Deck 23: Carbohydrates
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/15
Play
Full screen (f)
Deck 23: Carbohydrates
1
Which one of the following describes the furanose forms of carbohydrates?
A) a six-membered ring consisting of all carbons
B) a six-membered ring consisting of five carbons and one oxygen
C) a five-membered ring consisting of all carbons
D) a five-membered ring consisting of four carbons and one oxygen
A) a six-membered ring consisting of all carbons
B) a six-membered ring consisting of five carbons and one oxygen
C) a five-membered ring consisting of all carbons
D) a five-membered ring consisting of four carbons and one oxygen
a five-membered ring consisting of four carbons and one oxygen
2
What are the configurations of carbon atoms 2 and 3, respectively, of D-threose, shown below? 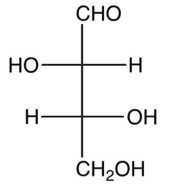
A) R,R
B) R,S
C) S,R
D) S,S

A) R,R
B) R,S
C) S,R
D) S,S
S,R
3
The major form of D-glucose in aqueous solution is
A) the open-chain form.
B) -D-glucopyranose.
C) -D-glucopyranose.
D) -D-glucofuranose.
A) the open-chain form.
B) -D-glucopyranose.
C) -D-glucopyranose.
D) -D-glucofuranose.
-D-glucopyranose.
4
The and forms of -glucopyranose can be described as
A) constitutional isomers.
B) enantiomers.
C) different conformations of the same compound.
D) anomers.
A) constitutional isomers.
B) enantiomers.
C) different conformations of the same compound.
D) anomers.

Unlock Deck
Unlock for access to all 15 flashcards in this deck.
Unlock Deck
k this deck
5
Identify the -pyranose form of -glucose.
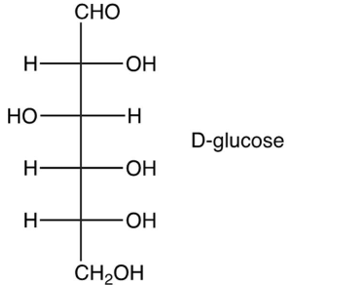
A)
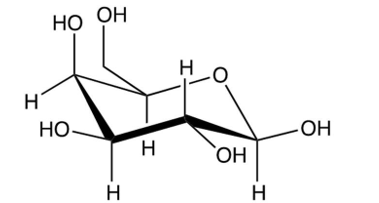
B)
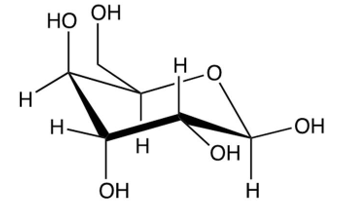
C)
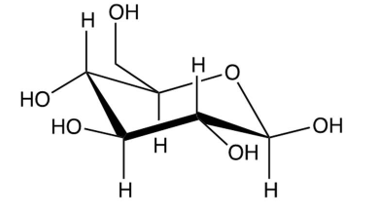
D)
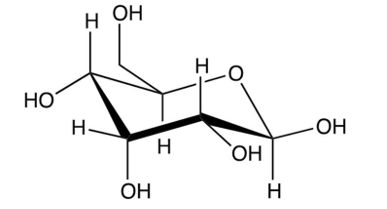

A)

B)

C)

D)


Unlock Deck
Unlock for access to all 15 flashcards in this deck.
Unlock Deck
k this deck
6
The - and -pyranose forms of -glucose interconvert by
A) "ring-flipping" between the two chair conformations.
B) ring-opening and ring-closing through the open-chain aldehyde form.
C) epimerization through the enol tautomer.
D) inversion with nucleophilic hydroxide ion.
A) "ring-flipping" between the two chair conformations.
B) ring-opening and ring-closing through the open-chain aldehyde form.
C) epimerization through the enol tautomer.
D) inversion with nucleophilic hydroxide ion.

