Deck 15: Oxidative Phosphorylation
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/20
Play
Full screen (f)
Deck 15: Oxidative Phosphorylation
1
The oxidation of FADH2 to FAD involves the transfer of _____; the oxidation of NADH to NAD+ involves the transfer of _____.
A) a hydrogen atom and an H+; a hydride
B) two hydrogen atoms; a hydride
C) a hydride and an H+; a hydrogen atom and an H+
D) a hydride; two hydrogen atoms
E) two hydrides; one hydride
A) a hydrogen atom and an H+; a hydride
B) two hydrogen atoms; a hydride
C) a hydride and an H+; a hydrogen atom and an H+
D) a hydride; two hydrogen atoms
E) two hydrides; one hydride
two hydrogen atoms; a hydride
2
Which of the following is the correct half reaction for the oxidation of acetaldehyde?
A) acetaldehyde + H2O acetate- + 3H+ + 2e-
B) acetate- + 3H+ + 2e- acetaldehyde + H2O
C) acetaldehyde + 2 H+ + 2e- ethanol
D) ethanol acetaldehyde + 2 H+ + 2e-
E) acetaldehyde + CO2 + H+ + 2e- pyruvate-
A) acetaldehyde + H2O acetate- + 3H+ + 2e-
B) acetate- + 3H+ + 2e- acetaldehyde + H2O
C) acetaldehyde + 2 H+ + 2e- ethanol
D) ethanol acetaldehyde + 2 H+ + 2e-
E) acetaldehyde + CO2 + H+ + 2e- pyruvate-
acetaldehyde + H2O acetate- + 3H+ + 2e-
3
If the following half-reactions are combined, what is the potential for the spontaneous reaction?
Oxaloacetate- + 2H+ + 2e- malate-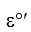 = -0.166
= -0.166
NAD+ + H+ + 2e- NADH 11ee7c6d_74e2_e452_ae82_f5f2b4bfa620_TBW1044_11= -0.315
A) -0.481 V
B) -0.149 V
C) 0.0523 V
D) 0.149 V
E) .0481 V
Oxaloacetate- + 2H+ + 2e- malate-
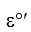 = -0.166
= -0.166NAD+ + H+ + 2e- NADH 11ee7c6d_74e2_e452_ae82_f5f2b4bfa620_TBW1044_11= -0.315
A) -0.481 V
B) -0.149 V
C) 0.0523 V
D) 0.149 V
E) .0481 V
0.149 V
4
If the reduction potential for NAD+ is -0.315 V and the reduction potential for oxygen is 0.815 V, what is the potential for the oxidation of NADH by oxygen?
A) -1.13 V
B) -0.50 V
C) 0.185 V
D) 0.50 V
E) 1.13 V
A) -1.13 V
B) -0.50 V
C) 0.185 V
D) 0.50 V
E) 1.13 V

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
5
In a muscle cell at 37  C, if the concentrations of pyruvate and lactate are 100 M and 50 M respectively, what is the actual reduction potential if
C, if the concentrations of pyruvate and lactate are 100 M and 50 M respectively, what is the actual reduction potential if 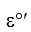 for pyruvate reduction is -0.185 V?
for pyruvate reduction is -0.185 V?
A) -0.194 V
B) -0.186 V
C) -0.176 V
D) 0.176 V
E) 0.194 V
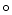 C, if the concentrations of pyruvate and lactate are 100 M and 50 M respectively, what is the actual reduction potential if
C, if the concentrations of pyruvate and lactate are 100 M and 50 M respectively, what is the actual reduction potential if 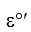 for pyruvate reduction is -0.185 V?
for pyruvate reduction is -0.185 V?A) -0.194 V
B) -0.186 V
C) -0.176 V
D) 0.176 V
E) 0.194 V

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
6
What is the G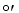 for the reduction of pyruvate to lactate by NADH given the following half reactions?
for the reduction of pyruvate to lactate by NADH given the following half reactions?
Pyruvate- + 2H+ + 2e- lactate-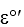 = -0.185
= -0.185
NAD+ + H+ + 2e- NADH 11ee7c6d_c899_8e54_ae82_0910aec0b20b_TBW1044_11= -0.315
A) -96.5 kJ/mol
B) -25.1 kJ/mol
C) -12.5 kJ/mol
D) 25.1 kJ/mol
E) 96.5 kJ/mol
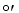 for the reduction of pyruvate to lactate by NADH given the following half reactions?
for the reduction of pyruvate to lactate by NADH given the following half reactions?Pyruvate- + 2H+ + 2e- lactate-
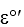 = -0.185
= -0.185NAD+ + H+ + 2e- NADH 11ee7c6d_c899_8e54_ae82_0910aec0b20b_TBW1044_11= -0.315
A) -96.5 kJ/mol
B) -25.1 kJ/mol
C) -12.5 kJ/mol
D) 25.1 kJ/mol
E) 96.5 kJ/mol

