Deck 11: Alcohols and Ethers
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/15
Play
Full screen (f)
Deck 11: Alcohols and Ethers
1
Which of the following reagent combinations give Markovnikov products?
A) Oxymercuration-Demercuration
B) Hydroboration-Oxidation
C) 1) MMPP 2) H3O+
D) A and B
E) A and C
F) B and C
G) all of the above
H) none of the above
A) Oxymercuration-Demercuration
B) Hydroboration-Oxidation
C) 1) MMPP 2) H3O+
D) A and B
E) A and C
F) B and C
G) all of the above
H) none of the above
Oxymercuration-Demercuration
2
What is the product of the following reaction? 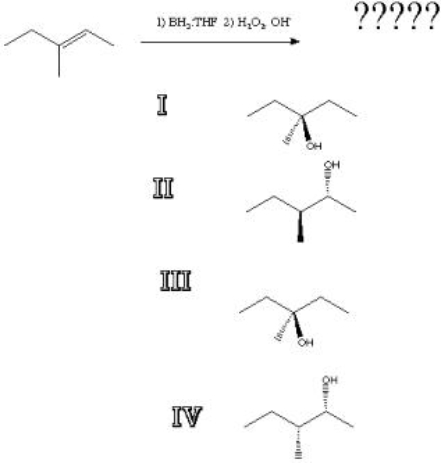
A) I
B) II
C) III
D) IV

A) I
B) II
C) III
D) IV
II
3
What is the product of the following reaction? 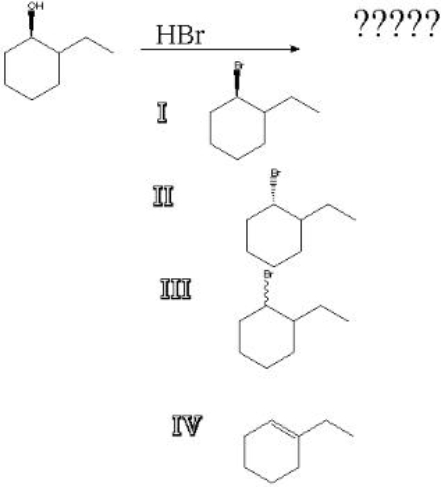
A) I
B) II
C) III
D) IV

A) I
B) II
C) III
D) IV
III
4
What is the product of the following reaction? 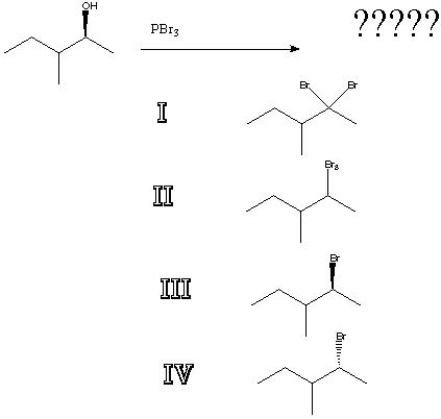
A) I
B) II
C) III
D) IV

A) I
B) II
C) III
D) IV

Unlock Deck
Unlock for access to all 15 flashcards in this deck.
Unlock Deck
k this deck
5
What is the product of the following reaction? 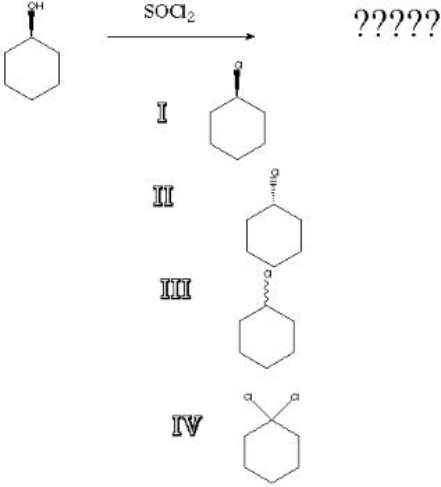
A) I
B) II
C) III
D) IV

