Deck 3: Protein Structure and Function
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/47
Play
Full screen (f)
Deck 3: Protein Structure and Function
1
What is the process component of the theory of chemical evolution?
A)Acid-base reactions resulted in the formation of large,complex organic molecules.
B)Kinetic energy was transformed into chemical energy.
C)During polymerization reactions,hydrolysis was completed with condensation.
D)The process occurred at black smokers,in the atmosphere and oceans,and in outer space.
A)Acid-base reactions resulted in the formation of large,complex organic molecules.
B)Kinetic energy was transformed into chemical energy.
C)During polymerization reactions,hydrolysis was completed with condensation.
D)The process occurred at black smokers,in the atmosphere and oceans,and in outer space.
B
2
Which of the following involves an increase in entropy?
A)hydrolysis
B)condensation
C)polymerization
D)chemical evolution
A)hydrolysis
B)condensation
C)polymerization
D)chemical evolution
A
3
How does the structure of an amino acid enable it to play its most important roles in cells?
A)It can serve a wide variety of functions in a cell,because it contains the atoms most commonly found in organisms (C,H,N,and O).
B)Because both carboxyl and amino groups are present,polymerization is exergonic.In addition,the presence of a side chain makes the molecule water soluble.
C)The presence of carboxyl and amino groups gives it the ability to form peptide bonds,and its side chain gives it unique chemical properties.
D)Because each amino acid contains a variety of functional groups,they can participate in a wide variety of chemical reactions.
A)It can serve a wide variety of functions in a cell,because it contains the atoms most commonly found in organisms (C,H,N,and O).
B)Because both carboxyl and amino groups are present,polymerization is exergonic.In addition,the presence of a side chain makes the molecule water soluble.
C)The presence of carboxyl and amino groups gives it the ability to form peptide bonds,and its side chain gives it unique chemical properties.
D)Because each amino acid contains a variety of functional groups,they can participate in a wide variety of chemical reactions.
C
4
In interstellar space,millions of ice-encrusted dust particles contain simple carbon-containing compounds.When particles like these are exposed to solar radiation,more complex organic molecules form on the surfaces of the dust.What is the significance of these findings?
A)Chemical evolution occurs only in outer space and was not possible on Earth.
B)Life began in outer space.
C)Life exists in outer space.
D)Chemical evolution can occur in outer space.
A)Chemical evolution occurs only in outer space and was not possible on Earth.
B)Life began in outer space.
C)Life exists in outer space.
D)Chemical evolution can occur in outer space.

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
5
Why are polymerization reactions endergonic?
A)They reduce entropy.
B)They release heat,making the reactant monomers move faster.
C)The condensation and hydrolysis reactions are equally spontaneous.
D)Polymers are energetically more stable and have lower potential energy than monomers do.
A)They reduce entropy.
B)They release heat,making the reactant monomers move faster.
C)The condensation and hydrolysis reactions are equally spontaneous.
D)Polymers are energetically more stable and have lower potential energy than monomers do.

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
6
At the pH found in cells (about 7.0),what happens to the amino group on an amino acid?
A)It acts as a base and gains a proton,giving it a positive charge.
B)It acts as an acid and loses a proton,giving it a negative charge.
C)It is reduced,and tends to act as an electron donor in redox reactions.
D)It remains neutral,like water,and does not have a charge.
A)It acts as a base and gains a proton,giving it a positive charge.
B)It acts as an acid and loses a proton,giving it a negative charge.
C)It is reduced,and tends to act as an electron donor in redox reactions.
D)It remains neutral,like water,and does not have a charge.

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
7
Which one of the following is not a component of each monomer used to make proteins?
A)a phosphorus atom,P
B)an amino functional group,NH₂
C)a side chain,R
D)a carboxyl group,COOH
A)a phosphorus atom,P
B)an amino functional group,NH₂
C)a side chain,R
D)a carboxyl group,COOH

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
8
At the pH found in cells (about 7.0),what happens to the carboxyl group on an amino acid?
A)It acts as a base and gains a proton,giving it a positive charge.
B)It acts as an acid and loses a proton,giving it a negative charge.
C)It is oxidized,and tends to act as an electron acceptor in redox reactions.
D)It remains neutral,like water,and does not have a charge.
A)It acts as a base and gains a proton,giving it a positive charge.
B)It acts as an acid and loses a proton,giving it a negative charge.
C)It is oxidized,and tends to act as an electron acceptor in redox reactions.
D)It remains neutral,like water,and does not have a charge.

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
9
Suppose you discovered a new amino acid.Its R-group contains only hydrogen and carbon atoms.Predict the behavior of this amino acid.
A)It is hydrophobic.
B)It is hydrophilic.
C)Relative to the amino acids found in organisms,its interactions with water will be intermediate.
D)Relative to the amino acids found in organisms,its interactions with water will be very high.
A)It is hydrophobic.
B)It is hydrophilic.
C)Relative to the amino acids found in organisms,its interactions with water will be intermediate.
D)Relative to the amino acids found in organisms,its interactions with water will be very high.

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
10
In solution,why do hydrolysis reactions occur more readily than condensation reactions?
A)Hydrolysis increases entropy and is exergonic.
B)Hydrolysis raises G,or Gibbs free energy.
C)Hydrolysis decreases entropy and is exergonic.
D)Hydrolysis increases entropy and is endergonic.
A)Hydrolysis increases entropy and is exergonic.
B)Hydrolysis raises G,or Gibbs free energy.
C)Hydrolysis decreases entropy and is exergonic.
D)Hydrolysis increases entropy and is endergonic.

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
11
In experiments that successfully simulate chemical evolution,why must at least some small,reduced molecules be present?
A)They act as proton donors in acid-base reactions.
B)They act as proton acceptors in acid-base reactions.
C)They act as electron acceptors in redox reactions.
D)They act as electron donors in redox reactions.
A)They act as proton donors in acid-base reactions.
B)They act as proton acceptors in acid-base reactions.
C)They act as electron acceptors in redox reactions.
D)They act as electron donors in redox reactions.

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
12
Which of the following best describes the first living entity?
A)It was a monomer.
B)It was large and extremely complex.
C)It could make a copy of itself.
A)It was a monomer.
B)It was large and extremely complex.
C)It could make a copy of itself.

