Deck 2: The Chemistry of Life
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/93
Play
Full screen (f)
Deck 2: The Chemistry of Life
1
Oil and water do not mix together well because
A) water is polar and oil is nonpolar.
B) only identical molecules of the same chemical can mix together easily.
C) water has hydrogen bonds and oil is polar.
D) water and oil are covalently bonded together.
A) water is polar and oil is nonpolar.
B) only identical molecules of the same chemical can mix together easily.
C) water has hydrogen bonds and oil is polar.
D) water and oil are covalently bonded together.
A
2
Covalent bonds are formed by
A) the sharing of valence electrons.
B) the transfer of valence electrons from one atom to another.
C) the sharing of electrons in the innermost shell.
D) the conversion of ionic bonds to covalent bonds.
A) the sharing of valence electrons.
B) the transfer of valence electrons from one atom to another.
C) the sharing of electrons in the innermost shell.
D) the conversion of ionic bonds to covalent bonds.
A
3
How many hydrogen atoms are in a molecule of C₈H₁₀N₄O₂?
A) 8
B) 10
C) 20
D) 24
A) 8
B) 10
C) 20
D) 24
B
4
An atom of the element with the atomic number of 11 (which can form an ion)always contains 11
A) neutrons.
B) nuclei.
C) electrons.
D) protons.
A) neutrons.
B) nuclei.
C) electrons.
D) protons.

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
5
Electrons are found
A) in the nucleus of an atom.
B) only in complex molecules.
C) in one or more shells that surround the atom's nucleus.
D) in both the nucleus and inner shell of an atom.
A) in the nucleus of an atom.
B) only in complex molecules.
C) in one or more shells that surround the atom's nucleus.
D) in both the nucleus and inner shell of an atom.

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
6
O₂,also termed atmospheric oxygen,is considered to be a molecule but not a compound; why not?
A) It contains no covalent bonds.
B) It contains a double covalent bond but not a single covalent bond.
C) To be considered a compound, there must be a minimum of three atoms; atmospheric oxygen is too small.
D) Compounds are defined as molecules constructed from two or more different elements.
A) It contains no covalent bonds.
B) It contains a double covalent bond but not a single covalent bond.
C) To be considered a compound, there must be a minimum of three atoms; atmospheric oxygen is too small.
D) Compounds are defined as molecules constructed from two or more different elements.

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
7
How many atoms are present in a single molecule of C₈H₁₀N₄O₂?
A) 4
B) 8
C) 12
D) 24
A) 4
B) 8
C) 12
D) 24

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
8
Which of the following combinations of atoms would form ionic bonds?
A) H+ and O
B) Na+ and K+
C) Na+ and Cl-
D) PO4- and I2-
A) H+ and O
B) Na+ and K+
C) Na+ and Cl-
D) PO4- and I2-

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
9
Radioisotopes are useful in scientific research and medicine because they
A) have a different number of protons than other isotopes of the same element.
B) give off high-energy radiation that can be detected by film and specialized scanning machines.
C) have the same atomic mass as other isotopes of the same element.
D) have a different number of electrons than other isotopes of the same element.
A) have a different number of protons than other isotopes of the same element.
B) give off high-energy radiation that can be detected by film and specialized scanning machines.
C) have the same atomic mass as other isotopes of the same element.
D) have a different number of electrons than other isotopes of the same element.

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
10
The smallest unit of a chemical element that displays the properties of that element is a(n)
A) amino acid.
B) molecule.
C) atom.
D) bond.
A) amino acid.
B) molecule.
C) atom.
D) bond.

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
11
Ionic bonds
A) result from the sharing of electrons between atoms.
B) form only between polar molecules.
C) form between atoms that develop opposite charges.
D) result from the natural repulsion that develops between protons.
A) result from the sharing of electrons between atoms.
B) form only between polar molecules.
C) form between atoms that develop opposite charges.
D) result from the natural repulsion that develops between protons.

