Deck 20: The Organic Chemistry of Carbohydrates
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/11
Play
Full screen (f)
Deck 20: The Organic Chemistry of Carbohydrates
1
Which of the following terms best describes an aldohexose?
1) A monosaccharide
2) A disaccharide
3) An aldehyde
4) A complex carbohydrate
A) 1 and 3
B) 2 and 3
C) 2 and 4
D) 1, 3, and 4
E) 2, 3, and 4
1) A monosaccharide
2) A disaccharide
3) An aldehyde
4) A complex carbohydrate
A) 1 and 3
B) 2 and 3
C) 2 and 4
D) 1, 3, and 4
E) 2, 3, and 4
1 and 3
2
D and L notations used in describing the stereochemistry of carbohydrates ____________.
A) are directly related to the R and S notation.
B) are directly related to the (+) and (?) notation.
C) are used to describe the asymmetric carbon farthest from the carbonyl group
D) are directly related to how the molecule rotates plane-polarized light
E) are used to describe the uppermost asymmetric carbon atom
A) are directly related to the R and S notation.
B) are directly related to the (+) and (?) notation.
C) are used to describe the asymmetric carbon farthest from the carbonyl group
D) are directly related to how the molecule rotates plane-polarized light
E) are used to describe the uppermost asymmetric carbon atom
are used to describe the asymmetric carbon farthest from the carbonyl group
3
Which of the following carbohydrates is D-glucose?
A)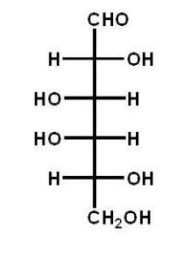
B)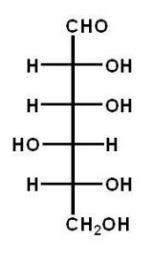
C)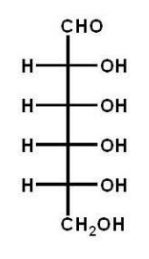
D)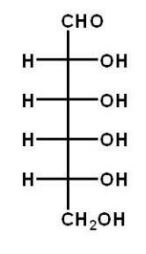
E)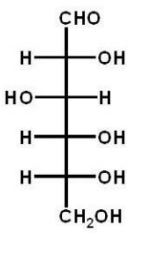
A)

B)

C)

D)

E)


4
How many stereoisomeric D-2-ketohexoses are possible?
A) 1
B) 2
C) 3
D) 4
E) 5
A) 1
B) 2
C) 3
D) 4
E) 5

Unlock Deck
Unlock for access to all 11 flashcards in this deck.
Unlock Deck
k this deck
5
Which reagent(s) will selectively react with aldoses but will not react with ketoses?
A) Br2/H2O
B) Ag+, NH3, HO?
C) HNO3
D) A and B
E) B and C
A) Br2/H2O
B) Ag+, NH3, HO?
C) HNO3
D) A and B
E) B and C

Unlock Deck
Unlock for access to all 11 flashcards in this deck.
Unlock Deck
k this deck
6
The Kiliani-Fischer synthesis (below) gives which two C-2 epimers? 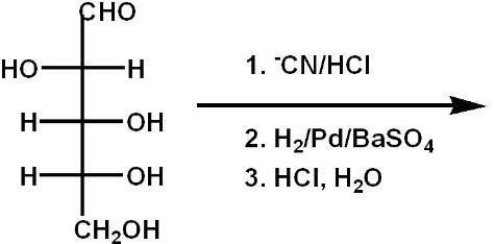
A) allose/altrose
B) gulose/idose
C) glucose/mannose
D) galactose/talose
E) sucrose/fructose

A) allose/altrose
B) gulose/idose
C) glucose/mannose
D) galactose/talose
E) sucrose/fructose

Unlock Deck
Unlock for access to all 11 flashcards in this deck.
Unlock Deck
k this deck
7
Which of the following D-hexoses can be converted to D-ribose by a Wohl degradation?
A) D-allose
B) D-glucose
C) D-galactose
D) D-talose
E) C and D
A) D-allose
B) D-glucose
C) D-galactose
D) D-talose
E) C and D

Unlock Deck
Unlock for access to all 11 flashcards in this deck.
Unlock Deck
k this deck
8
Which of the following Haworth projections is ?-D-glucose?
A)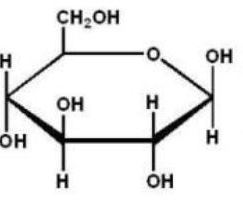
B)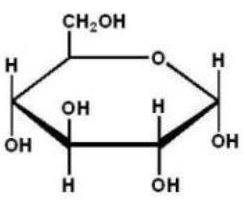
C)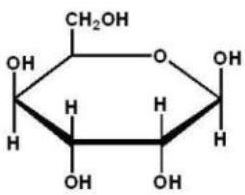
D)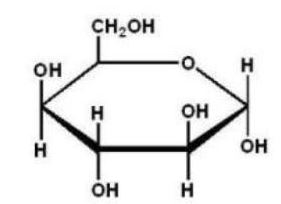
E)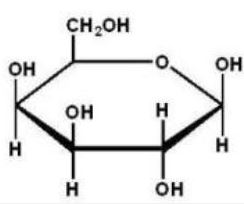
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 11 flashcards in this deck.
Unlock Deck
k this deck
9
Which D-aldohexose in its beta-D-pyranose form has only one OH group in the axial position?
A)D-allose
B) D-galactose
C)D-gulose
D)D-iodose
E)D-talose
A)D-allose
B) D-galactose
C)D-gulose
D)D-iodose
E)D-talose

Unlock Deck
Unlock for access to all 11 flashcards in this deck.
Unlock Deck
k this deck
10
Which of the following is a reducing sugar?
A)
B)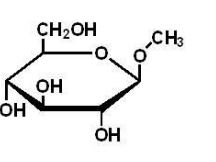
C)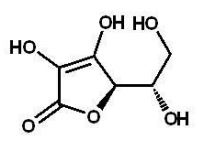
D)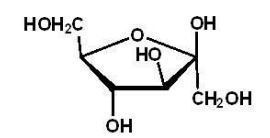
E) all of the above
A)

B)

C)

D)

E) all of the above

Unlock Deck
Unlock for access to all 11 flashcards in this deck.
Unlock Deck
k this deck
11
D-Glucose units joined by beta-1,4?-glycosidic linkages are not digestible by humans because____________.
A) humans lack the enzyme necessary to hydrolyze this linkage
B) all groups being equatorial in the glucose rings make the chains too stable to digest
C) it is a glycoside and, therefore, indigestible
D) it is unbranched and, therefore, indigestible
E) all the above
A) humans lack the enzyme necessary to hydrolyze this linkage
B) all groups being equatorial in the glucose rings make the chains too stable to digest
C) it is a glycoside and, therefore, indigestible
D) it is unbranched and, therefore, indigestible
E) all the above

Unlock Deck
Unlock for access to all 11 flashcards in this deck.
Unlock Deck
k this deck