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
13
Proteins in biological systems _____.
A)store genetic information
B)link together to form the plasma membrane
C)may be high-energy intermediates (for example,ATP)
D)may be enzymes that catalyze reactions
A)store genetic information
B)link together to form the plasma membrane
C)may be high-energy intermediates (for example,ATP)
D)may be enzymes that catalyze reactions

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
14
What is the pattern component of the theory of chemical evolution?
A)Both heat and electrical discharges are required for chemical evolution to occur.
B)Most chemical evolution occurred at black smokers.
C)The process occurred at black smokers,in the atmosphere and oceans,and in outer space.
D)Increasingly complex carbon-containing molecules formed early in Earth history.
A)Both heat and electrical discharges are required for chemical evolution to occur.
B)Most chemical evolution occurred at black smokers.
C)The process occurred at black smokers,in the atmosphere and oceans,and in outer space.
D)Increasingly complex carbon-containing molecules formed early in Earth history.

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
15
Consider the experiment that Stanley Miller did to simulate chemical evolution.Recall that a glass flask held the reduced gases NH₃,CH₄,and H₂ and that the gases were exposed to electrical sparks.What is the null hypothesis in the experiment?
A)Chemical evolution does not occur.
B)Chemical evolution requires the presence of reduced molecules.
C)Chemical evolution requires continuous heating.
D)Chemical evolution requires a source of kinetic energy.
E)Chemical evolution occurs only on Earth.
A)Chemical evolution does not occur.
B)Chemical evolution requires the presence of reduced molecules.
C)Chemical evolution requires continuous heating.
D)Chemical evolution requires a source of kinetic energy.
E)Chemical evolution occurs only on Earth.

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
16
What prediction does the chemical evolution hypothesis make?
A)Nothing will happen-meaning that no new types of molecules will appear.
B)Molecules with carbon-carbon bonds will form.
C)Proteins will be produced before any other macromolecule.
D)A self-replicating macromolecule cannot exist all need interaction with another macromolecule.
A)Nothing will happen-meaning that no new types of molecules will appear.
B)Molecules with carbon-carbon bonds will form.
C)Proteins will be produced before any other macromolecule.
D)A self-replicating macromolecule cannot exist all need interaction with another macromolecule.

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
17
Suppose that Miller repeated his chemical evolution experiment but without a source of electrical sparks.What would be the purpose?
A)to test if electrical energy is required for chemical evolution
B)to test the hypothesis that reduced molecules are required for chemical evolution
C)to test the hypothesis that both reduced molecules and electrical energy are required for chemical evolution
D)to make sure that the glassware had not been contaminated,and that any new molecules found were actually produced by chemical evolution
A)to test if electrical energy is required for chemical evolution
B)to test the hypothesis that reduced molecules are required for chemical evolution
C)to test the hypothesis that both reduced molecules and electrical energy are required for chemical evolution
D)to make sure that the glassware had not been contaminated,and that any new molecules found were actually produced by chemical evolution

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
18
You disrupt all hydrogen bonds in a protein.What level of structure will be preserved?
A)primary structure
B)secondary structure
C)tertiary structure
D)quaternary structure
A)primary structure
B)secondary structure
C)tertiary structure
D)quaternary structure

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
19
What aspects of amino acid structure vary among different amino acids?
A)the long carbon-hydrogen tails of the molecule
B)the presence of a central C atom
C)the components of the R-group
D)the glycerol molecule that forms the backbone of the amino acid
A)the long carbon-hydrogen tails of the molecule
B)the presence of a central C atom
C)the components of the R-group
D)the glycerol molecule that forms the backbone of the amino acid

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
20
The functional groups of amino acids _____.
A)are always charged
B)may be hydrophobic or hydrophilic
C)only contain C,H,and O
D)are identical in different types of amino acids
A)are always charged
B)may be hydrophobic or hydrophilic
C)only contain C,H,and O
D)are identical in different types of amino acids

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
21
Which of the following is true when comparing an uncatalyzed reaction to the same reaction with a catalyst?
A)The catalyzed reaction will be slower.
B)The catalyzed reaction will have the same ∆G.
C)The catalyzed reaction will have higher activation energy.
D)The catalyzed reaction will consume all of the catalyst.
A)The catalyzed reaction will be slower.
B)The catalyzed reaction will have the same ∆G.
C)The catalyzed reaction will have higher activation energy.
D)The catalyzed reaction will consume all of the catalyst.

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
22
What type of interaction is directly responsible for the formation of secondary structure?
A)peptide bonds between adjacent amino acids
B)peptide bonds between nonadjacent amino acids
C)hydrogen bonds between sections of the polypeptide backbone
D)hydrogen bonds between side chains of amino acids
A)peptide bonds between adjacent amino acids
B)peptide bonds between nonadjacent amino acids
C)hydrogen bonds between sections of the polypeptide backbone
D)hydrogen bonds between side chains of amino acids

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
23
How does primary protein structure affect the function of protein enzymes?
A)Substrates interact with R-groups at the enzyme's active site.
B)Substrates interact with R-groups at the enzyme's external surface.
C)Substrates interact with hydrophobic R-groups at any region of the enzyme.
D)Substrates permanently bind to R-groups at the enzyme's active site.
A)Substrates interact with R-groups at the enzyme's active site.
B)Substrates interact with R-groups at the enzyme's external surface.
C)Substrates interact with hydrophobic R-groups at any region of the enzyme.
D)Substrates permanently bind to R-groups at the enzyme's active site.

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
24
Aquaporins are proteins that control the passage of water molecules across the cell membrane.The protein forms a pore,or opening,in the membrane.You isolate what you think are two different molecules of aquaporin,and determine that one of the proteins has a larger pore diameter than the second.Which of the following do you conclude?
A)These two forms of aquaporin will have identical sequences of amino acids.
B)These two forms of aquaporin will have different sequences of amino acids.
C)You will have to sequence the proteins to compare their primary structure,because it should have no effect on pore diameter.
D)These molecules both can't have aquaporin because all proteins that do the same type of job (such as catalyze a reaction)have the exact same 3-D structure.
A)These two forms of aquaporin will have identical sequences of amino acids.
B)These two forms of aquaporin will have different sequences of amino acids.
C)You will have to sequence the proteins to compare their primary structure,because it should have no effect on pore diameter.
D)These molecules both can't have aquaporin because all proteins that do the same type of job (such as catalyze a reaction)have the exact same 3-D structure.