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
12
How many different elements would be needed to construct a molecule of C₈H₁₀N₄O₂?
A) 4
B) 8
C) 12
D) 24
A) 4
B) 8
C) 12
D) 24

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
13
In the following illustration,a positive ion is surrounded by water molecules. 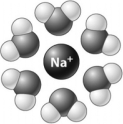 The water molecules orient as shown because the slightly ________ atoms in the water molecules are attracted to the positive charge of the ion.
The water molecules orient as shown because the slightly ________ atoms in the water molecules are attracted to the positive charge of the ion.
A) negative hydrogen
B) positive hydrogen
C) negative oxygen
D) positive oxygen
 The water molecules orient as shown because the slightly ________ atoms in the water molecules are attracted to the positive charge of the ion.
The water molecules orient as shown because the slightly ________ atoms in the water molecules are attracted to the positive charge of the ion.A) negative hydrogen
B) positive hydrogen
C) negative oxygen
D) positive oxygen

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
14
You are given an unknown substance and asked to determine whether it is polar or nonpolar.The easiest way to do this would be to
A) determine whether the compound is held together by hydrogen bonds.
B) determine the number of electrons in the compound's outer shell.
C) mix the compound with an ionic substance to see whether its bonds can withstand the pressure.
D) determine whether the compound dissolves in water.
A) determine whether the compound is held together by hydrogen bonds.
B) determine the number of electrons in the compound's outer shell.
C) mix the compound with an ionic substance to see whether its bonds can withstand the pressure.
D) determine whether the compound dissolves in water.

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
15
Examine the following illustration,a representation of a sodium ion with a charge of +1.Based on the information provided,determine the proton number for this atom. 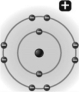
A) 8
B) 9
C) 10
D) 11

A) 8
B) 9
C) 10
D) 11

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
16
Individual water molecules orient toward each other because of the ________ bonds that form between them.
A) covalent
B) hydrogen
C) peptide
D) ionic
A) covalent
B) hydrogen
C) peptide
D) ionic

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
17
The outer electron shell of a nitrogen atom can hold up to eight electrons but contains only five.As a result,nitrogen can form ________ covalent bonds.
A) Zero
B) One
C) Three
D) Eight
A) Zero
B) One
C) Three
D) Eight

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
18
The following figure shows two hydrogen atoms.  How many covalent bonds will form between these two atoms?
How many covalent bonds will form between these two atoms?
A) One
B) Two
C) Three
D) None; these atoms will form an ionic bond.
 How many covalent bonds will form between these two atoms?
How many covalent bonds will form between these two atoms?A) One
B) Two
C) Three
D) None; these atoms will form an ionic bond.

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
19
A proton has ________ charge.
A) no
B) a neutral
C) a negative
D) a positive
A) no
B) a neutral
C) a negative
D) a positive

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
20
Chemists often represent the structure of atoms using p,n,and e to indicate the numbers of protons,neutrons,and electrons; which atom would have an atomic mass number of 30?
A) 10 p, 10 n, 10 e
B) 15 p, 15 n, 15 e
C) 15 p, 0 n, 15 e
D) 0 p, 15 n, 15 e
A) 10 p, 10 n, 10 e
B) 15 p, 15 n, 15 e
C) 15 p, 0 n, 15 e
D) 0 p, 15 n, 15 e

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
21
A molecule composed of amino acids is a
A) nucleotide.
B) lipid.
C) carbohydrate.
D) protein.
A) nucleotide.
B) lipid.
C) carbohydrate.
D) protein.

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
22
Based only on the following illustration,it could be predicted that ice floats on liquid water because 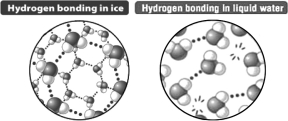
A) the crystal structure of ice is more regular than that seen in liquid water.
B) the distance between water molecules in ice is greater than in liquid water.
C) the cool temperature of ice reduces the extent of molecular motion relative to liquid water.
D) when ice forms, the hydrogen bond in the water molecule becomes nonpolar; ice behaves like oil.

A) the crystal structure of ice is more regular than that seen in liquid water.
B) the distance between water molecules in ice is greater than in liquid water.
C) the cool temperature of ice reduces the extent of molecular motion relative to liquid water.
D) when ice forms, the hydrogen bond in the water molecule becomes nonpolar; ice behaves like oil.

