Deck 6: Ionic Reactions
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/28
Play
Full screen (f)
Deck 6: Ionic Reactions
1
Which is the best nucleophile?
A) C-
B) N-
C) O-
D) F-
A) C-
B) N-
C) O-
D) F-
C-
2
The best leaving group produces a _________ base that is a(n) _________ anion.
A) weak, stable
B) strong, stable
C) weak, unstable
D) strong, unstable
A) weak, stable
B) strong, stable
C) weak, unstable
D) strong, unstable
weak, stable
3
Which is the best leaving group?
A) Br-
B) Cl-
C) F-
D) I-
A) Br-
B) Cl-
C) F-
D) I-
I-
4
SN2 reactions are ________ and have ________ kinetics.
A) unimolecular, 2nd order
B) unimolecular, 1st order
C) bimolecular, 2nd order
D) bimolecular, 1st order
A) unimolecular, 2nd order
B) unimolecular, 1st order
C) bimolecular, 2nd order
D) bimolecular, 1st order

Unlock Deck
Unlock for access to all 28 flashcards in this deck.
Unlock Deck
k this deck
5
In an SN2 reaction, if the [nucleophile] doubles, the reaction rate ________. In an SN2 reaction, if the [substrate] doubles, the reaction rate ________.
A) doubles, doubles
B) halves, halves
C) doubles, halves
D) halves, doubles
E) doubles, will not be effected
F) will not be effected, doubles
A) doubles, doubles
B) halves, halves
C) doubles, halves
D) halves, doubles
E) doubles, will not be effected
F) will not be effected, doubles

Unlock Deck
Unlock for access to all 28 flashcards in this deck.
Unlock Deck
k this deck
6
SN2 reactions proceed with a(n) ________ of configuration.
A) inversion
B) retention
C) racemization
A) inversion
B) retention
C) racemization

Unlock Deck
Unlock for access to all 28 flashcards in this deck.
Unlock Deck
k this deck
7
SN1 reactions are ________ and have ________ kinetics.
A) unimolecular, 2nd order
B) unimolecular, 1st order
C) bimolecular, 2nd order
D) bimolecular, 1st order
A) unimolecular, 2nd order
B) unimolecular, 1st order
C) bimolecular, 2nd order
D) bimolecular, 1st order

Unlock Deck
Unlock for access to all 28 flashcards in this deck.
Unlock Deck
k this deck
8
In an SN1 reaction, if the [nucleophile] doubles, the reaction rate ________. In an SN1 reaction, if the [substrate] doubles, the reaction rate ________.
A) doubles, doubles
B) halves, halves
C) doubles, halves
D) halves, doubles
E) doubles, will not be effected
F) will not be effected, doubles
A) doubles, doubles
B) halves, halves
C) doubles, halves
D) halves, doubles
E) doubles, will not be effected
F) will not be effected, doubles

Unlock Deck
Unlock for access to all 28 flashcards in this deck.
Unlock Deck
k this deck
9
The SN2 mechanism is characterized by the presence of a(n)________. The SN1 mechanism is characterized by the formation of a(n) _________ called a(n) ________
A) intermediate, transition state, carbocation
B) transition state, intermediate, carbanion
C) intermediate, intermediate, carbanion
D) transition state, intermediate, carbocation e) transition state, transition state, carbocation
A) intermediate, transition state, carbocation
B) transition state, intermediate, carbanion
C) intermediate, intermediate, carbanion
D) transition state, intermediate, carbocation e) transition state, transition state, carbocation

Unlock Deck
Unlock for access to all 28 flashcards in this deck.
Unlock Deck
k this deck
10
Which mechanism(s) is represented by the following?
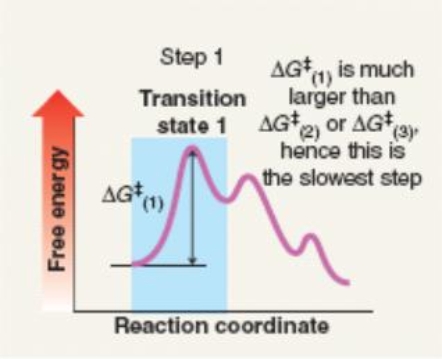
A) SN1
B) SN2
C) E1
D) E2
E) SN1 solvolysis
F) a and c
G) b and d
H) a, c and e