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
25
You are studying a protein that is shaped like a doughnut.The shape is a function of which level(s)of protein structure?
A)primary only
B)secondary only
C)tertiary only
D)secondary and tertiary only
E)primary,secondary,and tertiary
A)primary only
B)secondary only
C)tertiary only
D)secondary and tertiary only
E)primary,secondary,and tertiary

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
26
Which of the following would be an example of a cofactor?
A)an enzyme active site that contains an α-helix
B)the nonprotein heme group in a hemoglobin molecule
C)the disulfide bridge that forms between cysteine residues
D)a β-pleated sheet hidden on the inside of a protein's tertiary structure
A)an enzyme active site that contains an α-helix
B)the nonprotein heme group in a hemoglobin molecule
C)the disulfide bridge that forms between cysteine residues
D)a β-pleated sheet hidden on the inside of a protein's tertiary structure

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
27
An enzyme has a total of four active sites.When you denature the molecule and study its composition,you find that each active site occurs on a different polypeptide.Which of the following hypotheses does this observation support?
A)The enzyme is subject to allosteric regulation.
B)The enzyme requires a cofactor to function normally.
C)The protein's structure is affected by temperature and pH.
D)The protein has quaternary structure.
A)The enzyme is subject to allosteric regulation.
B)The enzyme requires a cofactor to function normally.
C)The protein's structure is affected by temperature and pH.
D)The protein has quaternary structure.

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
28
In cells,the activity of enzymes is often regulated by other molecules.Why is this necessary?
A)because all enzymes require some help from another molecule to function correctly
B)because other molecules are necessary to prevent enzymes from denaturing
C)because each enzyme has multiple functions
D)because it is unlikely that all reaction products are required all of the time
A)because all enzymes require some help from another molecule to function correctly
B)because other molecules are necessary to prevent enzymes from denaturing
C)because each enzyme has multiple functions
D)because it is unlikely that all reaction products are required all of the time

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
29
Several of the molecules called vitamins act as enzyme cofactors.Vitamin deficiencies cause disease.What is the most direct explanation for this?
A)Vitamins combine with nonprotein molecules to delay the onset of disease.
B)If cofactors are missing,enzymes cannot function properly,and important reaction products will be absent from cells.
C)Normal regulation cannot occur in the absence of cofactors.As a result,all enzymes will function all of the time.
D)Cofactors inhibit enzymes found in disease-causing bacteria and viruses.When cofactors are absent,these disease-causing agents multiply.
A)Vitamins combine with nonprotein molecules to delay the onset of disease.
B)If cofactors are missing,enzymes cannot function properly,and important reaction products will be absent from cells.
C)Normal regulation cannot occur in the absence of cofactors.As a result,all enzymes will function all of the time.
D)Cofactors inhibit enzymes found in disease-causing bacteria and viruses.When cofactors are absent,these disease-causing agents multiply.

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
30
A peptide bond _____.
A)forms between the functional groups of different amino acids
B)forms between the central carbon and the amino group of a single amino acid
C)forms the primary structure of proteins
D)does not play a role in maintaining the tertiary structure of proteins
A)forms between the functional groups of different amino acids
B)forms between the central carbon and the amino group of a single amino acid
C)forms the primary structure of proteins
D)does not play a role in maintaining the tertiary structure of proteins

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
31
You've discovered an enzyme that can catalyze two different chemical reactions.Which of the following is most likely to be correct?
A)The enzyme contains both α-helices and β-pleated sheets.
B)The enzyme is subject to both competitive inhibition and allosteric regulation.
C)Two types of allosteric regulation occur: The binding of one molecule activates the enzyme,while the binding of a different molecule inhibits it.
D)Either the enzyme has two distinct active sites,or the reactants involved in the two reactions are very similar in size and shape.
A)The enzyme contains both α-helices and β-pleated sheets.
B)The enzyme is subject to both competitive inhibition and allosteric regulation.
C)Two types of allosteric regulation occur: The binding of one molecule activates the enzyme,while the binding of a different molecule inhibits it.
D)Either the enzyme has two distinct active sites,or the reactants involved in the two reactions are very similar in size and shape.

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
32
Consider the HIV enzyme called protease.The amino acid residues at the active site are highly hydrophobic.In designing a drug that would bind to the active site and jam it,researchers should use which type of molecule?
A)hydrophobic
B)polar
C)charged
D)acidic
A)hydrophobic
B)polar
C)charged
D)acidic

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
33
You have isolated a previously unstudied protein,identified its complete structure in detail,and determined that it catalyzes the breakdown of a large substrate.You notice it has two binding sites.One of these is large,apparently the bonding site for the large substrate;the other is small,possibly a binding site for a regulatory molecule.What do these findings tell you about the mechanism of this protein?
A)It is probably a structural protein that is involved in cell-to-cell adhesion.
B)It is probably an enzyme that works through allosteric regulation.
C)It is probably an enzyme that works through competitive inhibition.
D)It is probably a cell membrane transport protein-like an ion channel.
E)It is probably a structural protein found in cartilage or skeletal tissue.
A)It is probably a structural protein that is involved in cell-to-cell adhesion.
B)It is probably an enzyme that works through allosteric regulation.
C)It is probably an enzyme that works through competitive inhibition.
D)It is probably a cell membrane transport protein-like an ion channel.
E)It is probably a structural protein found in cartilage or skeletal tissue.

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
34
The lock-and-key analogy for enzymes applies to the _____.
A)specificity of enzyme primary,secondary,and tertiary structure
B)specificity of enzyme tertiary subunits joining to form a quaternary structure
C)specificity of enzymes binding to their substrate
D)specificity of enzymes interacting with water
E)specificity of enzymes interacting with ions
A)specificity of enzyme primary,secondary,and tertiary structure
B)specificity of enzyme tertiary subunits joining to form a quaternary structure
C)specificity of enzymes binding to their substrate
D)specificity of enzymes interacting with water
E)specificity of enzymes interacting with ions

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
35
You've just sequenced a new protein found in mice and observe that sulfur-containing cysteine residues occur at regular intervals.What is the significance of this finding?
A)Cysteine residues are required for the formation of α-helices and β-pleated sheets.
B)It will be important to include cysteine in the diet of the mice.
C)Cysteine residues are involved in disulfide bridges that help form tertiary structure.
D)Cysteine causes bends,or angles,to occur in the tertiary structure of proteins.
A)Cysteine residues are required for the formation of α-helices and β-pleated sheets.
B)It will be important to include cysteine in the diet of the mice.
C)Cysteine residues are involved in disulfide bridges that help form tertiary structure.
D)Cysteine causes bends,or angles,to occur in the tertiary structure of proteins.

