Deck 8: Solution Chemistry: How Sweet Is Your Tea
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/90
Play
Full screen (f)
Deck 8: Solution Chemistry: How Sweet Is Your Tea
1
Of the following, which can serve as the solute in a solution?
A)A liquid
B)A solid
C)A gas
D)All of the above
A)A liquid
B)A solid
C)A gas
D)All of the above
All of the above
2
A homogeneous mixture consists of 12% ethanol, 28% methanol and 60% water. Which of these is the solvent for the mixture?
A)Ethanol
B)Methanol
C)Water
D)Ethanol and methanol
A)Ethanol
B)Methanol
C)Water
D)Ethanol and methanol
Water
3
A homogeneous mixture consists of 34% ethanol and 66% isopropanol. Which of these is the solute of the mixture?
A)Isopropanol
B)Ethanol
C)Both ethanol and isopropanol
D)Neither ethanol nor isopropanol
A)Isopropanol
B)Ethanol
C)Both ethanol and isopropanol
D)Neither ethanol nor isopropanol
Ethanol
4
In a solution, the solute is:
A)the substance in the greatest amount
B)the substance that is dissolved
C)always water
D)always a gas
A)the substance in the greatest amount
B)the substance that is dissolved
C)always water
D)always a gas

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
5
In a solution, the solvent is:
A)always water
B)the substance in the greatest amount
C)the substance that is dissolved
D)always a gas
A)always water
B)the substance in the greatest amount
C)the substance that is dissolved
D)always a gas

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
6
Which of the following statements best describes the phrase "like dissolves like"?
A)The only true solutions are formed when water dissolves a polar solute.
B)A solvent and a solute with similar intermolecular forces will easily make a solution.
C)The only true solutions are formed when water dissolves a nonpolar solute.
D)A solvent will dissolve a solute that has a similar mass.
A)The only true solutions are formed when water dissolves a polar solute.
B)A solvent and a solute with similar intermolecular forces will easily make a solution.
C)The only true solutions are formed when water dissolves a nonpolar solute.
D)A solvent will dissolve a solute that has a similar mass.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
7
Which of the following can be classified as a colloid?
A)Grape juice
B)Homogenized milk
C)Blood
D)Kool-Aid
A)Grape juice
B)Homogenized milk
C)Blood
D)Kool-Aid

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
8
Which of the following is an example of a true solution?
A)Blood
B)Homogenized milk
C)Apple juice
D)Mayonnaise
A)Blood
B)Homogenized milk
C)Apple juice
D)Mayonnaise

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
9
You have been given an unknown liquid that is soluble in water but not in benzene (a nonpolar solvent). Which type of intermolecular forces does this unknown have?
A)Polar
B)Nonpolar
C)Neither polar nor nonpolar
D)Both polar and nonpolar
A)Polar
B)Nonpolar
C)Neither polar nor nonpolar
D)Both polar and nonpolar

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
10
Which one of the following compounds will be most soluble in water?
A)CH3OH
B)CH3CH2OH
C)CH3CH2CH2OH
D)CH3CH2CH2CH2OH
A)CH3OH
B)CH3CH2OH
C)CH3CH2CH2OH
D)CH3CH2CH2CH2OH

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
11
Which one of the following compounds will be least soluble in water?
A)CH3NH2
B)CH3CH2OH
C)CH3CHO
D)CH3OCH3
A)CH3NH2
B)CH3CH2OH
C)CH3CHO
D)CH3OCH3

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
12
The exchange of oxygen and carbon dioxide in the lungs is an example of which law involving gases?
A)Boyle's law
B)Charles' law
C)Avogadro's law
D)Henry's law
A)Boyle's law
B)Charles' law
C)Avogadro's law
D)Henry's law

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
13
Which one of the following compounds will be most soluble in hexane, a nonpolar solvent?
A)CH3CH2 OH
B)CH3CH2CH2OH
C)CH3CH2CH2CH2CH3
D)CH3OH
A)CH3CH2 OH
B)CH3CH2CH2OH
C)CH3CH2CH2CH2CH3
D)CH3OH

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
14
A water solution of sucrose, table sugar, does not conduct electricity. Due to this property, sucrose is classified as a(n):
A)weak electrolyte
B)strong electrolyte
C)non-electrolyte
D)semi-electrolyte
A)weak electrolyte
B)strong electrolyte
C)non-electrolyte
D)semi-electrolyte

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
15
A water solution of acetic acid, vinegar, barely lights a light bulb. This property means that vinegar is a(n):
A)weak electrolyte
B)strong electrolyte
C)non-electrolyte
D)semi-electrolyte
A)weak electrolyte
B)strong electrolyte
C)non-electrolyte
D)semi-electrolyte

