Deck 13: Mass Spectrometry Infrared Spectroscopy Uv Vis Spectroscopy
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/10
Play
Full screen (f)
Deck 13: Mass Spectrometry Infrared Spectroscopy Uv Vis Spectroscopy
1
The tallest peak in a mass spectrum ____________.
A) is called the molecular ion
B) is called the base peak
C) has the highest charge
D) has the highest isotopic abundance
E) is never the molecular ion
A) is called the molecular ion
B) is called the base peak
C) has the highest charge
D) has the highest isotopic abundance
E) is never the molecular ion
is called the base peak
2
Which of the following is used to provide possible molecular formulas?
A) base peak
B) rule of 13
C) nitrogen rule
D) m/z value
E) M+1 peak
A) base peak
B) rule of 13
C) nitrogen rule
D) m/z value
E) M+1 peak
rule of 13
3
The way a molecule fragments in a mass spectrometer depends upon ____________.
A) the bond strengths
B) the stability of the fragment ions
C) the energy of the electron beam that causes ionization
D) the structure of the molecule
E) all of the above
A) the bond strengths
B) the stability of the fragment ions
C) the energy of the electron beam that causes ionization
D) the structure of the molecule
E) all of the above
all of the above
4
Which of the following molecules will undergo a McLafferty rearrangement?
A)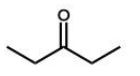
B)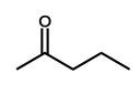
C)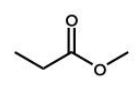
D)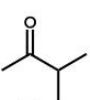
E)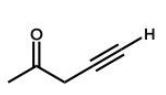
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 10 flashcards in this deck.
Unlock Deck
k this deck
5
Which C7H14O isomer will most likely give prominent peaks at m/z 71 and 43?
A) 2-heptanone
B) 3-heptanone
C) 4-heptanone
D) 2,2-dimethyl-3-pentanone
E) 4,4-dimethyl-2-pentanone
A) 2-heptanone
B) 3-heptanone
C) 4-heptanone
D) 2,2-dimethyl-3-pentanone
E) 4,4-dimethyl-2-pentanone

Unlock Deck
Unlock for access to all 10 flashcards in this deck.
Unlock Deck
k this deck
6
Many types of organic spectroscopy use electromagnetic radiation, several of which are listed below. Which type is the highest in energy?
A) radio waves
B) microwaves
C) infrared radiation
D) visible light
E) ultraviolet light
A) radio waves
B) microwaves
C) infrared radiation
D) visible light
E) ultraviolet light

Unlock Deck
Unlock for access to all 10 flashcards in this deck.
Unlock Deck
k this deck
7
The position and shape of an IR absorption band is affected by ____________.
A) electron delocalization
B) electron donation and withdrawal
C) hydrogen bonding
D) bond polarity
E) all of the above
A) electron delocalization
B) electron donation and withdrawal
C) hydrogen bonding
D) bond polarity
E) all of the above

Unlock Deck
Unlock for access to all 10 flashcards in this deck.
Unlock Deck
k this deck
8
What functional group is present in this IR spectrum?
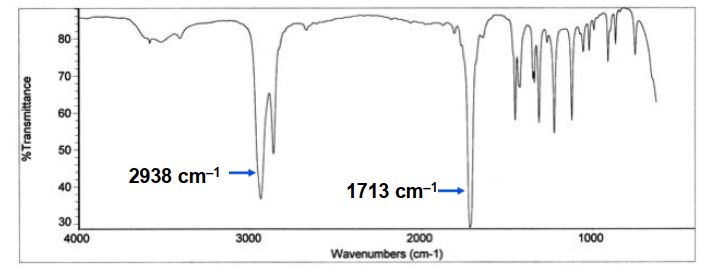
A) alcohol
B) alkyne
C) ketone
D) carboxylic acid
E) C?C

A) alcohol
B) alkyne
C) ketone
D) carboxylic acid
E) C?C

Unlock Deck
Unlock for access to all 10 flashcards in this deck.
Unlock Deck
k this deck
9
What functional group is present in this IR spectrum?
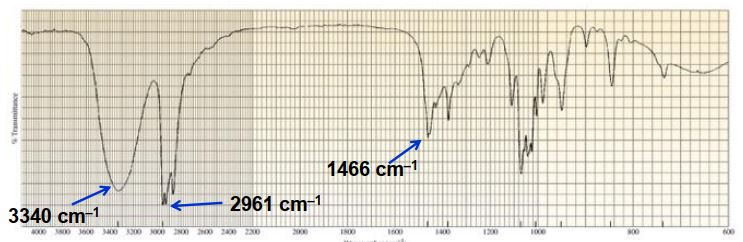
A) alcohol
B) alkyne
C) amine
D) carboxylic acid
E) Ester

A) alcohol
B) alkyne
C) amine
D) carboxylic acid
E) Ester

Unlock Deck
Unlock for access to all 10 flashcards in this deck.
Unlock Deck
k this deck
10
UV spectroscopy is based upon ____________.
A) stretching and bending of bonds
B) electronic transitions between ground and excited states
C) ionization of molecules by ultraviolet light
D) excitation of nuclei to higher spin states induced by a pulse of electromagnetic
Radiation
E) all of the above
A) stretching and bending of bonds
B) electronic transitions between ground and excited states
C) ionization of molecules by ultraviolet light
D) excitation of nuclei to higher spin states induced by a pulse of electromagnetic
Radiation
E) all of the above

Unlock Deck
Unlock for access to all 10 flashcards in this deck.
Unlock Deck
k this deck