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
36
Which of the following observations is the strongest argument in favor of the hypothesis that protein structure and function are correlated?
A)Proteins function best at certain temperatures.
B)Proteins have four distinct levels of structure and many functions.
C)Enzymes tend to be globular in shape.
D)Denatured (unfolded)proteins do not function normally.
A)Proteins function best at certain temperatures.
B)Proteins have four distinct levels of structure and many functions.
C)Enzymes tend to be globular in shape.
D)Denatured (unfolded)proteins do not function normally.

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
37
You determine the amino acid sequence of a protein and find it contains a long sequence of methionine,followed by a long sequence of proline,followed by a long sequence of valine.Using these data you predict the sequence of this protein's secondary structure will be _____.
A)beta sheets,then a region of no secondary structure,then beta sheets
B)alpha helices,then a region of no secondary structure,then alpha helices
C)beta sheets,then a region of no secondary structure,then alpha helices
D)alpha helices,then a region of no secondary structure,then beta sheets
A)beta sheets,then a region of no secondary structure,then beta sheets
B)alpha helices,then a region of no secondary structure,then alpha helices
C)beta sheets,then a region of no secondary structure,then alpha helices
D)alpha helices,then a region of no secondary structure,then beta sheets

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
38
When polymerization of a protein is complete,but the protein is still completely linear,what is the highest level of structure that has been completed?
A)primary
B)secondary
C)tertiary
D)quaternary
A)primary
B)secondary
C)tertiary
D)quaternary

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
39
A series of hydrophobic side chains will congregate together as a protein folds in an aqueous solution and be stabilized by _____.
A)disulfide bonds
B)van der Waals interaction
C)hydrogen bonds
D)quaternary structure bonds
A)disulfide bonds
B)van der Waals interaction
C)hydrogen bonds
D)quaternary structure bonds

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
40
HIV is the virus that causes AIDS.In the mid-1990s,researchers discovered an enzyme in HIV called protease.Once the enzyme's structure was known,researchers began looking for drugs that would fit into the active site and block it.If this strategy for stopping HIV infections were successful,it would be an example of what phenomenon?
A)vaccination
B)poisoning
C)allosteric regulation
D)competitive inhibition
A)vaccination
B)poisoning
C)allosteric regulation
D)competitive inhibition

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
41
Refer to the following paragraph and Figure 3.1 to answer the following questions.
![<strong>Refer to the following paragraph and Figure 3.1 to answer the following questions. Figure 3.1 Since structure correlates so well with function,biochemists are constantly looking for new ways to probe the complex structure of proteins in order to understand what they do and how they do it.One of the most powerful techniques in existence today is X-ray crystallography.The main difficulty with this technique is getting the protein to crystallize.Once crystallized,the protein is bombarded with X-rays to create a pattern that can be analyzed mathematically to determine the three-dimensional structure of the protein.This analysis has been performed by Krzysztof Palczewski on the protein rhodopsin,which is a light-sensitive protein found in species ranging from ancient bacteria (archaea)to humans.The structure (schematically shown above,where each letter represents an amino acid)is characterized by a single polypeptide chain with several α-helical segments that loop back and forth across the cell membrane.Another notable feature is the disulfide bond (-S-S-)that can be seen at the bottom of the third transmembrane segment.[Figure adapted from K.Palczewski et al. ,Science 289 (2000): 739.] How many times does the protein in Figure 3.1 cross the cell membrane?</strong> A)1 B)3 C)4 D)7](https://d2lvgg3v3hfg70.cloudfront.net/TB3734/11ea48b8_22eb_9c66_b057_b92d39768e23_TB3734_00_TB3734_00_TB3734_00_TB3734_00_TB3734_00_TB3734_00.jpg)
Figure 3.1
Since structure correlates so well with function,biochemists are constantly looking for new ways to probe the complex structure of proteins in order to understand what they do and how they do it.One of the most powerful techniques in existence today is X-ray crystallography.The main difficulty with this technique is getting the protein to crystallize.Once crystallized,the protein is bombarded with X-rays to create a pattern that can be analyzed mathematically to determine the three-dimensional structure of the protein.This analysis has been performed by Krzysztof Palczewski on the protein rhodopsin,which is a light-sensitive protein found in species ranging from ancient bacteria (archaea)to humans.The structure (schematically shown above,where each letter represents an amino acid)is characterized by a single polypeptide chain with several α-helical segments that loop back and forth across the cell membrane.Another notable feature is the disulfide bond (-S-S-)that can be seen at the bottom of the third transmembrane segment.[Figure adapted from K.Palczewski et al. ,Science 289 (2000): 739.]
How many times does the protein in Figure 3.1 cross the cell membrane?
A)1
B)3
C)4
D)7
![<strong>Refer to the following paragraph and Figure 3.1 to answer the following questions. Figure 3.1 Since structure correlates so well with function,biochemists are constantly looking for new ways to probe the complex structure of proteins in order to understand what they do and how they do it.One of the most powerful techniques in existence today is X-ray crystallography.The main difficulty with this technique is getting the protein to crystallize.Once crystallized,the protein is bombarded with X-rays to create a pattern that can be analyzed mathematically to determine the three-dimensional structure of the protein.This analysis has been performed by Krzysztof Palczewski on the protein rhodopsin,which is a light-sensitive protein found in species ranging from ancient bacteria (archaea)to humans.The structure (schematically shown above,where each letter represents an amino acid)is characterized by a single polypeptide chain with several α-helical segments that loop back and forth across the cell membrane.Another notable feature is the disulfide bond (-S-S-)that can be seen at the bottom of the third transmembrane segment.[Figure adapted from K.Palczewski et al. ,Science 289 (2000): 739.] How many times does the protein in Figure 3.1 cross the cell membrane?</strong> A)1 B)3 C)4 D)7](https://d2lvgg3v3hfg70.cloudfront.net/TB3734/11ea48b8_22eb_9c66_b057_b92d39768e23_TB3734_00_TB3734_00_TB3734_00_TB3734_00_TB3734_00_TB3734_00.jpg)
Figure 3.1
Since structure correlates so well with function,biochemists are constantly looking for new ways to probe the complex structure of proteins in order to understand what they do and how they do it.One of the most powerful techniques in existence today is X-ray crystallography.The main difficulty with this technique is getting the protein to crystallize.Once crystallized,the protein is bombarded with X-rays to create a pattern that can be analyzed mathematically to determine the three-dimensional structure of the protein.This analysis has been performed by Krzysztof Palczewski on the protein rhodopsin,which is a light-sensitive protein found in species ranging from ancient bacteria (archaea)to humans.The structure (schematically shown above,where each letter represents an amino acid)is characterized by a single polypeptide chain with several α-helical segments that loop back and forth across the cell membrane.Another notable feature is the disulfide bond (-S-S-)that can be seen at the bottom of the third transmembrane segment.[Figure adapted from K.Palczewski et al. ,Science 289 (2000): 739.]
How many times does the protein in Figure 3.1 cross the cell membrane?
A)1
B)3
C)4
D)7

