Deck 14: Reaction Systems
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/38
Play
Full screen (f)
Deck 14: Reaction Systems
1
Uses a reactor to make one large molecule out of two small molecules.
A) isomerization
B) alkylation
C) hydrodesulfurization
D) catcracking
A) isomerization
B) alkylation
C) hydrodesulfurization
D) catcracking
alkylation
2
Which of the following is not a chemical reaction:
A) replacement
B) neutralization
C) combustion
D) distillation
A) replacement
B) neutralization
C) combustion
D) distillation
distillation
3
Uses a multi-stage reactor system to boost yields of gasoline from crude oil.
A) fluid catalytic cracking
B) hydrocracking
C) hydrodesulfurization
D) fluid coking
A) fluid catalytic cracking
B) hydrocracking
C) hydrodesulfurization
D) fluid coking
hydrocracking
4
Suspend solids in a reactor by counter-current flow of gas. Particle segregation occurs over time as heavier components fall to bottom and lighter ones move to the top.
A) alkylation
B) fluidized bed reactor
C) regeneration
D) continuous batch reactor
A) alkylation
B) fluidized bed reactor
C) regeneration
D) continuous batch reactor

Unlock Deck
Unlock for access to all 38 flashcards in this deck.
Unlock Deck
k this deck
5
A three tube reactor surrounded by a heated jacket.
A) alkylation
B) isomerization
C) tubular reactor
D) hydrocracking
A) alkylation
B) isomerization
C) tubular reactor
D) hydrocracking

Unlock Deck
Unlock for access to all 38 flashcards in this deck.
Unlock Deck
k this deck
6
Sweetens products by removing sulfur.
A) catcracking
B) fluid coking
C) reformer
D) hydrodesulfurization
A) catcracking
B) fluid coking
C) reformer
D) hydrodesulfurization

Unlock Deck
Unlock for access to all 38 flashcards in this deck.
Unlock Deck
k this deck
7
Given the chemical equation: H3PO4 + 3 NaOH ----»Na3PO4 + 3H2O
If you are told by your supervisor to increase the set point from 98 GPM to 1470 GPM of phosphoric acid H3PO4 in the above equation, how many gallons per minute of sodium hydroxide NaOH will you need to keep the process balanced?
A) 3270 gallons
B) 810 gallons
C) 2460 gallons
D) 1800 gallons
If you are told by your supervisor to increase the set point from 98 GPM to 1470 GPM of phosphoric acid H3PO4 in the above equation, how many gallons per minute of sodium hydroxide NaOH will you need to keep the process balanced?
A) 3270 gallons
B) 810 gallons
C) 2460 gallons
D) 1800 gallons

Unlock Deck
Unlock for access to all 38 flashcards in this deck.
Unlock Deck
k this deck
8
_______________________ adds raw materials incrementally to the reactor. Finished products flow out while raw materials flow in and are exposed to catalysts, pressure, liquids, or heat.
A) batch operation
B) continuous reactor operation
A) batch operation
B) continuous reactor operation

Unlock Deck
Unlock for access to all 38 flashcards in this deck.
Unlock Deck
k this deck
9
2Na2O + 2HOCl --» 2NaOCl + H2O. Is this chemical equation balanced?
A) balanced
B) not balanced
A) balanced
B) not balanced

Unlock Deck
Unlock for access to all 38 flashcards in this deck.
Unlock Deck
k this deck
10
List the five basic reactor types:

Unlock Deck
Unlock for access to all 38 flashcards in this deck.
Unlock Deck
k this deck
11
2H3PO4 --» H2O + H4P2O7. Is this chemical equation balanced?
A) balanced
B) not balanced
A) balanced
B) not balanced

Unlock Deck
Unlock for access to all 38 flashcards in this deck.
Unlock Deck
k this deck
12
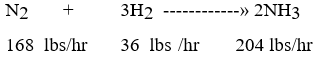 The Nitrogen Tk-100 has a capacity of 32,256 with a minimum cushion of 4,032. The hydrogen Tk-200 has a capacity of 6,912 with a minimum cushion of 864. The reactor can handle 39,168 lbs/hr before the catalyst needs to be regenerated. The Ammonia Tk-300 is protected with a safety valve and has a capacity of 34,200. TK-100 and TK-200 are refilled every 7 days. The limiting factor in this system is?
The Nitrogen Tk-100 has a capacity of 32,256 with a minimum cushion of 4,032. The hydrogen Tk-200 has a capacity of 6,912 with a minimum cushion of 864. The reactor can handle 39,168 lbs/hr before the catalyst needs to be regenerated. The Ammonia Tk-300 is protected with a safety valve and has a capacity of 34,200. TK-100 and TK-200 are refilled every 7 days. The limiting factor in this system is?A) TK-100
B) TK-200
C) The reactor
D) TK-300

Unlock Deck
Unlock for access to all 38 flashcards in this deck.
Unlock Deck
k this deck
13
The two most common forms of chemical bonding are:
A) covalent and ionic
B) covalent and numeric
C) ionic and electromagnetic
D) covalent and memonic
A) covalent and ionic
B) covalent and numeric
C) ionic and electromagnetic
D) covalent and memonic