A) I
B) II
C) III
D) IV

Unlock Deck
Unlock for access to all 15 flashcards in this deck.
Unlock Deck
k this deck
6
Which of the following give inversion of configuration when added to an alcohol?
A) NaH, Tosyl chloride
B) TBDMS-Cl, imidazole, DMF
C) PBr3
D) A and B
E) A and C
F) B and C
G) all of the above
H) none of the above
A) NaH, Tosyl chloride
B) TBDMS-Cl, imidazole, DMF
C) PBr3
D) A and B
E) A and C
F) B and C
G) all of the above
H) none of the above

Unlock Deck
Unlock for access to all 15 flashcards in this deck.
Unlock Deck
k this deck
7
What is the correct order of reagents needed for the following reaction? 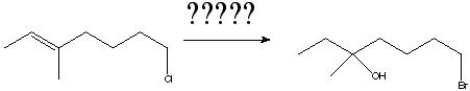
A) 1) Hg(OAc)2, THF:H2O 2) NaBH4, OH-3) TBDMS-Cl, imidazole, DMF 4) Potassium tert-butoxide 5) HBr/H2O2 6) TBAF/THF
B) 1) Potassium tert-butoxide 2) 1 eq. HBr/H2O2 3) Hg(OAc)2, THF:H2O 4) NaBH4, OH-(assume that terminalalkenes react in preference to internal alkenes)
C) 1) Hg(OAc)2, THF:H2O 2) NaBH4, OH- 3) TBDMS-Cl, imidazole, DMF
4) Potassium tert-butoxide 5) BH3:THF 6 ) H2O2, OH- 7)PBr3 8) TBAF/THF
D) all of the above

A) 1) Hg(OAc)2, THF:H2O 2) NaBH4, OH-3) TBDMS-Cl, imidazole, DMF 4) Potassium tert-butoxide 5) HBr/H2O2 6) TBAF/THF
B) 1) Potassium tert-butoxide 2) 1 eq. HBr/H2O2 3) Hg(OAc)2, THF:H2O 4) NaBH4, OH-(assume that terminalalkenes react in preference to internal alkenes)
C) 1) Hg(OAc)2, THF:H2O 2) NaBH4, OH- 3) TBDMS-Cl, imidazole, DMF
4) Potassium tert-butoxide 5) BH3:THF 6 ) H2O2, OH- 7)PBr3 8) TBAF/THF
D) all of the above

Unlock Deck
Unlock for access to all 15 flashcards in this deck.
Unlock Deck
k this deck
8
What is the product of the following reaction? 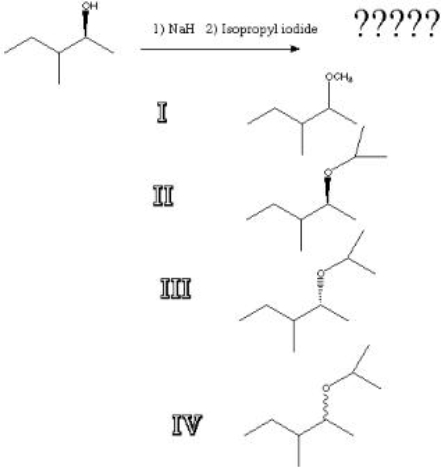
A) I
B) II
C) III
D) IV

A) I
B) II
C) III
D) IV

Unlock Deck
Unlock for access to all 15 flashcards in this deck.
Unlock Deck
k this deck
9
What is the correct order of reagents needed for the following reaction? 
A) 1) Hg(OAc)2, THF:H2O 2) NaBH4, OH-
B) 1) BH3:THF 2) H2O2, OH-
C) 1) Hg(O2CCF3)2, THF:CH3CH2OH 2) NaBH4, OH-
D) 1) MMPP 2) H+ 3) NaH 4) Ethyl iodide

A) 1) Hg(OAc)2, THF:H2O 2) NaBH4, OH-
B) 1) BH3:THF 2) H2O2, OH-
C) 1) Hg(O2CCF3)2, THF:CH3CH2OH 2) NaBH4, OH-
D) 1) MMPP 2) H+ 3) NaH 4) Ethyl iodide

