the value of K3.
I. NO(g) + ½ O2(g) NO2(g) K1 = 1.3 x 106
NO2(g) K1 = 1.3 x 106
II. ½ N2(g) + ½ O2(g) NO(g) K2 = 6.5 x 10-16
NO(g) K2 = 6.5 x 10-16
N2(g) + 2 O2(g) 2 NO2(g) K3 =?
2 NO2(g) K3 =?
I. NO(g) + ½ O2(g)
 NO2(g) K1 = 1.3 x 106
NO2(g) K1 = 1.3 x 106II. ½ N2(g) + ½ O2(g)
 NO(g) K2 = 6.5 x 10-16
NO(g) K2 = 6.5 x 10-16N2(g) + 2 O2(g)
 2 NO2(g) K3 =?
2 NO2(g) K3 =? 
Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
42
Calculate the equilibrium constant for the oxychlorination of ethylene to vinyl chloride, CH2CHCl, under standard conditions at 100oC given the thermodynamic data below collected at 25oC:
G° = 53.6(CH2CHCl), -95.3(HCl), 68.49(CH2CH2), -228.7(H2O) kJ/mol
H° = 37.2(CH2CHCl), -92.3(HCl), 52.4(CH2CH2), -241.8(H2O) kJ/mol
The equation of reaction is:
CH2CH2 (g) + HCl (g) + O2 (g) CH2CHCl (g) + H2O (g)
G° = 53.6(CH2CHCl), -95.3(HCl), 68.49(CH2CH2), -228.7(H2O) kJ/mol
H° = 37.2(CH2CHCl), -92.3(HCl), 52.4(CH2CH2), -241.8(H2O) kJ/mol
The equation of reaction is:
CH2CH2 (g) + HCl (g) + O2 (g) CH2CHCl (g) + H2O (g)

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
43
The dehydration of benzyl alcohol to benzaldehyde is shown below:C6H5CH2OH(g)  C6H5CHO(g) + H2(g)The equilibrium constant for this process is 0.558 at 525 K. 0.2 moles of benzyl alcohol is placed in a 2L flask with 0.15 moles benzaldehyde and 0.1 moles of H2 gas. Determine Q for this mixture and which direction the reaction will shift to reach equilibrium.
C6H5CHO(g) + H2(g)The equilibrium constant for this process is 0.558 at 525 K. 0.2 moles of benzyl alcohol is placed in a 2L flask with 0.15 moles benzaldehyde and 0.1 moles of H2 gas. Determine Q for this mixture and which direction the reaction will shift to reach equilibrium.
 C6H5CHO(g) + H2(g)The equilibrium constant for this process is 0.558 at 525 K. 0.2 moles of benzyl alcohol is placed in a 2L flask with 0.15 moles benzaldehyde and 0.1 moles of H2 gas. Determine Q for this mixture and which direction the reaction will shift to reach equilibrium.
C6H5CHO(g) + H2(g)The equilibrium constant for this process is 0.558 at 525 K. 0.2 moles of benzyl alcohol is placed in a 2L flask with 0.15 moles benzaldehyde and 0.1 moles of H2 gas. Determine Q for this mixture and which direction the reaction will shift to reach equilibrium.
Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
44
Calcite, CaCO3(s), can be converted to CaO(s) and CO2(g). Determine the equilibrium constant at 25oC and predict whether equilibrium favours the products or the reactants. 


Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
45
Calcite, CaCO3(s), can be converted to CaO(s) and CO2(g). What is the equilibrium pressure of CO2 at 1150ºK? 


Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
46
Determine the equilibrium constant at 0oC for the following unbalanced reaction:Ba(OH)2.08H2O(s) + NH4NO3(s) Ba(NO3)2(s) + H2O(l) + NH3(aq) 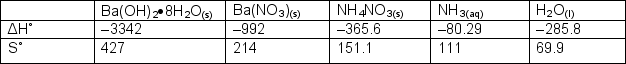


Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
47
The "water gas shift reaction" is shown below. Calculate K at (a) 1000ºK and (b) find the temperature at which the equilibrium constant is 1.CO (g) + H2O (g)  CO2 (g) + H2 (g)
CO2 (g) + H2 (g) 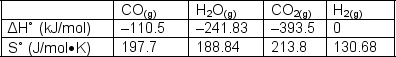
 CO2 (g) + H2 (g)
CO2 (g) + H2 (g) 

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
48
Consider the dissociation of mono-hydrogen carbonate to carbonate and aqueous hydrogen ion:H2O(l) + HCO3- (aq) ![Consider the dissociation of mono-hydrogen carbonate to carbonate and aqueous hydrogen ion:H<sub>2</sub>O<sub>(</sub><sub>l)</sub><sub> </sub>+ HCO<sub>3</sub><sup>-</sup> (aq) H<sub>3</sub>O<sup>+</sup> (aq) + CO<sub>3</sub><sup>2-</sup> (aq)ΔG˚ = (-237.1) (-586.77) (-237.1) (-527.8) kJ/mol a) Calculate ∆G° for this reaction. b) Calculate ∆G for the same reaction, where [CO<sub>3</sub><sup>2-</sup>]=[HCO<sub>3</sub><sup>-</sup>] = 1 M and [H<sup>+</sup>] = 1 x 10<sup>-11</sup> M. Under which conditions will this reaction be spontaneous?](https://d2lvgg3v3hfg70.cloudfront.net/TB9687/11ee726d_d43e_537c_827e_ed882efe9646_TB9687_11.jpg) H3O+ (aq) + CO32- (aq)ΔG˚ = (-237.1) (-586.77) (-237.1) (-527.8) kJ/mol
H3O+ (aq) + CO32- (aq)ΔG˚ = (-237.1) (-586.77) (-237.1) (-527.8) kJ/mol
a) Calculate ∆G° for this reaction.
b) Calculate ∆G for the same reaction, where [CO32-]=[HCO3-] = 1 M and [H+] = 1 x 10-11 M. Under which conditions will this reaction be spontaneous?
![Consider the dissociation of mono-hydrogen carbonate to carbonate and aqueous hydrogen ion:H<sub>2</sub>O<sub>(</sub><sub>l)</sub><sub> </sub>+ HCO<sub>3</sub><sup>-</sup> (aq) H<sub>3</sub>O<sup>+</sup> (aq) + CO<sub>3</sub><sup>2-</sup> (aq)ΔG˚ = (-237.1) (-586.77) (-237.1) (-527.8) kJ/mol a) Calculate ∆G° for this reaction. b) Calculate ∆G for the same reaction, where [CO<sub>3</sub><sup>2-</sup>]=[HCO<sub>3</sub><sup>-</sup>] = 1 M and [H<sup>+</sup>] = 1 x 10<sup>-11</sup> M. Under which conditions will this reaction be spontaneous?](https://d2lvgg3v3hfg70.cloudfront.net/TB9687/11ee726d_d43e_537c_827e_ed882efe9646_TB9687_11.jpg) H3O+ (aq) + CO32- (aq)ΔG˚ = (-237.1) (-586.77) (-237.1) (-527.8) kJ/mol
H3O+ (aq) + CO32- (aq)ΔG˚ = (-237.1) (-586.77) (-237.1) (-527.8) kJ/mol a) Calculate ∆G° for this reaction.
b) Calculate ∆G for the same reaction, where [CO32-]=[HCO3-] = 1 M and [H+] = 1 x 10-11 M. Under which conditions will this reaction be spontaneous?

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
49
Water undergoes dissociation as shown below:
2 H2O (aq) H3O+ (aq) + OH- (aq)
H3O+ (aq) + OH- (aq)
?G? = (-237.1) (-237.1) (-157.244) kJ/mole
Find K for this reaction at 25?C and 99?C.
2 H2O (aq)
 H3O+ (aq) + OH- (aq)
H3O+ (aq) + OH- (aq)?G? = (-237.1) (-237.1) (-157.244) kJ/mole
Find K for this reaction at 25?C and 99?C.