Unlock Deck
Unlock for access to all 38 flashcards in this deck.
Unlock Deck
k this deck
14
The major difference between the two most common forms of chemical bonding are:
A) neutral vs electrically charged
B) endothermic vs. exothermic
C) reactive vs. equilibrium
D) all of the above
A) neutral vs electrically charged
B) endothermic vs. exothermic
C) reactive vs. equilibrium
D) all of the above

Unlock Deck
Unlock for access to all 38 flashcards in this deck.
Unlock Deck
k this deck
15
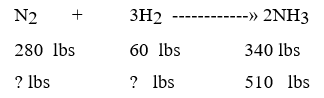 What are the new settings for nitrogen and hydrogen?
What are the new settings for nitrogen and hydrogen?A) 420 nitrogen, 90 hydrogen
B) 187 nitrogen, 40 hydrogen
C) 28 nitrogen, 6 hydrogen
D) 630 nitrogen, 135 hydrogen

Unlock Deck
Unlock for access to all 38 flashcards in this deck.
Unlock Deck
k this deck
16
How does heat and pressure effect a chemical reaction?
A) enhances
B) slows down
C) no effect
D) a & b
A) enhances
B) slows down
C) no effect
D) a & b

Unlock Deck
Unlock for access to all 38 flashcards in this deck.
Unlock Deck
k this deck
17
Alkylate is a superior _____________ product that is used in blending unleaded gasoline.
A) anti-knock
B) cleaning agent
C) high-octane
D) a & c
A) anti-knock
B) cleaning agent
C) high-octane
D) a & c

Unlock Deck
Unlock for access to all 38 flashcards in this deck.
Unlock Deck
k this deck
18
An exothermic reaction:
A) requires heat
B) requires energy
C) gives off heat
D) destroys matter
A) requires heat
B) requires energy
C) gives off heat
D) destroys matter

Unlock Deck
Unlock for access to all 38 flashcards in this deck.
Unlock Deck
k this deck
19
During _______________________, operation gas oil enters the reactor and is mixed with a superheated powdered catalyst. The term cracking is applied to the process because during vaporization the molecules literally split and are sent to a fractionation tower for further processing.
A) hydrocracking
B) fluid catalytic cracking
C) fluid coking
D) Chemical synthesis
A) hydrocracking
B) fluid catalytic cracking
C) fluid coking
D) Chemical synthesis

Unlock Deck
Unlock for access to all 38 flashcards in this deck.
Unlock Deck
k this deck
20
All of the following are active catalysts except:
A) intermediate type
B) adsorption
C) inhibitor
D) poisoned
A) intermediate type
B) adsorption
C) inhibitor
D) poisoned

Unlock Deck
Unlock for access to all 38 flashcards in this deck.
Unlock Deck
k this deck
21
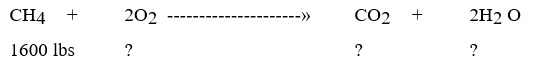 Find?
Find?A) 7400, 5400, 4600
B) 6600, 4600, 3800
C) 5400, 3400, 2600
D) 6400, 4400, 3600

Unlock Deck
Unlock for access to all 38 flashcards in this deck.
Unlock Deck
k this deck
22
Given the chemical equation: H3PO4 (98 GPM) + 3 NaOH (120 GPM) ----»Na3PO4 (164 GPM) + 3H2O (54 GPM)
If you change (3 NaOH) to 1800 GPM, how much water will be produced?
A) 108 GPM
B) 270 GPM
C) 540 GPM
D) 810 GPM
If you change (3 NaOH) to 1800 GPM, how much water will be produced?
A) 108 GPM
B) 270 GPM
C) 540 GPM
D) 810 GPM

Unlock Deck
Unlock for access to all 38 flashcards in this deck.
Unlock Deck
k this deck
23
A process that uses a reactor to scrape the bottom of the barrel and squeeze light products out of reside.
A) alkylation
B) hydrocracking
C) hydrodesulfurization
D) fluid coking
A) alkylation
B) hydrocracking
C) hydrodesulfurization
D) fluid coking

Unlock Deck
Unlock for access to all 38 flashcards in this deck.
Unlock Deck
k this deck
24
During reactor operation process technicians are responsible for all of the following except:
A) establishing correct flow or feed rates to the reactor
B) ensuring correct temperature, pressures and level
C) monitoring and controlling reaction rates (time)
D) ensure specified mixing or agitation is occurring (pumps or mechanical agitators)
E) monitor and maintain auxiliary equipment
F) calculate complex raw materials mass relationships and product economics
A) establishing correct flow or feed rates to the reactor
B) ensuring correct temperature, pressures and level
C) monitoring and controlling reaction rates (time)
D) ensure specified mixing or agitation is occurring (pumps or mechanical agitators)
E) monitor and maintain auxiliary equipment
F) calculate complex raw materials mass relationships and product economics