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
7
What does the reduction potential of 0.815 V for the reduction of water to oxygen indicate?
A) water is a very strong reducing agent
B) water is a very strong reducing agent and oxygen is a very strong oxidizing agent
C) oxygen is a very strong oxidizing agent
D) water will be spontaneously reduced to oxygen
E) none of the above
A) water is a very strong reducing agent
B) water is a very strong reducing agent and oxygen is a very strong oxidizing agent
C) oxygen is a very strong oxidizing agent
D) water will be spontaneously reduced to oxygen
E) none of the above

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
8
What genes are found in the mitochondrial genome?
A) electron transport proteins
B) citric acid cycle enzymes
C) fatty acid oxidation enzymes
D) outer membrane transport proteins
E) none of the above
A) electron transport proteins
B) citric acid cycle enzymes
C) fatty acid oxidation enzymes
D) outer membrane transport proteins
E) none of the above

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
9
Which of the following transport mechanisms is used in the inner mitochondrial membrane?
IMS = intermembrane space
A) antiport of ATP from IMS to matrix, ADP from matrix to IMS
B) symport of ATP and H+ from matrix to IMS
C) symport of Pi and H+ from IMS to matrix
D) antiport of ADP from IMS to matrix, Pi from matrix to IMS
E) symport of ADP and H+ from IMS to matrix
IMS = intermembrane space
A) antiport of ATP from IMS to matrix, ADP from matrix to IMS
B) symport of ATP and H+ from matrix to IMS
C) symport of Pi and H+ from IMS to matrix
D) antiport of ADP from IMS to matrix, Pi from matrix to IMS
E) symport of ADP and H+ from IMS to matrix

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
10
Which of the following is a source of ubiquinol?
A) Complex I
B) fatty acyl-CoA dehydrogenase
C) succinate dehydrogenase
D) mitochondrial dehydrogenase
E) all of the above
A) Complex I
B) fatty acyl-CoA dehydrogenase
C) succinate dehydrogenase
D) mitochondrial dehydrogenase
E) all of the above

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
11
The cytochromes undergo which of the following oxidation reactions?
A) Fe3+ Fe2+
B) Fe0 Fe2+
C) Fe2+ Fe3+
D) Cu1+ Cu2+
E) Cu2+ Cu1+
A) Fe3+ Fe2+
B) Fe0 Fe2+
C) Fe2+ Fe3+
D) Cu1+ Cu2+
E) Cu2+ Cu1+

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
12
How many protons are moved across the inner mitochondrial membrane at Complex III?
A) 1
B) 2
C) 3
D) 4
E) none of the above
A) 1
B) 2
C) 3
D) 4
E) none of the above

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
13
Ubiquinone is a _____ molecule that serves as a _____ electron carrier; cytochrome c is a _____ molecule that serves as a _____ electron carrier.
A) hydrophobic; 2; hydrophilic; 1
B) hydrophobic; 1; hydrophilic; 1
C) hydrophilic; 2; hydrophobic; 2
D) hydrophilic; 2; hydrophobic; 1
E) hydrophobic; 1; hydrophilic; 2
A) hydrophobic; 2; hydrophilic; 1
B) hydrophobic; 1; hydrophilic; 1
C) hydrophilic; 2; hydrophobic; 2
D) hydrophilic; 2; hydrophobic; 1
E) hydrophobic; 1; hydrophilic; 2

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
14
What free radical is thought to be commonly produced by the electron transport chain?
A) hydroxyl
B) peroxyl
C) superoxide
D) hydrogen peroxyl
E) all of the above
A) hydroxyl
B) peroxyl
C) superoxide
D) hydrogen peroxyl
E) all of the above

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
15
How many ATP can be produced from the complete oxidation of glucose if the malate-aspartate shuttle is used?
A) 2
B) 19.5
C) 28
D) 30
E) 32
A) 2
B) 19.5
C) 28
D) 30
E) 32

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
16
If an ATP synthase has 12 c subunits, how many protons must move through the enzyme to produce one ATP?
A) 2.5
B) 3
C) 4
D) 10
E) 12
A) 2.5
B) 3
C) 4
D) 10
E) 12

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
17
Which subunit of ATP synthase is responsible for the catalysis of ATP formation?
A) a
B) c
C)
D)
E)
A) a
B) c
C)
D)
E)

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
18
What is the P/O ratio for the oxidation of ubiquinone if three protons are required to synthesize one ATP?
A) 1
B) 1.5
C) 2
D) 3
E) 3.3
A) 1
B) 1.5
C) 2
D) 3
E) 3.3

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
19
Under normal metabolic conditions, the difference in pH between the matrix and intermembrane space is ___ suggesting that the processes of electron transport and oxidative phosphorylation ___.

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
20
The F0 component of ATP synthase contains ___ subunits; the F1 component contains ___ subunits.

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck



