Deck 9: Acidûbase Equilibria
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/11
Play
Full screen (f)
Deck 9: Acidûbase Equilibria
1
Ka = 6.6´10-4 for the reaction:
HF + H2O H3O+ + F-
H3O+ + F-
What is Kb for the reaction?
F- + H2O HF + OH-
HF + OH-
A) 6.6´10-4
B) 1.6´104
C) 6.6´10-18
D) 1.5´10-11
HF + H2O
 H3O+ + F-
H3O+ + F-What is Kb for the reaction?
F- + H2O
 HF + OH-
HF + OH-A) 6.6´10-4
B) 1.6´104
C) 6.6´10-18
D) 1.5´10-11
1.6´104
2
Which of the following is the titration curve of strong acid being titrated by a strong base
A)
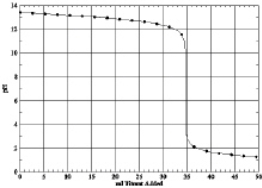
B)
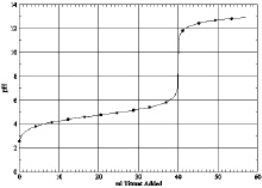
C)
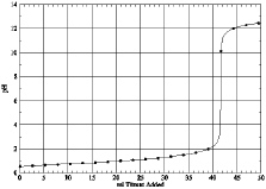
D)
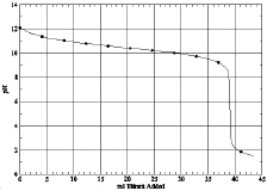
A)

B)

C)

D)


3
Which of the following is the titration curve of weak base being titrated by a strong acid
A)
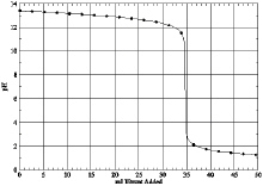
B)
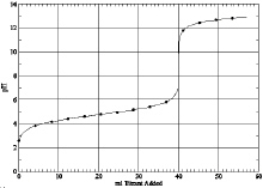
C)
D)
A)

B)

C)

D)


4
A student has 100.0 ml of 0.65 M HCl. What is the pH of this solution?
A) 1.39
B) 0.187
C) 6.50
D) 13.8
E) None of the above
A) 1.39
B) 0.187
C) 6.50
D) 13.8
E) None of the above

Unlock Deck
Unlock for access to all 11 flashcards in this deck.
Unlock Deck
k this deck
5
A student has 50.0 ml of 0.30 M NaOH. What is the pH of this solution?
A) 1.82
B) 11.00
C) 0.523
D) 2.0
E) None of the above
A) 1.82
B) 11.00
C) 0.523
D) 2.0
E) None of the above

Unlock Deck
Unlock for access to all 11 flashcards in this deck.
Unlock Deck
k this deck
6
Based on the plot below, estimate the value of Ka for the weak acid. 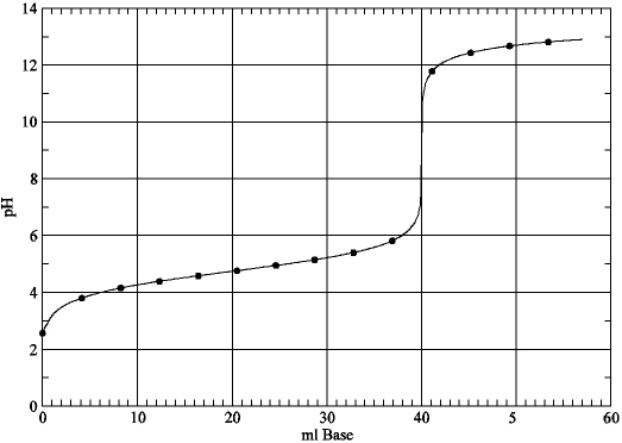
A) 4.7
B) 2.0´10-5
C) 2.6
D) 2.5´10-3
E) None of the above