Unlock Deck
Unlock for access to all 15 flashcards in this deck.
Unlock Deck
k this deck
7
Which of the following statements is(are) true concerning the equilibrium between the - and -pyranose forms of D-glucose?
I. The interconversion is acid-catalyzed.
II. Both anomers are equally stable.
III. Both anomers are optically active.
A) only III
B) II and III
C) I and III
D) I, II, and III
I. The interconversion is acid-catalyzed.
II. Both anomers are equally stable.
III. Both anomers are optically active.
A) only III
B) II and III
C) I and III
D) I, II, and III

Unlock Deck
Unlock for access to all 15 flashcards in this deck.
Unlock Deck
k this deck
8
Identify the anomeric carbon in the following carbohydrate.
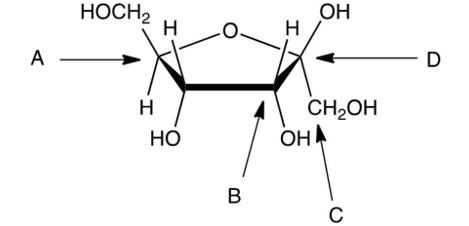
A) A
B) B
C) C
D) D

A) A
B) B
C) C
D) D

Unlock Deck
Unlock for access to all 15 flashcards in this deck.
Unlock Deck
k this deck
9
The principal difference between amylose (from starch) and cellulose is
A) amylose has exclusively -linked glucose and cellulose has exclusively -linked glucose.
B) amylose has exclusively -linked glucose and cellulose has exclusively -linked glucose.
C) amylose has -linked glucose with branches and cellulose has exclusively -linked glucose.
D) amylose has exclusively -linked glucose and cellulose has -linked glucose with branches.
A) amylose has exclusively -linked glucose and cellulose has exclusively -linked glucose.
B) amylose has exclusively -linked glucose and cellulose has exclusively -linked glucose.
C) amylose has -linked glucose with branches and cellulose has exclusively -linked glucose.
D) amylose has exclusively -linked glucose and cellulose has -linked glucose with branches.

Unlock Deck
Unlock for access to all 15 flashcards in this deck.
Unlock Deck
k this deck
10
Which of the following give an optically inactive compound when reacted with sodium borohydride, ? 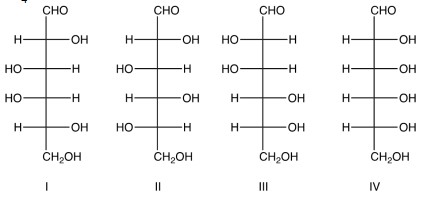
A) I and II
B) II and III
C) III and IV
D) I and IV

A) I and II
B) II and III
C) III and IV
D) I and IV

Unlock Deck
Unlock for access to all 15 flashcards in this deck.
Unlock Deck
k this deck
11
The following series of reactions was done on a monosaccharide. What information does this give about the monosaccharide?
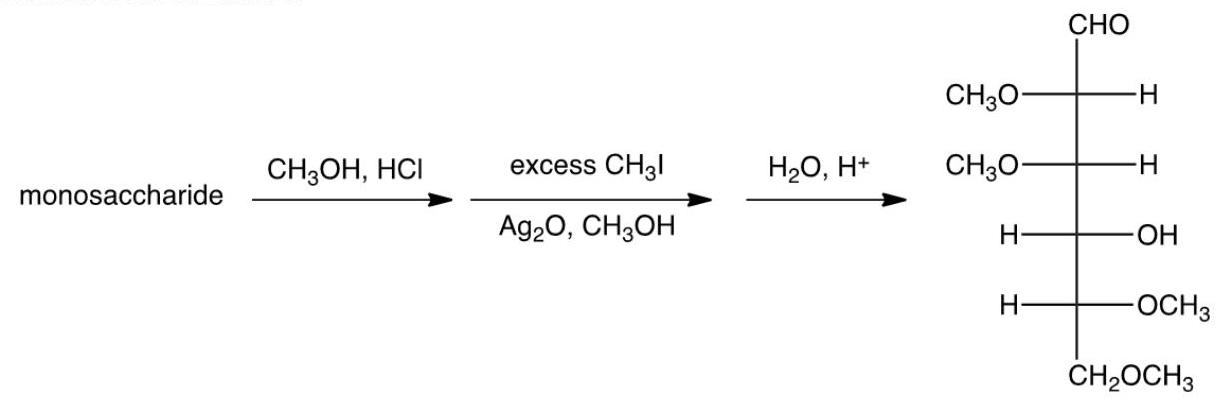
A) The monosaccharide was in the open-chain form.
B) The monosaccharide was in the furanose form.
C) The monosaccharide was in the pyranose form.
D) The monosaccharide was the anomer.