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
42
Refer to the following paragraph and Figure 3.1 to answer the following questions.
![<strong>Refer to the following paragraph and Figure 3.1 to answer the following questions. Figure 3.1 Since structure correlates so well with function,biochemists are constantly looking for new ways to probe the complex structure of proteins in order to understand what they do and how they do it.One of the most powerful techniques in existence today is X-ray crystallography.The main difficulty with this technique is getting the protein to crystallize.Once crystallized,the protein is bombarded with X-rays to create a pattern that can be analyzed mathematically to determine the three-dimensional structure of the protein.This analysis has been performed by Krzysztof Palczewski on the protein rhodopsin,which is a light-sensitive protein found in species ranging from ancient bacteria (archaea)to humans.The structure (schematically shown above,where each letter represents an amino acid)is characterized by a single polypeptide chain with several α-helical segments that loop back and forth across the cell membrane.Another notable feature is the disulfide bond (-S-S-)that can be seen at the bottom of the third transmembrane segment.[Figure adapted from K.Palczewski et al. ,Science 289 (2000): 739.] If you were reading off the sequence of amino acids in Figure 3.1 to a biologist friend,what should the first three letters be?</strong> A)M-N-G B)A-P-A C)It doesn't matter,since the protein has no polarity or directionality.](https://d2lvgg3v3hfg70.cloudfront.net/TB3734/11ea48b8_22eb_9c66_b057_b92d39768e23_TB3734_00_TB3734_00_TB3734_00_TB3734_00_TB3734_00_TB3734_00.jpg)
Figure 3.1
Since structure correlates so well with function,biochemists are constantly looking for new ways to probe the complex structure of proteins in order to understand what they do and how they do it.One of the most powerful techniques in existence today is X-ray crystallography.The main difficulty with this technique is getting the protein to crystallize.Once crystallized,the protein is bombarded with X-rays to create a pattern that can be analyzed mathematically to determine the three-dimensional structure of the protein.This analysis has been performed by Krzysztof Palczewski on the protein rhodopsin,which is a light-sensitive protein found in species ranging from ancient bacteria (archaea)to humans.The structure (schematically shown above,where each letter represents an amino acid)is characterized by a single polypeptide chain with several α-helical segments that loop back and forth across the cell membrane.Another notable feature is the disulfide bond (-S-S-)that can be seen at the bottom of the third transmembrane segment.[Figure adapted from K.Palczewski et al. ,Science 289 (2000): 739.]
If you were reading off the sequence of amino acids in Figure 3.1 to a biologist friend,what should the first three letters be?
A)M-N-G
B)A-P-A
C)It doesn't matter,since the protein has no polarity or directionality.
![<strong>Refer to the following paragraph and Figure 3.1 to answer the following questions. Figure 3.1 Since structure correlates so well with function,biochemists are constantly looking for new ways to probe the complex structure of proteins in order to understand what they do and how they do it.One of the most powerful techniques in existence today is X-ray crystallography.The main difficulty with this technique is getting the protein to crystallize.Once crystallized,the protein is bombarded with X-rays to create a pattern that can be analyzed mathematically to determine the three-dimensional structure of the protein.This analysis has been performed by Krzysztof Palczewski on the protein rhodopsin,which is a light-sensitive protein found in species ranging from ancient bacteria (archaea)to humans.The structure (schematically shown above,where each letter represents an amino acid)is characterized by a single polypeptide chain with several α-helical segments that loop back and forth across the cell membrane.Another notable feature is the disulfide bond (-S-S-)that can be seen at the bottom of the third transmembrane segment.[Figure adapted from K.Palczewski et al. ,Science 289 (2000): 739.] If you were reading off the sequence of amino acids in Figure 3.1 to a biologist friend,what should the first three letters be?</strong> A)M-N-G B)A-P-A C)It doesn't matter,since the protein has no polarity or directionality.](https://d2lvgg3v3hfg70.cloudfront.net/TB3734/11ea48b8_22eb_9c66_b057_b92d39768e23_TB3734_00_TB3734_00_TB3734_00_TB3734_00_TB3734_00_TB3734_00.jpg)
Figure 3.1
Since structure correlates so well with function,biochemists are constantly looking for new ways to probe the complex structure of proteins in order to understand what they do and how they do it.One of the most powerful techniques in existence today is X-ray crystallography.The main difficulty with this technique is getting the protein to crystallize.Once crystallized,the protein is bombarded with X-rays to create a pattern that can be analyzed mathematically to determine the three-dimensional structure of the protein.This analysis has been performed by Krzysztof Palczewski on the protein rhodopsin,which is a light-sensitive protein found in species ranging from ancient bacteria (archaea)to humans.The structure (schematically shown above,where each letter represents an amino acid)is characterized by a single polypeptide chain with several α-helical segments that loop back and forth across the cell membrane.Another notable feature is the disulfide bond (-S-S-)that can be seen at the bottom of the third transmembrane segment.[Figure adapted from K.Palczewski et al. ,Science 289 (2000): 739.]
If you were reading off the sequence of amino acids in Figure 3.1 to a biologist friend,what should the first three letters be?
A)M-N-G
B)A-P-A
C)It doesn't matter,since the protein has no polarity or directionality.