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
23
Chemical reactions do not change the identity of the participating atoms; all atoms present at the beginning of the reaction must be present at the end.Balance the chemical reaction by indicating the number of molecules necessary for each reactant and product: ________ Cl₂ + ________ NaBr à________ Br₂ + ________ NaCl.
A) 1; 1; 1; 1
B) 1; 2; 1; 1
C) 1; 2; 1; 2
D) 2; 2; 1; 2
A) 1; 1; 1; 1
B) 1; 2; 1; 1
C) 1; 2; 1; 2
D) 2; 2; 1; 2

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
24
The chemical reaction that represents the combustion of glucose is C₆H₁₂O₆ + O₂ à CO₂ + H₂O + E; the reactants are
A) CO₂ and H2O.
B) O₂ and H2O.
C) C6H12O6 and O₂ .
D) C6H12O6 and CO₂ .
A) CO₂ and H2O.
B) O₂ and H2O.
C) C6H12O6 and O₂ .
D) C6H12O6 and CO₂ .

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
25
A molecule with the general formula CH₂O is a(n)
A) protein.
B) carbohydrate.
C) amino acid.
D) nucleic acid.
A) protein.
B) carbohydrate.
C) amino acid.
D) nucleic acid.

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
26
Macromolecules are typically formed by repetitively adding small monomers together; which macromolecule is properly matched with the appropriate monomer?
A) polypeptide-amino acid
B) nucleic acid-amino acid
C) polysaccharide-nucleotide
D) triglyceride-cholesterol
A) polypeptide-amino acid
B) nucleic acid-amino acid
C) polysaccharide-nucleotide
D) triglyceride-cholesterol

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
27
Which of the following levels of protein structure involves more than one polypeptide chain?
A) primary
B) secondary
C) tertiary
D) quaternary
A) primary
B) secondary
C) tertiary
D) quaternary

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
28
Inside a cell,the cytoplasm is generally maintained at a pH around 7.This might be so because
A) buffers work best when the pH is close to 7.
B) humans are largely made up of water by weight.
C) most chemical reactions that occur in the cytoplasm can proceed optimally at pH 7.
D) ionic bonds cannot form at pH 7.
A) buffers work best when the pH is close to 7.
B) humans are largely made up of water by weight.
C) most chemical reactions that occur in the cytoplasm can proceed optimally at pH 7.
D) ionic bonds cannot form at pH 7.

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
29
The sugar glucose has an important role
A) in the formation of proteins.
B) in short-term energy storage.
C) in the formation of membranes.
D) as a building block of nucleotides.
A) in the formation of proteins.
B) in short-term energy storage.
C) in the formation of membranes.
D) as a building block of nucleotides.

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
30
A solution with a pH of 3 is
A) acidic.
B) nonpolar.
C) basic.
D) neutral.
A) acidic.
B) nonpolar.
C) basic.
D) neutral.

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
31
An acid is a polar substance that dissolves in water and
A) becomes nonpolar.
B) leaves behind an OH- ion.
C) accepts hydrogen ions from the solution.
D) donates hydrogen ions to the solution.
A) becomes nonpolar.
B) leaves behind an OH- ion.
C) accepts hydrogen ions from the solution.
D) donates hydrogen ions to the solution.

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
32
Of the following pH values,which indicates the most basic pH?
A) 3
B) 7
C) 8
D) 10
A) 3
B) 7
C) 8
D) 10

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
33
One of the symptoms of kidney disease is the presence of proteins in a patient's urine.To quickly test for kidney disease using a urine sample,a doctor might add a chemical that causes a color change when
A) nitrogen is present, but not oxygen.
B) nitrogen is present, but not phosphorus.
C) only oxygen and hydrogen are present.
D) only carbon and hydrogen are present.
A) nitrogen is present, but not oxygen.
B) nitrogen is present, but not phosphorus.
C) only oxygen and hydrogen are present.
D) only carbon and hydrogen are present.