Unlock Deck
Unlock for access to all 38 flashcards in this deck.
Unlock Deck
k this deck
25
Reaction rates are impacted by:
A) heat and surface area
B) concentration
C) pressure & flow rates
D) all the above
A) heat and surface area
B) concentration
C) pressure & flow rates
D) all the above

Unlock Deck
Unlock for access to all 38 flashcards in this deck.
Unlock Deck
k this deck
26
_________________ take two small molecules of iso-butane and olefin (propylene, butylenes or pentylenes) and combines them into one large molecule of high-octane liquid called alkylate.
A) alkylation
B) alkylate isomerization
C) poly-olefin unit
D) iso-butane olefin unit
A) alkylation
B) alkylate isomerization
C) poly-olefin unit
D) iso-butane olefin unit

Unlock Deck
Unlock for access to all 38 flashcards in this deck.
Unlock Deck
k this deck
27
The reactor bed in a hydrocracker is ____________.
A) fixed
B) fluidized
A) fixed
B) fluidized

Unlock Deck
Unlock for access to all 38 flashcards in this deck.
Unlock Deck
k this deck
28
A 3500 pound barrel has a 37% catalyst solution. What is the weight of the catalyst?
A) 129.5 lbs
B) 1295 lbs
C) 2205 lbs
D) 1400 lbs
A) 129.5 lbs
B) 1295 lbs
C) 2205 lbs
D) 1400 lbs

Unlock Deck
Unlock for access to all 38 flashcards in this deck.
Unlock Deck
k this deck
29
What is the primary purpose of a reactor?
A) to combine chemicals and make new products
B) to make, break, or make and break chemical bonds
C) produce heat and pressure
D) a & b
A) to combine chemicals and make new products
B) to make, break, or make and break chemical bonds
C) produce heat and pressure
D) a & b

Unlock Deck
Unlock for access to all 38 flashcards in this deck.
Unlock Deck
k this deck
30
In a ________________ raw materials are carefully weighed and added to a reactor. The raw materials are mixed and allowed to react. After a pre-determined amount of time the batch is dumped and a new batch mixed.
A) continuous batch operation
B) batch operation
A) continuous batch operation
B) batch operation

Unlock Deck
Unlock for access to all 38 flashcards in this deck.
Unlock Deck
k this deck
31
The fixed medium in a reactor remains in place as raw materials pass over it.
A) fixed bed reactor
B) continuous batch
C) batch reactor
D) fluid coking
A) fixed bed reactor
B) continuous batch
C) batch reactor
D) fluid coking

Unlock Deck
Unlock for access to all 38 flashcards in this deck.
Unlock Deck
k this deck
32
A good rule of thumb for chemical reactions is that the speed with which two chemicals react:
A) doubles for each 5ºF increase in temperature
B) Triples for each 10ºC increase in temperature
C) doubles for each 10ºF increase in temperature
D) doubles for each 10ºC increase in temperature
A) doubles for each 5ºF increase in temperature
B) Triples for each 10ºC increase in temperature
C) doubles for each 10ºF increase in temperature
D) doubles for each 10ºC increase in temperature

Unlock Deck
Unlock for access to all 38 flashcards in this deck.
Unlock Deck
k this deck
33
If 10% of the chemicals in a reaction form products in one hour at 50ºC, then ________% will form products at 60ºC in one hour.
A) 5%
B) 10%
C) 20%
D) 50%
A) 5%
B) 10%
C) 20%
D) 50%

Unlock Deck
Unlock for access to all 38 flashcards in this deck.
Unlock Deck
k this deck
34
An inhibitor is a substance that:
A) reacts with catalyst making it unstable
B) limits or prevents the reaction of two or more chemicals
C) may hinder the reaction by reacting with some raw materials before reaction can take place.
D) all the above
A) reacts with catalyst making it unstable
B) limits or prevents the reaction of two or more chemicals
C) may hinder the reaction by reacting with some raw materials before reaction can take place.
D) all the above

Unlock Deck
Unlock for access to all 38 flashcards in this deck.
Unlock Deck
k this deck
35
In a liquid phase reaction, pressure can ______________ the reaction rate.
A) increases
B) linked to readiness of reactants/products to vaporize
C) decreases
D) all the above
A) increases
B) linked to readiness of reactants/products to vaporize
C) decreases
D) all the above

Unlock Deck
Unlock for access to all 38 flashcards in this deck.
Unlock Deck
k this deck
36
In a gaseous reaction, pressure forces molecules to ______________.
A) move closer together
B) collide more frequently
C) decreases
D) a & b
A) move closer together
B) collide more frequently
C) decreases
D) a & b

Unlock Deck
Unlock for access to all 38 flashcards in this deck.
Unlock Deck
k this deck
37
In a gaseous reaction, the higher the pressure the ________ the reaction rate.
A) slower
B) higher
C) more eratic
D) b & c
A) slower
B) higher
C) more eratic
D) b & c

Unlock Deck
Unlock for access to all 38 flashcards in this deck.
Unlock Deck
k this deck
38
Reactors are equipped with a number of safety features. Please list four.

Unlock Deck
Unlock for access to all 38 flashcards in this deck.
Unlock Deck
k this deck