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
50
Lead chloride is not very soluble in water:
PbCl2 (s) Pb2+(aq) + 2 Cl- (aq)
Pb2+(aq) + 2 Cl- (aq) 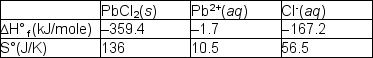 Using the tabulated data, find the equilibrium constants for dissolution of PbCl2 at 298º and 372ºK, respectively.
Using the tabulated data, find the equilibrium constants for dissolution of PbCl2 at 298º and 372ºK, respectively.
PbCl2 (s)
 Pb2+(aq) + 2 Cl- (aq)
Pb2+(aq) + 2 Cl- (aq)  Using the tabulated data, find the equilibrium constants for dissolution of PbCl2 at 298º and 372ºK, respectively.
Using the tabulated data, find the equilibrium constants for dissolution of PbCl2 at 298º and 372ºK, respectively.
Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
51
H2PO4-1 is commonly used in making buffer solutions with a pH near your body's pH. Given that Ka = 6.2 x10-8 at 25˚C for the following acid-base reaction, determine ΔG˚:H2PO4-1(aq) + H2O(l)  HPO4-2(aq) + H3O+(aq)
HPO4-2(aq) + H3O+(aq)
 HPO4-2(aq) + H3O+(aq)
HPO4-2(aq) + H3O+(aq)
Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
52
Predict whether each equilibrium reaction will shift toward products or reactants with a decrease in temperature.(a) CH4 + H2O  CO + 3 H2 ∆H° = 206 kJ(b) 2 SO2 + O2
CO + 3 H2 ∆H° = 206 kJ(b) 2 SO2 + O2  2 SO3 ∆H° = - 198 kJ
2 SO3 ∆H° = - 198 kJ
 CO + 3 H2 ∆H° = 206 kJ(b) 2 SO2 + O2
CO + 3 H2 ∆H° = 206 kJ(b) 2 SO2 + O2  2 SO3 ∆H° = - 198 kJ
2 SO3 ∆H° = - 198 kJ
Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
53
What will be the shift in equilibrium for the following reaction upon increasing temperature?N2 (g) + O2 (g)  2 NO (g) ∆H° = 90.3 kJ
2 NO (g) ∆H° = 90.3 kJ
 2 NO (g) ∆H° = 90.3 kJ
2 NO (g) ∆H° = 90.3 kJ
Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
54
A system containing nitrogen, ammonia and hydrogen is at equilibrium. If the partial pressures of the gases remain the same immediately upon addition of Ar, what will the equilibrium do to re-establish equilibrium?
N2 + 3 H2 2 NH3
2 NH3
N2 + 3 H2
 2 NH3
2 NH3
Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
55
Water undergoes self dissociation as below:
2 H2O (aq) H3O+ (aq) + OH- (aq)
H3O+ (aq) + OH- (aq)
If K increases as temperature increases, determine if this reaction is endothermic or exothermic and explain.
2 H2O (aq)
 H3O+ (aq) + OH- (aq)
H3O+ (aq) + OH- (aq)If K increases as temperature increases, determine if this reaction is endothermic or exothermic and explain.