A) SN1
B) SN2
C) E1
D) E2
E) SN1 solvolysis
F) a and c
G) b and d
H) a, c and e

Unlock Deck
Unlock for access to all 28 flashcards in this deck.
Unlock Deck
k this deck
11
The order for stability of carbocations is __________
A) methyl > tertiary > secondary > primary
B) methyl > primary > secondary > tertiary
C) tertiary > secondary > primary > methyl
D) secondary > tertiary > primary > methyl
A) methyl > tertiary > secondary > primary
B) methyl > primary > secondary > tertiary
C) tertiary > secondary > primary > methyl
D) secondary > tertiary > primary > methyl

Unlock Deck
Unlock for access to all 28 flashcards in this deck.
Unlock Deck
k this deck
12
Carbocations have __________ geometry
A) tetrahedral
B) octahedral
C) bent
D) linear
E) trigonal pyramidal
F) trigonal planar
A) tetrahedral
B) octahedral
C) bent
D) linear
E) trigonal pyramidal
F) trigonal planar

Unlock Deck
Unlock for access to all 28 flashcards in this deck.
Unlock Deck
k this deck
13
SN1 reactions produce ________ products
A) inverted
B) identical
C) racemic
A) inverted
B) identical
C) racemic

Unlock Deck
Unlock for access to all 28 flashcards in this deck.
Unlock Deck
k this deck
14
In an SN2 reaction, reaction rates are affected by th__________ of the substrate. In an SN1 reaction, reaction rates are determined by the __________ of the carbocation.
A) stability, substrate
B) steric hindrance, stability
C) substrate, concentration
D) stability, steric hindrance
A) stability, substrate
B) steric hindrance, stability
C) substrate, concentration
D) stability, steric hindrance

Unlock Deck
Unlock for access to all 28 flashcards in this deck.
Unlock Deck
k this deck
15
SN1 reactions run best in __________ solvents and SN2 reactions run best in _________ solvents.
A) polar protic, polar protic
B) polar protic, polar aprotic
C) polar aprotic, polar aprotic
D) polar aprotic, polar protic
A) polar protic, polar protic
B) polar protic, polar aprotic
C) polar aprotic, polar aprotic
D) polar aprotic, polar protic

Unlock Deck
Unlock for access to all 28 flashcards in this deck.
Unlock Deck
k this deck
16
Which of the following is a polar protic solvent?
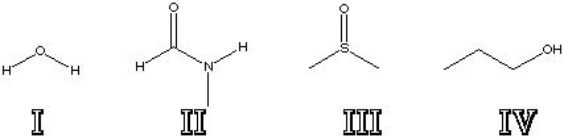
A) I
B) II
C) III
D) IV
E) I and IV
F) I, II and IV
G) all of the above

A) I
B) II
C) III
D) IV
E) I and IV
F) I, II and IV
G) all of the above

Unlock Deck
Unlock for access to all 28 flashcards in this deck.
Unlock Deck
k this deck
17
Which mechanism(s) is represented by the following?
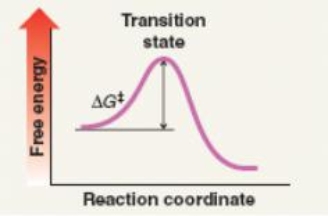
A) SN1
B) SN2
C) E1
D) E2
E) SN1 solvolysis
F) a and c
G) b and d
H) a, c and e

A) SN1
B) SN2
C) E1
D) E2
E) SN1 solvolysis
F) a and c
G) b and d
H) a, c and e

Unlock Deck
Unlock for access to all 28 flashcards in this deck.
Unlock Deck
k this deck
18
Which mechanism(s) is represented by the following?
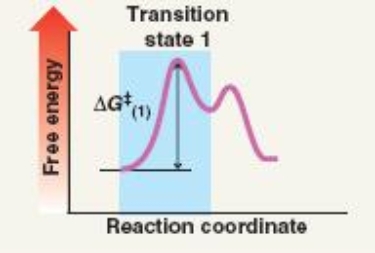
A) SN1
B) SN2
C) E1
D) E2
E) SN1 solvolysis
F) a and c
G) b and d
H) a, c and e