Unlock Deck
Unlock for access to all 15 flashcards in this deck.
Unlock Deck
k this deck
10
What is the product of the following reaction? 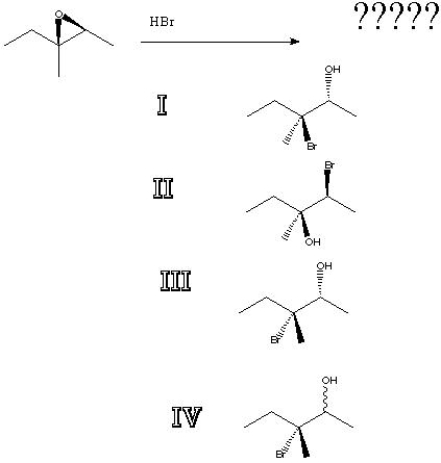
A) I
B) II
C) III
D) IV

A) I
B) II
C) III
D) IV

Unlock Deck
Unlock for access to all 15 flashcards in this deck.
Unlock Deck
k this deck
11
What is the correct order of reagents needed for the following reaction? 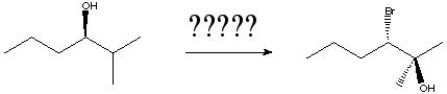
A) 1) Sodium Methoxide/Methanol 2) MMPP 3) NaBr
B) 1) NaH, Ts-Cl 2) Sodium Methoxide/Methanol 3) MMPP 4) NaBr
C) 1) NaH, Ts-Cl 2) Sodium Methoxide/Methanol 3) MMPP 4) HBr D) 1) Sodium Methoxide/Methanol 2) MMPP 3) HBr

A) 1) Sodium Methoxide/Methanol 2) MMPP 3) NaBr
B) 1) NaH, Ts-Cl 2) Sodium Methoxide/Methanol 3) MMPP 4) NaBr
C) 1) NaH, Ts-Cl 2) Sodium Methoxide/Methanol 3) MMPP 4) HBr D) 1) Sodium Methoxide/Methanol 2) MMPP 3) HBr

Unlock Deck
Unlock for access to all 15 flashcards in this deck.
Unlock Deck
k this deck
12
Which of the following reagent combinations, when added to an alkene, give regiospecific and stereospecific products?
A) Oxymercuration-Demercuration
B) Hydroboration-Oxidation
C) 1) MMPP 2) H3O+
D) A and B
E) A and C
F) B and C
G) all of the above
H) none of the above
A) Oxymercuration-Demercuration
B) Hydroboration-Oxidation
C) 1) MMPP 2) H3O+
D) A and B
E) A and C
F) B and C
G) all of the above
H) none of the above

Unlock Deck
Unlock for access to all 15 flashcards in this deck.
Unlock Deck
k this deck
13
Which of the following reagent combinations give Markovnikov products?
A) 1) MMPP 2) HBr
B) 1) MMPP 2) NaBr
C) 1) MMPP 2) H3O+
D) A and B
E) A and C
F) B and C
G) all of the above
H) none of the above
A) 1) MMPP 2) HBr
B) 1) MMPP 2) NaBr
C) 1) MMPP 2) H3O+
D) A and B
E) A and C
F) B and C
G) all of the above
H) none of the above

Unlock Deck
Unlock for access to all 15 flashcards in this deck.
Unlock Deck
k this deck
14
What is the correct order of reagents needed for the following reaction? 
A) KMnO4, OH-, H2O, cold
B) 1) OsO4, pyridine 2) NaHSO3, H2O
C) 1) MMPP 2) H3O+
D) all of the above
E) none of the above

A) KMnO4, OH-, H2O, cold
B) 1) OsO4, pyridine 2) NaHSO3, H2O
C) 1) MMPP 2) H3O+
D) all of the above
E) none of the above

Unlock Deck
Unlock for access to all 15 flashcards in this deck.
Unlock Deck
k this deck
15
Crown ethers are examples of phase-transfer catalysts (PTC). PTC takes the ionic nucleophile and brings it into the organic layer. There they pick up the leaving group anion and bring it back into the aqueous layer. The leaving group is transferred for another nucleophile and the whole process continues. PTC allows SN2 reactions to occur in the best possible solvent, ___ .
A) polar protic
B) polar aprotic
C) nonpolar protic
D) nonpolar aprotic
A) polar protic
B) polar aprotic
C) nonpolar protic
D) nonpolar aprotic

Unlock Deck
Unlock for access to all 15 flashcards in this deck.
Unlock Deck
k this deck