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
34
Fevers in young children are a particular concern because oxygen is less effectively transported by hemoglobin at high temperature.How might this be explained?
A) The hemoglobin becomes denatured and cannot transport the oxygen.
B) The oxygen becomes denatured and cannot bind to the hemoglobin.
C) Oxygen has too much thermal energy to be bound by hemoglobin.
D) Oxygen evaporates at high temperature and is not available for binding with hemoglobin.
A) The hemoglobin becomes denatured and cannot transport the oxygen.
B) The oxygen becomes denatured and cannot bind to the hemoglobin.
C) Oxygen has too much thermal energy to be bound by hemoglobin.
D) Oxygen evaporates at high temperature and is not available for binding with hemoglobin.

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
35
Carbon dioxide is carried to the lungs in the blood.When it is dissolved in water,an acid is created.How does the body prevent your blood from becoming too acidic on the way to the lungs?
A) Buffers in the blood release OH- ions to make the blood more basic.
B) Buffers in the blood accept H+ ions to make the blood less acidic.
C) Buffers in the blood release H+ to make the blood more basic.
D) Buffers in the blood accept OH- to make the blood less acidic.
A) Buffers in the blood release OH- ions to make the blood more basic.
B) Buffers in the blood accept H+ ions to make the blood less acidic.
C) Buffers in the blood release H+ to make the blood more basic.
D) Buffers in the blood accept OH- to make the blood less acidic.

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
36
After adding a small amount of Solution A to Solution B,the pH of Solution B declines from 8 to 3.Solution A must contain
A) a salt.
B) an acid.
C) water only.
D) a base.
A) a salt.
B) an acid.
C) water only.
D) a base.

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
37
In the equation 3H₂ + N₂ à 2NH₃,how many molecules of hydrogen gas (H₂)are present?
A) 2
B) 3
C) 6
D) 12
A) 2
B) 3
C) 6
D) 12

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
38
In the equation 2 H₂O₂ à 2 H₂O + O₂,the H₂O₂ molecules are the ________ and the H₂O + O₂ molecules are the ________.
A) products; products
B) reactants; products
C) products; reactants
D) reactants; reactants
A) products; products
B) reactants; products
C) products; reactants
D) reactants; reactants

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
39
In organic compounds,carbon atoms are bound to each other by
A) ionic bonds.
B) polar bonds.
C) hydrogen bonds.
D) covalent bonds.
A) ionic bonds.
B) polar bonds.
C) hydrogen bonds.
D) covalent bonds.

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
40
A solution with a pH of ________ is ________ times more acidic than a solution with a pH of ________.
A) 3; 10,000; 7
B) 12; 100; 10
C) 7; 1,000; 9
D) 4; 10; 3
A) 3; 10,000; 7
B) 12; 100; 10
C) 7; 1,000; 9
D) 4; 10; 3

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
41
The uncharged component in the core of an atom is a(n)________.

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
42
A scientist observed a chemical that changes to bright red in the presence of organic compounds containing nitrogen and phosphorus.To test this chemical,a set of test tubes is prepared,with each tube containing a purified sample of one of the following organic compounds.The chemical is then added to each tube.The test tube containing ________ will always turn bright red.
A) nucleic acids
B) proteins
C) carbohydrates
D) phospholipids
A) nucleic acids
B) proteins
C) carbohydrates
D) phospholipids

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
43
Which statement below is consistent with the facts that one function of nucleotides is energy transfer and that carbohydrates can be used to store energy?
A) If we humans could not store and transfer energy, we would have to match our energy input (eating) exactly to our energy requirements, even while sleeping.
B) There are not enough kinds of amino acids for proteins to be used as energy storage molecules.
C) Energy transfer and storage are processes that are unique to humans and, therefore, they are used to determine the classification of people.
D) Energy transfer is how we take the energy we gather from photosynthesis and transfer it into water molecules for later use when we need energy.
A) If we humans could not store and transfer energy, we would have to match our energy input (eating) exactly to our energy requirements, even while sleeping.
B) There are not enough kinds of amino acids for proteins to be used as energy storage molecules.
C) Energy transfer and storage are processes that are unique to humans and, therefore, they are used to determine the classification of people.
D) Energy transfer is how we take the energy we gather from photosynthesis and transfer it into water molecules for later use when we need energy.