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
16
Which of the following is a typical chemical equation that represents a hydration reaction?
A)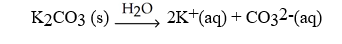
B)C6H12O6 (s)+ 6 O2 (g)? 6 CO2 (g)+ 6 H2O (l)
C)2 KClO3 (s)? 2 KCl (s)+ 3 O2 (g)
D)HCl (aq)+ NaOH (aq)? NaCl (aq)+ H2O (l)
A)
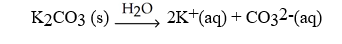
B)C6H12O6 (s)+ 6 O2 (g)? 6 CO2 (g)+ 6 H2O (l)
C)2 KClO3 (s)? 2 KCl (s)+ 3 O2 (g)
D)HCl (aq)+ NaOH (aq)? NaCl (aq)+ H2O (l)

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
17
What is the correct balanced hydration equation for (NH4)2CO3?
A)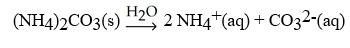
B)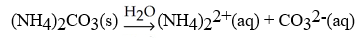
C)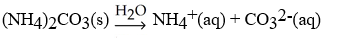
D)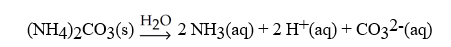
A)
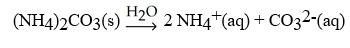
B)
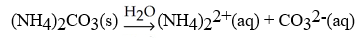
C)
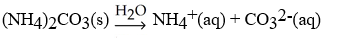
D)

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
18
Which one of these substances will exist in solution only as molecules?
A)CH3OH
B)LiOH
C)NH4OH
D)CH3NH2
A)CH3OH
B)LiOH
C)NH4OH
D)CH3NH2

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
19
Which of the following is an example of a weak electrolyte?
A)MgCl2
B)HC2H3O2
C)HNO3
D)Na2CO3
A)MgCl2
B)HC2H3O2
C)HNO3
D)Na2CO3

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
20
Which of the following is an example of a nonelectrolyte?
A)MgCl2
B)HC2H3O2
C)C12H22O11
D)Na2SO4
A)MgCl2
B)HC2H3O2
C)C12H22O11
D)Na2SO4

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
21
How many equivalents of Li+ are present in a solution that contains 5.75 mol of Li+?
A)0)575 eq
B)5)75 eq
C)2)88 eq
D)11.5 eq
A)0)575 eq
B)5)75 eq
C)2)88 eq
D)11.5 eq

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
22
How many equivalents of Mg+2 are present in a solution that contains 2.75 mol of Mg+2?
A)1)38 eq
B)2)75 eq
C)5)50 eq
D)27.5 eq
A)1)38 eq
B)2)75 eq
C)5)50 eq
D)27.5 eq

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
23
Which of the following equation is a correct balanced hydration equation for the hydration of Na2SO4?
A)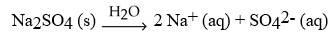
B)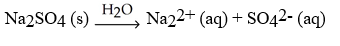
C)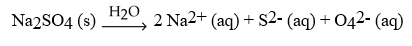
D)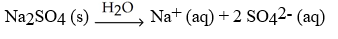
A)
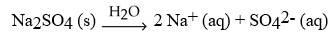
B)
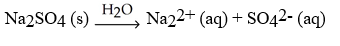
C)
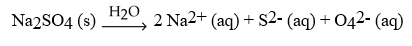
D)
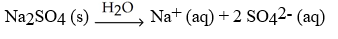

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
24
What is 75% of 375?
A)281
B)28,125
C)2)81
D)0)281
A)281
B)28,125
C)2)81
D)0)281

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
25
45 is what percent of 150?
A)0)30
B)30
C)0)0030
D)3000
A)0)30
B)30
C)0)0030
D)3000

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
26
Calculate the mass percent of a solution prepared by dissolving 17.2 g of NaCl in 149 g of water.
A)10.3%
B)11.5%
C)0)103%
D)0)115%
A)10.3%
B)11.5%
C)0)103%
D)0)115%

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
27
What is the percent mass/volume (%m/v)of a solution that is made from putting 25.0 g of NaCl in water to make 275 mL of solution?
A)0)0909%m/v
B)9)09%m/v
C)8)33%m/v
D)10.9%m/v
A)0)0909%m/v
B)9)09%m/v
C)8)33%m/v
D)10.9%m/v

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
28
What is the %v/v concentration of a sample of wine that contains 15 mL of ethyl alcohol in 200 mL of wine?
A)0)075%v/v
B)7)0%v/v
C)7)5%v/v
D)0)070%v/v
A)0)075%v/v
B)7)0%v/v
C)7)5%v/v
D)0)070%v/v