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
56
A sample of 3.00 x 10-1 mole of pure phosgene gas, COCl2, was placed in a 15.0 L container and heated to 800ºK. At equilibrium, the partial pressure of CO was found to be 0.497 bar. Calculate the equilibrium constant for the following reaction:COCl2 (g)  CO (g) + Cl2 (g)
CO (g) + Cl2 (g)
 CO (g) + Cl2 (g)
CO (g) + Cl2 (g)
Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
57
A sample of pure phosgene, COCl2, is placed in a 15.0 L container which is subsequently heated to 800ºK. The equilibrium constant for the decomposition of phosgene at 800ºK is 0.297, and the equilibrium mixture contains 1.1 x 10-1 moles of Cl2(g), what are the equilibrium partial pressures of phosgene and carbon monoxide and how many moles of phosgene were placed in the original sample?COCl2 (g)  CO (g) + Cl2 (g)
CO (g) + Cl2 (g)
 CO (g) + Cl2 (g)
CO (g) + Cl2 (g)
Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
58
The conversion of nitrogen and hydrogen to ammonia is an important industrial reaction:N2 (g) + 3 H2 (g)  2 NH3 (g)If a tank initially containing only nitrogen at 1.0 atm and hydrogen at 3.0 atm converts 13.0 % of the nitrogen to ammonia, what is the value of the equilibrium constant for the reaction at this temperature?
2 NH3 (g)If a tank initially containing only nitrogen at 1.0 atm and hydrogen at 3.0 atm converts 13.0 % of the nitrogen to ammonia, what is the value of the equilibrium constant for the reaction at this temperature?
 2 NH3 (g)If a tank initially containing only nitrogen at 1.0 atm and hydrogen at 3.0 atm converts 13.0 % of the nitrogen to ammonia, what is the value of the equilibrium constant for the reaction at this temperature?
2 NH3 (g)If a tank initially containing only nitrogen at 1.0 atm and hydrogen at 3.0 atm converts 13.0 % of the nitrogen to ammonia, what is the value of the equilibrium constant for the reaction at this temperature?
Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
59
Heating isopropyl alcohol causes it to break down to acetone and hydrogen gas:(CH3)2CHOH(g)  (CH3)2CO(g) + H2(g)At 180˚C, the equilibrium constant is 0.444 for this dehydrogenation reaction. If 10.0 grams of isopropyl alcohol is placed in a 10.0-L vessel and heated, what will be the partial pressure of acetone when equilibrium is reached?
(CH3)2CO(g) + H2(g)At 180˚C, the equilibrium constant is 0.444 for this dehydrogenation reaction. If 10.0 grams of isopropyl alcohol is placed in a 10.0-L vessel and heated, what will be the partial pressure of acetone when equilibrium is reached?
 (CH3)2CO(g) + H2(g)At 180˚C, the equilibrium constant is 0.444 for this dehydrogenation reaction. If 10.0 grams of isopropyl alcohol is placed in a 10.0-L vessel and heated, what will be the partial pressure of acetone when equilibrium is reached?
(CH3)2CO(g) + H2(g)At 180˚C, the equilibrium constant is 0.444 for this dehydrogenation reaction. If 10.0 grams of isopropyl alcohol is placed in a 10.0-L vessel and heated, what will be the partial pressure of acetone when equilibrium is reached?
Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
60
Heating isopropyl alcohol causes it to break down to acetone and hydrogen gas:(CH3)2CHOH(g)  (CH3)2CO(g) + H2(g)If 20.0 g of isopropyl alcohol is placed in a 20.0 L vessel and heated to 180oC, the partial pressure of acetone stabilizes at 0.35 atm. What is the equilibrium constant?
(CH3)2CO(g) + H2(g)If 20.0 g of isopropyl alcohol is placed in a 20.0 L vessel and heated to 180oC, the partial pressure of acetone stabilizes at 0.35 atm. What is the equilibrium constant?
 (CH3)2CO(g) + H2(g)If 20.0 g of isopropyl alcohol is placed in a 20.0 L vessel and heated to 180oC, the partial pressure of acetone stabilizes at 0.35 atm. What is the equilibrium constant?
(CH3)2CO(g) + H2(g)If 20.0 g of isopropyl alcohol is placed in a 20.0 L vessel and heated to 180oC, the partial pressure of acetone stabilizes at 0.35 atm. What is the equilibrium constant?
Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
61
Heating isopropyl alcohol causes it to break down to acetone and hydrogen gas:(CH3)2CHOH(g)  (CH3)2CO(g) + H2(g)If 20.0 g of isopropyl alcohol is placed in a 20.0 L vessel and heated to 180oC. The final pressure in the vessel is 0.96 atm, what is the equilibrium constant?
(CH3)2CO(g) + H2(g)If 20.0 g of isopropyl alcohol is placed in a 20.0 L vessel and heated to 180oC. The final pressure in the vessel is 0.96 atm, what is the equilibrium constant?