A) SN1
B) SN2
C) E1
D) E2
E) SN1 solvolysis
F) a and c
G) b and d
H) a, c and e

Unlock Deck
Unlock for access to all 28 flashcards in this deck.
Unlock Deck
k this deck
19
__________ increases the likelihood of elimination occurring instead of substitution.
A) Increased pressure
B) Increased temperature
C) Decreased pressure
D) Decreased temperature
A) Increased pressure
B) Increased temperature
C) Decreased pressure
D) Decreased temperature

Unlock Deck
Unlock for access to all 28 flashcards in this deck.
Unlock Deck
k this deck
20
SN2 reactions proceed fastest with ________ substrates
A) Primary
B) Secondary
C) Tertiary
A) Primary
B) Secondary
C) Tertiary

Unlock Deck
Unlock for access to all 28 flashcards in this deck.
Unlock Deck
k this deck
21
Primary substrates always give SN2 reactions with all
Bases except for ________, which will give E2 reactions due to its steric hindrance.
A) Sodium methoxide
B) Sodium ethoxide
C) Potassium hydroxide
D) Potassium methoxide
E) Potassium tert-butoxide
Bases except for ________, which will give E2 reactions due to its steric hindrance.
A) Sodium methoxide
B) Sodium ethoxide
C) Potassium hydroxide
D) Potassium methoxide
E) Potassium tert-butoxide

Unlock Deck
Unlock for access to all 28 flashcards in this deck.
Unlock Deck
k this deck
22
What is the major product of the following reaction?
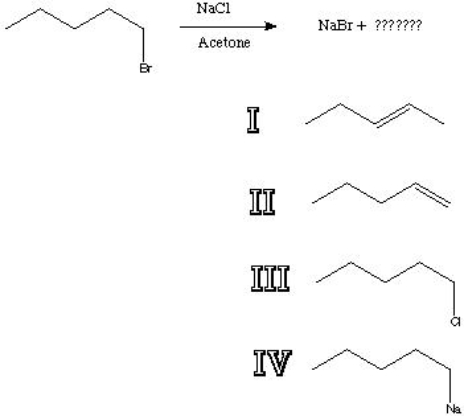
A) I
B) II
C) III
D) IV

A) I
B) II
C) III
D) IV

Unlock Deck
Unlock for access to all 28 flashcards in this deck.
Unlock Deck
k this deck
23
What is the major product of the following reaction?
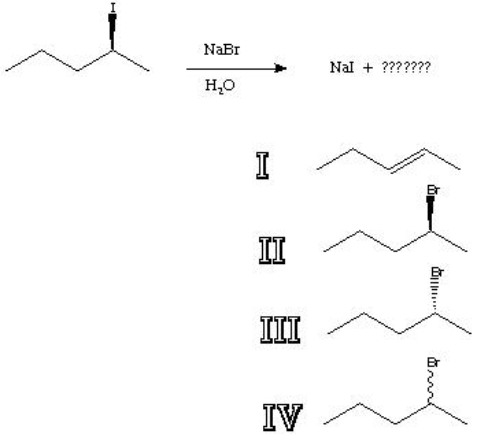
A) I
B) II
C) III
D) IV

A) I
B) II
C) III
D) IV

Unlock Deck
Unlock for access to all 28 flashcards in this deck.
Unlock Deck
k this deck
24
What is the major product of the following reaction?
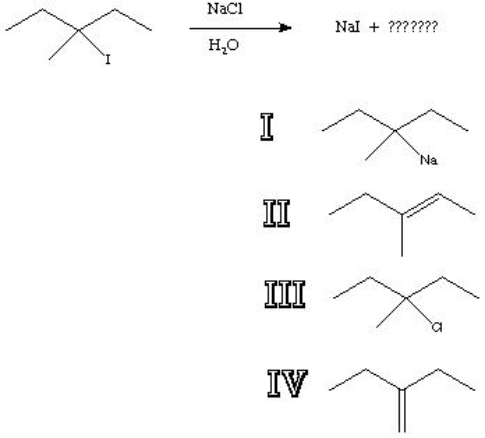
A) I
B) II
C) III
D) IV