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
29
What is the %v/v concentration of a solution made by adding 25 mL of acetone to 75 mL of water?
A)0.25%v/v
B)25%v/v
C).33%v/v
D)33%v/v
A)0.25%v/v
B)25%v/v
C).33%v/v
D)33%v/v

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
30
What is the glucose concentration in ppm of a solution made by mixing 23.2 mg glucose with 2.000 L?
A)8)62 ppm
B)11.6 ppm
C)1)16 ppm
D)0)862 ppm
A)8)62 ppm
B)11.6 ppm
C)1)16 ppm
D)0)862 ppm

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
31
What is the lead concentration in ppb of a water sample that contains 28 ?g of lead in 44 L?
A)0)064 ppb
B)0)64 ppb
C)64 ppb
D)640 ppb
A)0)064 ppb
B)0)64 ppb
C)64 ppb
D)640 ppb

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
32
Calculate the volume (in L)of a solution that contains 3.12 moles of NaCl if the concentration of this solution is 6.67 M NaCl?
A)2)14 L
B)20.8 L
C)46.8 L
D)0)468 L
A)2)14 L
B)20.8 L
C)46.8 L
D)0)468 L

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
33
What is the molarity of a solution formed by dissolving 45 g NaOH in water to give a final volume of 250 mL?
A)0)0045 M
B)0)18 M
C)4)5 M
D)9)0 M
A)0)0045 M
B)0)18 M
C)4)5 M
D)9)0 M

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
34
What is the molarity of a solution that contains 36 mEq Ca2+ per liter?
A)0)018 M
B)0)036 M
C)18 M
D)36 M
A)0)018 M
B)0)036 M
C)18 M
D)36 M

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
35
Calculate the volume (in mL)of a 2.75 M solution that must be used to make 1.25 L of a 0.150 M solution.
A)0)0682 mL
B)68.2 mL
C)0)0330 mL
D)33.0 mL
A)0)0682 mL
B)68.2 mL
C)0)0330 mL
D)33.0 mL

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
36
What volume of 8.25 M NaOH solution must be diluted to prepare 2.40 L of 0.500 M NaOH solution?
A)39.6 L
B)0)356 L
C)438 mL
D)145 mL
A)39.6 L
B)0)356 L
C)438 mL
D)145 mL

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
37
What is the molarity of the solution obtained by diluting 125 mL of 2.50 M NaOH to 575 mL?
A)0)272 M
B)0)543 M
C)1)84 M
D)11.5 M
A)0)272 M
B)0)543 M
C)1)84 M
D)11.5 M

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
38
How much water must be added when 125 mL of a 2.00 M solution of HCl is diluted to a final concentration of 0.400 M?
A)125 mL
B)500 mL
C)625 mL
D)750 mL
A)125 mL
B)500 mL
C)625 mL
D)750 mL

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
39
In order to prevent the process of either crenation or hemolysis with red blood cells an intravenous solution concentration should be ________ to the concentration of blood.
A)hypotonic
B)hypertonic
C)isotonic
D)all of these
A)hypotonic
B)hypertonic
C)isotonic
D)all of these

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
40
The process of crenation occurs when the concentration outside a cell is ________ and is said to be ________.
A)lower in concentration; hypotonic
B)lower in concentration; hypertonic
C)higher in concentration; hypotonic
D)higher in concentration; hypertonic
A)lower in concentration; hypotonic
B)lower in concentration; hypertonic
C)higher in concentration; hypotonic
D)higher in concentration; hypertonic

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
41
The transport of nitrogen molecules across a cell membrane occurs via:
A)passive diffusion
B)facilitated transport
C)active transport
D)all of the above
A)passive diffusion
B)facilitated transport
C)active transport
D)all of the above

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
42
The transport of glucose molecules across a cell membrane occurs via:
A)passive diffusion
B)facilitated transport
C)active transport
D)all of the above
A)passive diffusion
B)facilitated transport
C)active transport
D)all of the above

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
43
A solution is also known as a ________ mixture.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
44
In a solution the particles are ________ distributed.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
45
Blood is not a colloid but a ________.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
46
Homogenized milk is not a solution but a ________.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
47
In a colloid the suspended particles do or do not settle out over time?

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
48
In a suspension the particles do or do not settle out over time?

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
49
A solution consists of 150 mL of isopropanol and 50 mL of water. Which is the solute?

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
50
The golden rule of solubility states that, "like dissolves ________."