 (CH3)2CO(g) + H2(g)If 20.0 g of isopropyl alcohol is placed in a 20.0 L vessel and heated to 180oC. The final pressure in the vessel is 0.96 atm, what is the equilibrium constant?
(CH3)2CO(g) + H2(g)If 20.0 g of isopropyl alcohol is placed in a 20.0 L vessel and heated to 180oC. The final pressure in the vessel is 0.96 atm, what is the equilibrium constant?
Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
62
The water gas shift reaction is used to remove CO from the mixture of gases in ammonia production:CO (g) + H2O (g)  CO2 (g) + H2 (g)At 300°C, the equilibrium constant for this reaction has K = 36. If a tank with a volume of 30.0 L is charged with 1.0 mole of CO and 2.2 mols of H2O and the temperature brought to 300°C, what are the equilibrium pressures of CO, H2, H2O and CO2 in the tank?
CO2 (g) + H2 (g)At 300°C, the equilibrium constant for this reaction has K = 36. If a tank with a volume of 30.0 L is charged with 1.0 mole of CO and 2.2 mols of H2O and the temperature brought to 300°C, what are the equilibrium pressures of CO, H2, H2O and CO2 in the tank?
 CO2 (g) + H2 (g)At 300°C, the equilibrium constant for this reaction has K = 36. If a tank with a volume of 30.0 L is charged with 1.0 mole of CO and 2.2 mols of H2O and the temperature brought to 300°C, what are the equilibrium pressures of CO, H2, H2O and CO2 in the tank?
CO2 (g) + H2 (g)At 300°C, the equilibrium constant for this reaction has K = 36. If a tank with a volume of 30.0 L is charged with 1.0 mole of CO and 2.2 mols of H2O and the temperature brought to 300°C, what are the equilibrium pressures of CO, H2, H2O and CO2 in the tank?
Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
63
At 1033ºK, K = 33.3 M for the equilibrium reaction:PCl5 (g)  PCl3 (g) + Cl2 (g)If a mixture of 0.100 mole of PCl5 and 0.300 mole of PCl3 is placed in a 2.00 L reaction vessel and heated to 1033ºK, what are the numbers of mols of each component at equilibrium?
PCl3 (g) + Cl2 (g)If a mixture of 0.100 mole of PCl5 and 0.300 mole of PCl3 is placed in a 2.00 L reaction vessel and heated to 1033ºK, what are the numbers of mols of each component at equilibrium?
 PCl3 (g) + Cl2 (g)If a mixture of 0.100 mole of PCl5 and 0.300 mole of PCl3 is placed in a 2.00 L reaction vessel and heated to 1033ºK, what are the numbers of mols of each component at equilibrium?
PCl3 (g) + Cl2 (g)If a mixture of 0.100 mole of PCl5 and 0.300 mole of PCl3 is placed in a 2.00 L reaction vessel and heated to 1033ºK, what are the numbers of mols of each component at equilibrium?
Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
64
HCO3-1 is used by your body to maintain pH levels in the blood. If the original concentration of [HCO3-1] = 0.0020M, determine the concentration of H3O+ given Keq = 4.7 x10-11.HCO3-1(aq) + H2O(l) ![HCO<sub>3</sub><sup>-1</sup> is used by your body to maintain pH levels in the blood. If the original concentration of [HCO<sub>3</sub><sup>-1</sup>] = 0.0020M, determine the concentration of H<sub>3</sub>O<sup>+</sup> given K<sub>eq</sub> = 4.7 x10<sup>-11</sup>.HCO<sub>3</sub><sup>-1</sup><sub>(</sub><sub>aq</sub><sub>)</sub> + H<sub>2</sub>O<sub>(</sub><sub>l)</sub><sub> </sub> H<sub>3</sub>O<sup>+</sup><sub>(</sub><sub>aq</sub><sub>)</sub> + CO<sub>3</sub><sup>-2</sup><sub>(</sub><sub>aq</sub><sub>)</sub>](https://d2lvgg3v3hfg70.cloudfront.net/TB9687/11ee726d_d43f_da32_827e_8d4bedfd17d0_TB9687_11.jpg) H3O+(aq) + CO3-2(aq)
H3O+(aq) + CO3-2(aq)
![HCO<sub>3</sub><sup>-1</sup> is used by your body to maintain pH levels in the blood. If the original concentration of [HCO<sub>3</sub><sup>-1</sup>] = 0.0020M, determine the concentration of H<sub>3</sub>O<sup>+</sup> given K<sub>eq</sub> = 4.7 x10<sup>-11</sup>.HCO<sub>3</sub><sup>-1</sup><sub>(</sub><sub>aq</sub><sub>)</sub> + H<sub>2</sub>O<sub>(</sub><sub>l)</sub><sub> </sub> H<sub>3</sub>O<sup>+</sup><sub>(</sub><sub>aq</sub><sub>)</sub> + CO<sub>3</sub><sup>-2</sup><sub>(</sub><sub>aq</sub><sub>)</sub>](https://d2lvgg3v3hfg70.cloudfront.net/TB9687/11ee726d_d43f_da32_827e_8d4bedfd17d0_TB9687_11.jpg) H3O+(aq) + CO3-2(aq)
H3O+(aq) + CO3-2(aq)
Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
65
A solution contains 0.01 M Ag and 0.1 M Pb2+. NaCl is gradually added to precipitate the Ag+ as AgCl, and the Pb2+ as PbCl2. Given the following Ksp values: AgCl (Ksp = 1.6 x 10-10) PbCl2 (Ksp = 2.4 x 10-4). What is the concentration of Ag+ when the Pb2+ begins to precipitate?