A) I
B) II
C) III
D) IV

Unlock Deck
Unlock for access to all 28 flashcards in this deck.
Unlock Deck
k this deck
25
What is the major product of the following reaction?
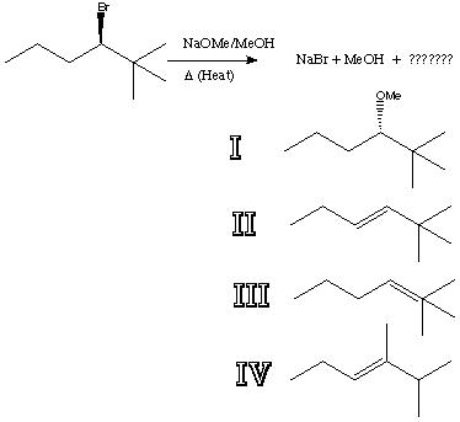
A) I
B) II
C) III
D) IV

A) I
B) II
C) III
D) IV

Unlock Deck
Unlock for access to all 28 flashcards in this deck.
Unlock Deck
k this deck
26
What is the major product of the following reaction?
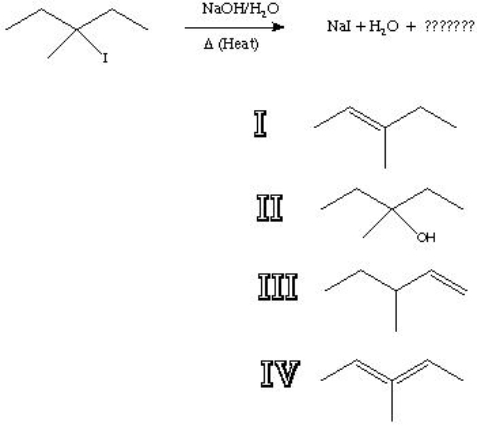
A) I
B) II
C) III
D) IV

A) I
B) II
C) III
D) IV

Unlock Deck
Unlock for access to all 28 flashcards in this deck.
Unlock Deck
k this deck
27
What is the major product of the following reaction?
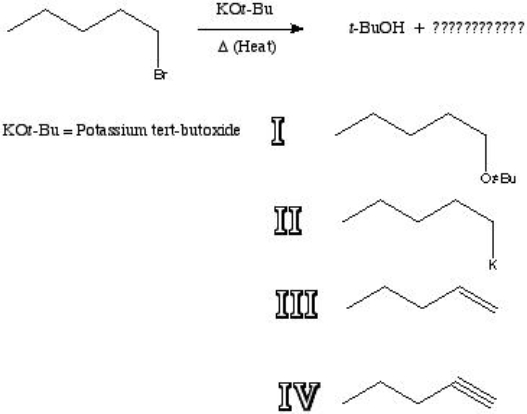
A) I
B) II
C) III
D) IV

A) I
B) II
C) III
D) IV

Unlock Deck
Unlock for access to all 28 flashcards in this deck.
Unlock Deck
k this deck
28
What is the major product of the following reaction?
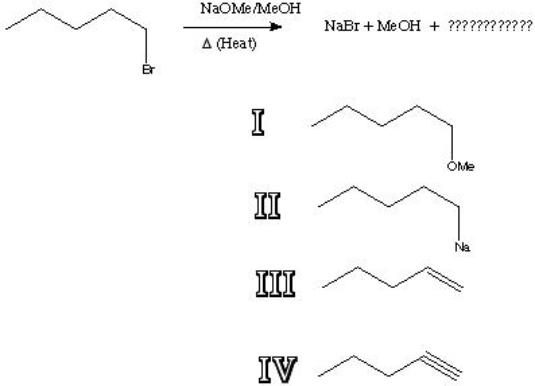
A) I
B) II
C) III
D) IV

A) I
B) II
C) III
D) IV

Unlock Deck
Unlock for access to all 28 flashcards in this deck.
Unlock Deck
k this deck