A) The monosaccharide was in the open-chain form.
B) The monosaccharide was in the furanose form.
C) The monosaccharide was in the pyranose form.
D) The monosaccharide was the anomer.

Unlock Deck
Unlock for access to all 15 flashcards in this deck.
Unlock Deck
k this deck
12
The - and -pyranose forms of -glucose can be described as
A) cyclic acetals formed between the aldehyde and the hydroxy group on carbon-5.
B) cyclic hemiacetals formed between the aldehyde and the hydroxy group on carbon-5.
C) cyclic acetals formed between the aldehyde and the hydroxy group on carbon-6.
D) cyclic hemiacetals formed between the aldehyde and the hydroxy group on carbon-6.
A) cyclic acetals formed between the aldehyde and the hydroxy group on carbon-5.
B) cyclic hemiacetals formed between the aldehyde and the hydroxy group on carbon-5.
C) cyclic acetals formed between the aldehyde and the hydroxy group on carbon-6.
D) cyclic hemiacetals formed between the aldehyde and the hydroxy group on carbon-6.

Unlock Deck
Unlock for access to all 15 flashcards in this deck.
Unlock Deck
k this deck
13
Which one of the following is L-(-)-glucose?
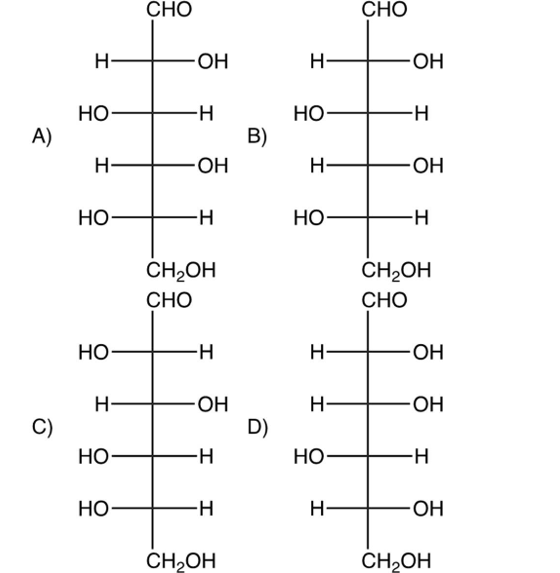
A) I
B) II
C) III
D) IV

A) I
B) II
C) III
D) IV

Unlock Deck
Unlock for access to all 15 flashcards in this deck.
Unlock Deck
k this deck
14
Which statement is not true of the and stereoisomers of any given sugar?
A) they have equal but opposite optical rotations
B) they both form cyclic hemiacetals in solution
C) they both undergo the same enzymatic digestion process
D) they can both undergo the - and -anomer equilibration process
A) they have equal but opposite optical rotations
B) they both form cyclic hemiacetals in solution
C) they both undergo the same enzymatic digestion process
D) they can both undergo the - and -anomer equilibration process