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
43
Refer to the following paragraph and Figure 3.1 to answer the following questions.
![<strong>Refer to the following paragraph and Figure 3.1 to answer the following questions. Figure 3.1 Since structure correlates so well with function,biochemists are constantly looking for new ways to probe the complex structure of proteins in order to understand what they do and how they do it.One of the most powerful techniques in existence today is X-ray crystallography.The main difficulty with this technique is getting the protein to crystallize.Once crystallized,the protein is bombarded with X-rays to create a pattern that can be analyzed mathematically to determine the three-dimensional structure of the protein.This analysis has been performed by Krzysztof Palczewski on the protein rhodopsin,which is a light-sensitive protein found in species ranging from ancient bacteria (archaea)to humans.The structure (schematically shown above,where each letter represents an amino acid)is characterized by a single polypeptide chain with several α-helical segments that loop back and forth across the cell membrane.Another notable feature is the disulfide bond (-S-S-)that can be seen at the bottom of the third transmembrane segment.[Figure adapted from K.Palczewski et al. ,Science 289 (2000): 739.] Refer to Figure 3.1.Which level of structure is being maintained by the disulfide bond?</strong> A)primary B)secondary C)tertiary D)quaternary](https://d2lvgg3v3hfg70.cloudfront.net/TB3734/11ea48b8_22eb_9c66_b057_b92d39768e23_TB3734_00_TB3734_00_TB3734_00_TB3734_00_TB3734_00_TB3734_00.jpg)
Figure 3.1
Since structure correlates so well with function,biochemists are constantly looking for new ways to probe the complex structure of proteins in order to understand what they do and how they do it.One of the most powerful techniques in existence today is X-ray crystallography.The main difficulty with this technique is getting the protein to crystallize.Once crystallized,the protein is bombarded with X-rays to create a pattern that can be analyzed mathematically to determine the three-dimensional structure of the protein.This analysis has been performed by Krzysztof Palczewski on the protein rhodopsin,which is a light-sensitive protein found in species ranging from ancient bacteria (archaea)to humans.The structure (schematically shown above,where each letter represents an amino acid)is characterized by a single polypeptide chain with several α-helical segments that loop back and forth across the cell membrane.Another notable feature is the disulfide bond (-S-S-)that can be seen at the bottom of the third transmembrane segment.[Figure adapted from K.Palczewski et al. ,Science 289 (2000): 739.]
Refer to Figure 3.1.Which level of structure is being maintained by the disulfide bond?
A)primary
B)secondary
C)tertiary
D)quaternary
![<strong>Refer to the following paragraph and Figure 3.1 to answer the following questions. Figure 3.1 Since structure correlates so well with function,biochemists are constantly looking for new ways to probe the complex structure of proteins in order to understand what they do and how they do it.One of the most powerful techniques in existence today is X-ray crystallography.The main difficulty with this technique is getting the protein to crystallize.Once crystallized,the protein is bombarded with X-rays to create a pattern that can be analyzed mathematically to determine the three-dimensional structure of the protein.This analysis has been performed by Krzysztof Palczewski on the protein rhodopsin,which is a light-sensitive protein found in species ranging from ancient bacteria (archaea)to humans.The structure (schematically shown above,where each letter represents an amino acid)is characterized by a single polypeptide chain with several α-helical segments that loop back and forth across the cell membrane.Another notable feature is the disulfide bond (-S-S-)that can be seen at the bottom of the third transmembrane segment.[Figure adapted from K.Palczewski et al. ,Science 289 (2000): 739.] Refer to Figure 3.1.Which level of structure is being maintained by the disulfide bond?</strong> A)primary B)secondary C)tertiary D)quaternary](https://d2lvgg3v3hfg70.cloudfront.net/TB3734/11ea48b8_22eb_9c66_b057_b92d39768e23_TB3734_00_TB3734_00_TB3734_00_TB3734_00_TB3734_00_TB3734_00.jpg)
Figure 3.1
Since structure correlates so well with function,biochemists are constantly looking for new ways to probe the complex structure of proteins in order to understand what they do and how they do it.One of the most powerful techniques in existence today is X-ray crystallography.The main difficulty with this technique is getting the protein to crystallize.Once crystallized,the protein is bombarded with X-rays to create a pattern that can be analyzed mathematically to determine the three-dimensional structure of the protein.This analysis has been performed by Krzysztof Palczewski on the protein rhodopsin,which is a light-sensitive protein found in species ranging from ancient bacteria (archaea)to humans.The structure (schematically shown above,where each letter represents an amino acid)is characterized by a single polypeptide chain with several α-helical segments that loop back and forth across the cell membrane.Another notable feature is the disulfide bond (-S-S-)that can be seen at the bottom of the third transmembrane segment.[Figure adapted from K.Palczewski et al. ,Science 289 (2000): 739.]
Refer to Figure 3.1.Which level of structure is being maintained by the disulfide bond?
A)primary
B)secondary
C)tertiary
D)quaternary