A) 4.7
B) 2.0´10-5
C) 2.6
D) 2.5´10-3
E) None of the above

Unlock Deck
Unlock for access to all 11 flashcards in this deck.
Unlock Deck
k this deck
7
A student dissolves 30.00 grams of sodium acetate in exactly one liter of water. What will be the pH of the resulting solution?
A) 9.68
B) 9.24
C) 9.16
D) 4.84
A) 9.68
B) 9.24
C) 9.16
D) 4.84

Unlock Deck
Unlock for access to all 11 flashcards in this deck.
Unlock Deck
k this deck
8
pertain to a solution made by combining benzoic acid and sodium benzoate. The final volume of the solution is 1.00 L and the initial concentrations of benzoic acid and sodium benzoate are 0.50 and 0.70 respectively.
-Refer to Exhibit 15-3. When adding strong acid to this solution:
A) the benzoic acid concentration should rise and the benzoate concentration should fall
B) the benzoic acid concentration should fall and the benzoate concentration should rise
C) both benzoic acid and sodium benzoate concentrations should fall
D) the concentration of all ions should remain roughly constant
E) None of the above
-Refer to Exhibit 15-3. When adding strong acid to this solution:
A) the benzoic acid concentration should rise and the benzoate concentration should fall
B) the benzoic acid concentration should fall and the benzoate concentration should rise
C) both benzoic acid and sodium benzoate concentrations should fall
D) the concentration of all ions should remain roughly constant
E) None of the above

Unlock Deck
Unlock for access to all 11 flashcards in this deck.
Unlock Deck
k this deck
9
pertain to a solution made by combining benzoic acid and sodium benzoate. The final volume of the solution is 1.00 L and the initial concentrations of benzoic acid and sodium benzoate are 0.50 and 0.70 respectively.
-Refer to Exhibit 15-3. Calculate the pH after the addition of an additional 250 ml of 1.00 M HCl.
A) 0.339
B) 1.54
C) 2.98
D) 5.38
E) None of the above
-Refer to Exhibit 15-3. Calculate the pH after the addition of an additional 250 ml of 1.00 M HCl.
A) 0.339
B) 1.54
C) 2.98
D) 5.38
E) None of the above

Unlock Deck
Unlock for access to all 11 flashcards in this deck.
Unlock Deck
k this deck
10
pertain to a solution prepared by combining 1.0 mole of ammonia and 50.0 grams of ammonium nitrate in enough water to give a final volume of 2.00 liters.
-Refer to Exhibit 15-4. When adding strong acid to this solution:
A) the ammonia concentration should rise
B) the ammonium ion concentration should fall
C) both a and b
D) None of the above
-Refer to Exhibit 15-4. When adding strong acid to this solution:
A) the ammonia concentration should rise
B) the ammonium ion concentration should fall
C) both a and b
D) None of the above

Unlock Deck
Unlock for access to all 11 flashcards in this deck.
Unlock Deck
k this deck
11
Which of the following titration curves schematically represents a diprotic acid being titrated by a strong base?
A)
B)
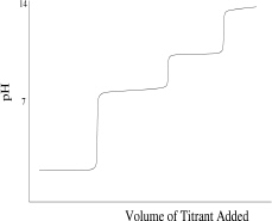
C)
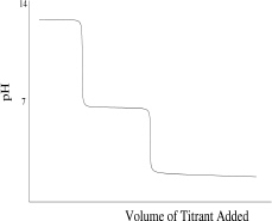
D)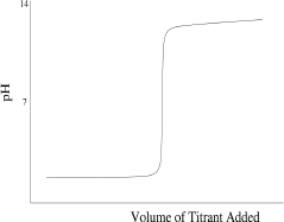
E) None of the above
A)

B)

C)

D)

E) None of the above

Unlock Deck
Unlock for access to all 11 flashcards in this deck.
Unlock Deck
k this deck