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
51
Henry's law states that the solubility of a gas in a liquid ________ with increasing ________.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
52
The solubility of a gas in water ________ with increasing temperature.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
53
A solution that contains all the solute that it can contain is called a ________ solution.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
54
Identify the following as weak, strong or non-electrolytes.
-KNO3
A)strong
B)weak
C)Non
-KNO3
A)strong
B)weak
C)Non

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
55
Identify the following as weak, strong or non-electrolytes.
-HCl
A)strong
B)weak
C)Non
-HCl
A)strong
B)weak
C)Non

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
56
Identify the following as weak, strong or non-electrolytes.
-C2H5OH
A)strong
B)weak
C)Non
-C2H5OH
A)strong
B)weak
C)Non

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
57
Identify the following as weak, strong or non-electrolytes.
-NH3(aq)
A)strong
B)weak
C)Non
-NH3(aq)
A)strong
B)weak
C)Non

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
58
Identify the following as weak, strong or non-electrolytes.
-NaOH
A)strong
B)weak
C)Non
-NaOH
A)strong
B)weak
C)Non

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
59
Identify the following as weak, strong or non-electrolytes.
-C2H5COOH
A)strong
B)weak
C)Non
-C2H5COOH
A)strong
B)weak
C)Non

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
60
Identify the following as weak, strong or non-electrolytes.
-C12H22O11
A)strong
B)weak
C)Non
-C12H22O11
A)strong
B)weak
C)Non

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
61
Soluble covalent compounds do not conduct electricity and are therefore called ________.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
62
K2CO3 dissolves in water and produces ________ and ________ ions.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
63
NH2CONH2 is a ________electrolyte.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
64
A solution that contains 3.0 moles of Mg2+ ions contains ________ Eq Mg2+.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
65
Ethylene glycol (C2H6O2), a nonelectrolyte, is used as antifreeze in car radiators. A solution containing 86.0 g of ethylene glycol dissolved in 465 g of water has a density of 1.35 g/mL at 25 °C. Calculate the following:
A)the %m/m of the ethylene glycol in this solution.
B)the molarity of the ethylene glycol in this solution.
A)the %m/m of the ethylene glycol in this solution.
B)the molarity of the ethylene glycol in this solution.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
66
An aqueous solution is made by dissolving 45.0 g of H2SO4 in 850. g of water. The density of water is 1.00 g/mL.
A)What is the concentration of the starting solution in percent by mass (%m/m)?
B)What is the molarity of the H2SO4 solution?
C)If 25.0 mL of this solution is diluted to 500. mL, what is the resulting molarity?
A)What is the concentration of the starting solution in percent by mass (%m/m)?
B)What is the molarity of the H2SO4 solution?
C)If 25.0 mL of this solution is diluted to 500. mL, what is the resulting molarity?

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
67
Calculate the molarity of a solution prepared by dissolving 14.7 g of Ca(NO3)2 in enough water to make 750. mL of solution.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
68
Calculate the grams of solute in 25.0 mL of a 2.50%m/v solution of fructose.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
69
What is the %m/m of a solution made from 21.0 g NaCl in 5.00 × 102 mL of solution? (Density of the solution is 1.14 g/mL)

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
70
A cup of coffee may contain as much as 300. mg of caffeine, C8H10O2. Calculate the molarity of caffeine in one cup of coffee. (4 cups = 1 L)

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
71
An intravenous electrolyte solution has a Na+ ion concentration of 154 mEq/L. If a patient in the hospital receives 1500 mL of this solution how many equivalents have they received?

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
72
An intravenous maintenance solution that is 5.0% dextrose contains 15 mEq/L HPO42-. If a patient receives 1750 mL of this solution to maintain their electrolyte solution, how many equivalents of the HPO42- are in this amount?

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
73
What is the concentration in ppm and ppb of a solution that contains 45 mg of lead in 1750 mL of solution?

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
74
How much of a 2.75 M solution of AgNO3 in mL is needed to make 1.25 L of a 0.150 M solution of this same compound?

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
75
50.0 mL of a 0.250 M sucrose solution was diluted to 4.00 × 102 mL. What is the concentration of the new solution?

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
76
Define the terms isotonic, hypotonic and hypertonic solutions in relationship to a cell.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
77
Osmosis is the passage of water molecules through a semi-permeable membrane from a solution of ________ concentration to ________ concentration.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
78
Define the term "semipermeable."

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
79
Why do your fingers wrinkle after you spend a lot of time swimming in the ocean?

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
80
If physiological saline has a concentration of 0.90% (m/v)NaCl, identify the following solutions as isotonic, hypotonic or hypertonic:
A)0.25% (m/v)NaCl
B)0.90% (m/v)NaCl
C)1.8% (m/v)NaCl
A)0.25% (m/v)NaCl
B)0.90% (m/v)NaCl
C)1.8% (m/v)NaCl

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck