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
44
You collect data on the effect of pH on the function of the enzyme catalase in human cells.Which of the following graphs would you expect?
A)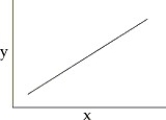
B)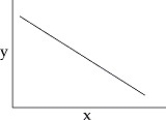
C)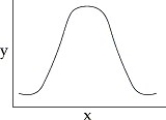
D)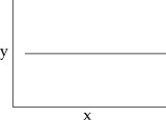
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
45
Refer to the following paragraph and Figure 3.1 to answer the following questions.
![<strong>Refer to the following paragraph and Figure 3.1 to answer the following questions. Figure 3.1 Since structure correlates so well with function,biochemists are constantly looking for new ways to probe the complex structure of proteins in order to understand what they do and how they do it.One of the most powerful techniques in existence today is X-ray crystallography.The main difficulty with this technique is getting the protein to crystallize.Once crystallized,the protein is bombarded with X-rays to create a pattern that can be analyzed mathematically to determine the three-dimensional structure of the protein.This analysis has been performed by Krzysztof Palczewski on the protein rhodopsin,which is a light-sensitive protein found in species ranging from ancient bacteria (archaea)to humans.The structure (schematically shown above,where each letter represents an amino acid)is characterized by a single polypeptide chain with several α-helical segments that loop back and forth across the cell membrane.Another notable feature is the disulfide bond (-S-S-)that can be seen at the bottom of the third transmembrane segment.[Figure adapted from K.Palczewski et al. ,Science 289 (2000): 739.] What is the location of the C-terminus of the protein in Figure 3.1?</strong> A)extracellular B)cytoplasm C)embedded within the membrane D)nucleus](https://d2lvgg3v3hfg70.cloudfront.net/TB3734/11ea48b8_22eb_9c66_b057_b92d39768e23_TB3734_00_TB3734_00_TB3734_00_TB3734_00_TB3734_00_TB3734_00.jpg)
Figure 3.1
Since structure correlates so well with function,biochemists are constantly looking for new ways to probe the complex structure of proteins in order to understand what they do and how they do it.One of the most powerful techniques in existence today is X-ray crystallography.The main difficulty with this technique is getting the protein to crystallize.Once crystallized,the protein is bombarded with X-rays to create a pattern that can be analyzed mathematically to determine the three-dimensional structure of the protein.This analysis has been performed by Krzysztof Palczewski on the protein rhodopsin,which is a light-sensitive protein found in species ranging from ancient bacteria (archaea)to humans.The structure (schematically shown above,where each letter represents an amino acid)is characterized by a single polypeptide chain with several α-helical segments that loop back and forth across the cell membrane.Another notable feature is the disulfide bond (-S-S-)that can be seen at the bottom of the third transmembrane segment.[Figure adapted from K.Palczewski et al. ,Science 289 (2000): 739.]
What is the location of the C-terminus of the protein in Figure 3.1?
A)extracellular
B)cytoplasm
C)embedded within the membrane
D)nucleus
![<strong>Refer to the following paragraph and Figure 3.1 to answer the following questions. Figure 3.1 Since structure correlates so well with function,biochemists are constantly looking for new ways to probe the complex structure of proteins in order to understand what they do and how they do it.One of the most powerful techniques in existence today is X-ray crystallography.The main difficulty with this technique is getting the protein to crystallize.Once crystallized,the protein is bombarded with X-rays to create a pattern that can be analyzed mathematically to determine the three-dimensional structure of the protein.This analysis has been performed by Krzysztof Palczewski on the protein rhodopsin,which is a light-sensitive protein found in species ranging from ancient bacteria (archaea)to humans.The structure (schematically shown above,where each letter represents an amino acid)is characterized by a single polypeptide chain with several α-helical segments that loop back and forth across the cell membrane.Another notable feature is the disulfide bond (-S-S-)that can be seen at the bottom of the third transmembrane segment.[Figure adapted from K.Palczewski et al. ,Science 289 (2000): 739.] What is the location of the C-terminus of the protein in Figure 3.1?</strong> A)extracellular B)cytoplasm C)embedded within the membrane D)nucleus](https://d2lvgg3v3hfg70.cloudfront.net/TB3734/11ea48b8_22eb_9c66_b057_b92d39768e23_TB3734_00_TB3734_00_TB3734_00_TB3734_00_TB3734_00_TB3734_00.jpg)
Figure 3.1
Since structure correlates so well with function,biochemists are constantly looking for new ways to probe the complex structure of proteins in order to understand what they do and how they do it.One of the most powerful techniques in existence today is X-ray crystallography.The main difficulty with this technique is getting the protein to crystallize.Once crystallized,the protein is bombarded with X-rays to create a pattern that can be analyzed mathematically to determine the three-dimensional structure of the protein.This analysis has been performed by Krzysztof Palczewski on the protein rhodopsin,which is a light-sensitive protein found in species ranging from ancient bacteria (archaea)to humans.The structure (schematically shown above,where each letter represents an amino acid)is characterized by a single polypeptide chain with several α-helical segments that loop back and forth across the cell membrane.Another notable feature is the disulfide bond (-S-S-)that can be seen at the bottom of the third transmembrane segment.[Figure adapted from K.Palczewski et al. ,Science 289 (2000): 739.]
What is the location of the C-terminus of the protein in Figure 3.1?
A)extracellular
B)cytoplasm
C)embedded within the membrane
D)nucleus

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
46
Refer to the following paragraph and Figure 3.1 to answer the following questions.
![<strong>Refer to the following paragraph and Figure 3.1 to answer the following questions. Figure 3.1 Since structure correlates so well with function,biochemists are constantly looking for new ways to probe the complex structure of proteins in order to understand what they do and how they do it.One of the most powerful techniques in existence today is X-ray crystallography.The main difficulty with this technique is getting the protein to crystallize.Once crystallized,the protein is bombarded with X-rays to create a pattern that can be analyzed mathematically to determine the three-dimensional structure of the protein.This analysis has been performed by Krzysztof Palczewski on the protein rhodopsin,which is a light-sensitive protein found in species ranging from ancient bacteria (archaea)to humans.The structure (schematically shown above,where each letter represents an amino acid)is characterized by a single polypeptide chain with several α-helical segments that loop back and forth across the cell membrane.Another notable feature is the disulfide bond (-S-S-)that can be seen at the bottom of the third transmembrane segment.[Figure adapted from K.Palczewski et al. ,Science 289 (2000): 739.] Which term best describes the type of membrane protein in Figure 3.1?</strong> A)peripheral B)external C)internal D)integral](https://d2lvgg3v3hfg70.cloudfront.net/TB3734/11ea48b8_22eb_9c66_b057_b92d39768e23_TB3734_00_TB3734_00_TB3734_00_TB3734_00_TB3734_00_TB3734_00.jpg)
Figure 3.1
Since structure correlates so well with function,biochemists are constantly looking for new ways to probe the complex structure of proteins in order to understand what they do and how they do it.One of the most powerful techniques in existence today is X-ray crystallography.The main difficulty with this technique is getting the protein to crystallize.Once crystallized,the protein is bombarded with X-rays to create a pattern that can be analyzed mathematically to determine the three-dimensional structure of the protein.This analysis has been performed by Krzysztof Palczewski on the protein rhodopsin,which is a light-sensitive protein found in species ranging from ancient bacteria (archaea)to humans.The structure (schematically shown above,where each letter represents an amino acid)is characterized by a single polypeptide chain with several α-helical segments that loop back and forth across the cell membrane.Another notable feature is the disulfide bond (-S-S-)that can be seen at the bottom of the third transmembrane segment.[Figure adapted from K.Palczewski et al. ,Science 289 (2000): 739.]
Which term best describes the type of membrane protein in Figure 3.1?
A)peripheral
B)external
C)internal
D)integral
![<strong>Refer to the following paragraph and Figure 3.1 to answer the following questions. Figure 3.1 Since structure correlates so well with function,biochemists are constantly looking for new ways to probe the complex structure of proteins in order to understand what they do and how they do it.One of the most powerful techniques in existence today is X-ray crystallography.The main difficulty with this technique is getting the protein to crystallize.Once crystallized,the protein is bombarded with X-rays to create a pattern that can be analyzed mathematically to determine the three-dimensional structure of the protein.This analysis has been performed by Krzysztof Palczewski on the protein rhodopsin,which is a light-sensitive protein found in species ranging from ancient bacteria (archaea)to humans.The structure (schematically shown above,where each letter represents an amino acid)is characterized by a single polypeptide chain with several α-helical segments that loop back and forth across the cell membrane.Another notable feature is the disulfide bond (-S-S-)that can be seen at the bottom of the third transmembrane segment.[Figure adapted from K.Palczewski et al. ,Science 289 (2000): 739.] Which term best describes the type of membrane protein in Figure 3.1?</strong> A)peripheral B)external C)internal D)integral](https://d2lvgg3v3hfg70.cloudfront.net/TB3734/11ea48b8_22eb_9c66_b057_b92d39768e23_TB3734_00_TB3734_00_TB3734_00_TB3734_00_TB3734_00_TB3734_00.jpg)
Figure 3.1
Since structure correlates so well with function,biochemists are constantly looking for new ways to probe the complex structure of proteins in order to understand what they do and how they do it.One of the most powerful techniques in existence today is X-ray crystallography.The main difficulty with this technique is getting the protein to crystallize.Once crystallized,the protein is bombarded with X-rays to create a pattern that can be analyzed mathematically to determine the three-dimensional structure of the protein.This analysis has been performed by Krzysztof Palczewski on the protein rhodopsin,which is a light-sensitive protein found in species ranging from ancient bacteria (archaea)to humans.The structure (schematically shown above,where each letter represents an amino acid)is characterized by a single polypeptide chain with several α-helical segments that loop back and forth across the cell membrane.Another notable feature is the disulfide bond (-S-S-)that can be seen at the bottom of the third transmembrane segment.[Figure adapted from K.Palczewski et al. ,Science 289 (2000): 739.]
Which term best describes the type of membrane protein in Figure 3.1?
A)peripheral
B)external
C)internal
D)integral