Unlock Deck
Unlock for access to all 15 flashcards in this deck.
Unlock Deck
k this deck
15
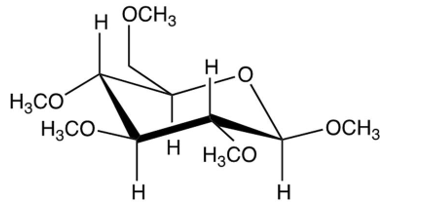
What results from the reaction shown?
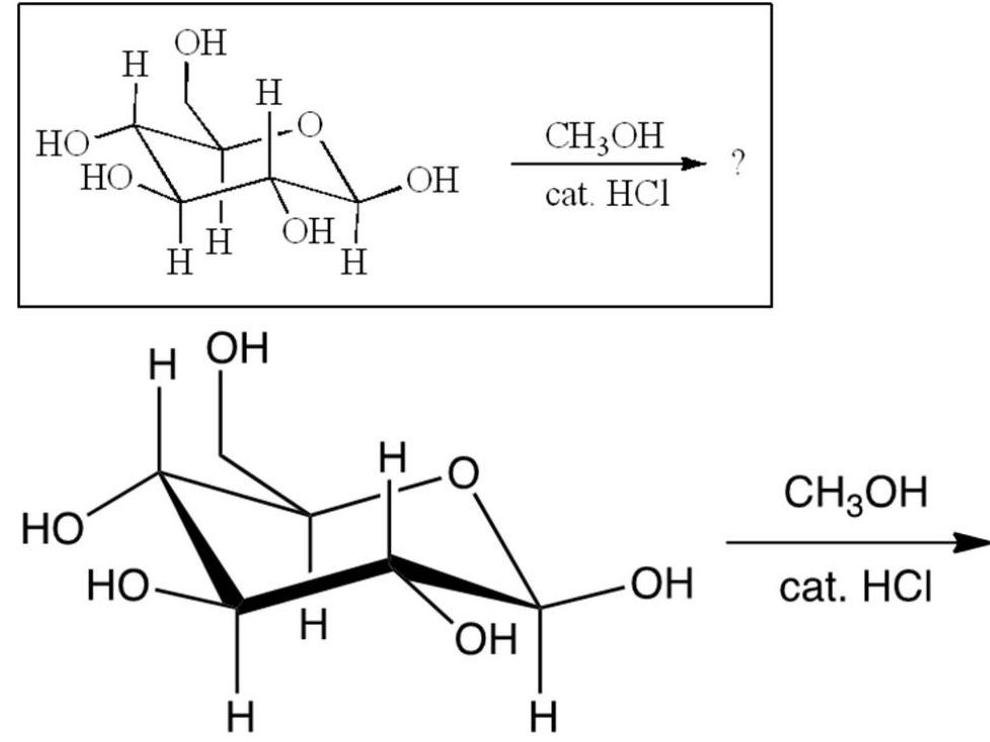
A)
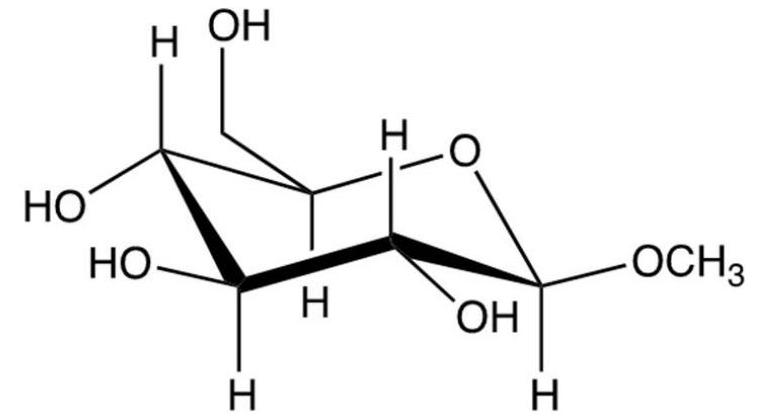
B)
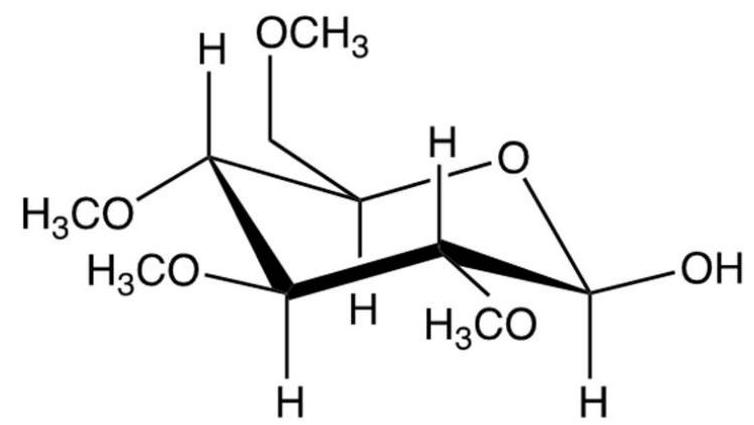
C)
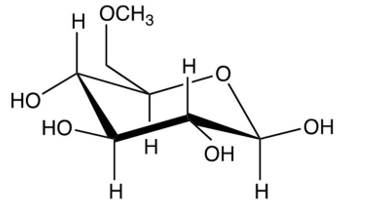
D)

A)

B)

C)

D)


Unlock Deck
Unlock for access to all 15 flashcards in this deck.
Unlock Deck
k this deck