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
66
The Ksp of PbBr2 is 8.9 x 10-6. If enough PbBr2 salt is added to saturate the following solutions, determine the equilibrium concentration of Pb2+ in pure water and in 0.20 M KBr.

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
67
Solid CaF2 is added to a 0.10 M solution of CaCl2 until no more will dissolve. If the Ksp of CaF2 is 3.9 x 10-11 M3, what are the concentrations of Ca2+ (aq) and F-(aq) at equilibrium?

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
68
A sample of 0.134 g of CuCl2•2H2O is added to 0.10 L of an 1.0 M ammonia solution. It completely dissolves to give aqueous Cu2+ ions which react with the ammonia to form the coppertetraammine complex ion:Cu2+ (aq) + 4 NH3 (aq)  Cu(NH3)42+(aq) Kf = 5.0 x 1013What are the concentrations of copper (II), ammonia, and the complex ion, Cu(NH3) 42+, at equilibrium?
Cu(NH3)42+(aq) Kf = 5.0 x 1013What are the concentrations of copper (II), ammonia, and the complex ion, Cu(NH3) 42+, at equilibrium?
 Cu(NH3)42+(aq) Kf = 5.0 x 1013What are the concentrations of copper (II), ammonia, and the complex ion, Cu(NH3) 42+, at equilibrium?
Cu(NH3)42+(aq) Kf = 5.0 x 1013What are the concentrations of copper (II), ammonia, and the complex ion, Cu(NH3) 42+, at equilibrium?
Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
69
What are the major species present upon dissolving glucose (C6H12O6) in water?

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
70
Solid silver chloride is added to two beakers, one containing 0.5 M NaNO3 and the other 0.5 M NaCl. In which will the silver ion concentration be higher?

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck