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
47
Refer to the following paragraph and Figure 3.1 to answer the following questions.
![<strong>Refer to the following paragraph and Figure 3.1 to answer the following questions. Figure 3.1 Since structure correlates so well with function,biochemists are constantly looking for new ways to probe the complex structure of proteins in order to understand what they do and how they do it.One of the most powerful techniques in existence today is X-ray crystallography.The main difficulty with this technique is getting the protein to crystallize.Once crystallized,the protein is bombarded with X-rays to create a pattern that can be analyzed mathematically to determine the three-dimensional structure of the protein.This analysis has been performed by Krzysztof Palczewski on the protein rhodopsin,which is a light-sensitive protein found in species ranging from ancient bacteria (archaea)to humans.The structure (schematically shown above,where each letter represents an amino acid)is characterized by a single polypeptide chain with several α-helical segments that loop back and forth across the cell membrane.Another notable feature is the disulfide bond (-S-S-)that can be seen at the bottom of the third transmembrane segment.[Figure adapted from K.Palczewski et al. ,Science 289 (2000): 739.] Identify the location of the disulfide bond in Figure 3.1.What is the name of the amino acids that are forming this bond?</strong> A)cytosine B)aspartic acid C)cysteine D)glycine](https://d2lvgg3v3hfg70.cloudfront.net/TB3734/11ea48b8_22eb_9c66_b057_b92d39768e23_TB3734_00_TB3734_00_TB3734_00_TB3734_00_TB3734_00_TB3734_00.jpg)
Figure 3.1
Since structure correlates so well with function,biochemists are constantly looking for new ways to probe the complex structure of proteins in order to understand what they do and how they do it.One of the most powerful techniques in existence today is X-ray crystallography.The main difficulty with this technique is getting the protein to crystallize.Once crystallized,the protein is bombarded with X-rays to create a pattern that can be analyzed mathematically to determine the three-dimensional structure of the protein.This analysis has been performed by Krzysztof Palczewski on the protein rhodopsin,which is a light-sensitive protein found in species ranging from ancient bacteria (archaea)to humans.The structure (schematically shown above,where each letter represents an amino acid)is characterized by a single polypeptide chain with several α-helical segments that loop back and forth across the cell membrane.Another notable feature is the disulfide bond (-S-S-)that can be seen at the bottom of the third transmembrane segment.[Figure adapted from K.Palczewski et al. ,Science 289 (2000): 739.]
Identify the location of the disulfide bond in Figure 3.1.What is the name of the amino acids that are forming this bond?
A)cytosine
B)aspartic acid
C)cysteine
D)glycine
![<strong>Refer to the following paragraph and Figure 3.1 to answer the following questions. Figure 3.1 Since structure correlates so well with function,biochemists are constantly looking for new ways to probe the complex structure of proteins in order to understand what they do and how they do it.One of the most powerful techniques in existence today is X-ray crystallography.The main difficulty with this technique is getting the protein to crystallize.Once crystallized,the protein is bombarded with X-rays to create a pattern that can be analyzed mathematically to determine the three-dimensional structure of the protein.This analysis has been performed by Krzysztof Palczewski on the protein rhodopsin,which is a light-sensitive protein found in species ranging from ancient bacteria (archaea)to humans.The structure (schematically shown above,where each letter represents an amino acid)is characterized by a single polypeptide chain with several α-helical segments that loop back and forth across the cell membrane.Another notable feature is the disulfide bond (-S-S-)that can be seen at the bottom of the third transmembrane segment.[Figure adapted from K.Palczewski et al. ,Science 289 (2000): 739.] Identify the location of the disulfide bond in Figure 3.1.What is the name of the amino acids that are forming this bond?</strong> A)cytosine B)aspartic acid C)cysteine D)glycine](https://d2lvgg3v3hfg70.cloudfront.net/TB3734/11ea48b8_22eb_9c66_b057_b92d39768e23_TB3734_00_TB3734_00_TB3734_00_TB3734_00_TB3734_00_TB3734_00.jpg)
Figure 3.1
Since structure correlates so well with function,biochemists are constantly looking for new ways to probe the complex structure of proteins in order to understand what they do and how they do it.One of the most powerful techniques in existence today is X-ray crystallography.The main difficulty with this technique is getting the protein to crystallize.Once crystallized,the protein is bombarded with X-rays to create a pattern that can be analyzed mathematically to determine the three-dimensional structure of the protein.This analysis has been performed by Krzysztof Palczewski on the protein rhodopsin,which is a light-sensitive protein found in species ranging from ancient bacteria (archaea)to humans.The structure (schematically shown above,where each letter represents an amino acid)is characterized by a single polypeptide chain with several α-helical segments that loop back and forth across the cell membrane.Another notable feature is the disulfide bond (-S-S-)that can be seen at the bottom of the third transmembrane segment.[Figure adapted from K.Palczewski et al. ,Science 289 (2000): 739.]
Identify the location of the disulfide bond in Figure 3.1.What is the name of the amino acids that are forming this bond?
A)cytosine
B)aspartic acid
C)cysteine
D)glycine

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck