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
44
Which of the following is NOT a role of fatty acids in living organisms?
A) energy storage
B) storage of genetic information
C) membrane construction
D) building blocks of fats
A) energy storage
B) storage of genetic information
C) membrane construction
D) building blocks of fats

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
45
ATP is a universal fuel for living organisms.The energy that ATP molecules deliver in chemical reactions is stored in
A) covalent bonds between the molecule's phosphate groups.
B) covalent bonds between the molecule's sugar and phosphate groups.
C) hydrogen bonds between the bases of two of these molecules.
D) ionic bonds between the molecule's sugar and base.
A) covalent bonds between the molecule's phosphate groups.
B) covalent bonds between the molecule's sugar and phosphate groups.
C) hydrogen bonds between the bases of two of these molecules.
D) ionic bonds between the molecule's sugar and base.

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
46
When phospholipids are added to water,they arrange themselves so that
A) their hydrophobic tails are on the inside of a lipid droplet.
B) their hydrophilic tails are on the outside of a lipid droplet.
C) their hydrophobic heads are facing the water.
D) their hydrophilic heads are on the inside of a lipid droplet.
A) their hydrophobic tails are on the inside of a lipid droplet.
B) their hydrophilic tails are on the outside of a lipid droplet.
C) their hydrophobic heads are facing the water.
D) their hydrophilic heads are on the inside of a lipid droplet.

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
47
An atom that becomes charged due to the gain or loss of an electron is called a(n)________.

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
48
Both trans fats and saturated fats have been linked to comparable groups of undesirable health complications.What do the two types of molecules share in common that may account for the similarity in their health impacts?
A) Both molecules form solid assemblies at body temperature and clog small blood vessels.
B) Both molecules are rich in hydrogen that can easily form hydrogen ions and lower the pH to harmful values.
C) Both molecules are linear; for reasons not currently understood, linear fatty acids appear to be more difficult to metabolize and have high biological activities.
D) Both molecules are rapidly converted to signal molecules called prostaglandins, creating discordant signaling within the body.
A) Both molecules form solid assemblies at body temperature and clog small blood vessels.
B) Both molecules are rich in hydrogen that can easily form hydrogen ions and lower the pH to harmful values.
C) Both molecules are linear; for reasons not currently understood, linear fatty acids appear to be more difficult to metabolize and have high biological activities.
D) Both molecules are rapidly converted to signal molecules called prostaglandins, creating discordant signaling within the body.

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
49
The process of partial hydrogenation turns liquid plant lipids into semisolid lipids by
A) adding antioxidants that prevent lipid oxidation.
B) creating hydrocarbon chains that are more kinked than those in natural fats.
C) substituting nitrogen for carbon in fatty acid chains.
D) removing double bonds and adding hydrogen to the fatty acid chains of plant lipids.
A) adding antioxidants that prevent lipid oxidation.
B) creating hydrocarbon chains that are more kinked than those in natural fats.
C) substituting nitrogen for carbon in fatty acid chains.
D) removing double bonds and adding hydrogen to the fatty acid chains of plant lipids.

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
50
You purchase a laundry product that claims to use natural enzymes to remove stains from clothing.After spilling grape juice on your favorite shirt,you apply the product and wash your shirt (following the directions,of course).When you pull the shirt out of the washer,the stain is still there! Which of the following might explain why the stain remover did not work?
A) The stain remover and the grape juice are both hydrophilic, so the enzymes could not interact with the stain.
B) The pH of the water in your house has a pH of 7.0, which prevents the enzymes from working properly.
C) Before you got home from the store, you stopped at the mall and left your stain remover in the trunk of your car on a hot day, denaturing the enzymes.
D) The stain remover and the grape juice are both hydrophobic, so the enzymes could not interact with the stain.
A) The stain remover and the grape juice are both hydrophilic, so the enzymes could not interact with the stain.
B) The pH of the water in your house has a pH of 7.0, which prevents the enzymes from working properly.
C) Before you got home from the store, you stopped at the mall and left your stain remover in the trunk of your car on a hot day, denaturing the enzymes.
D) The stain remover and the grape juice are both hydrophobic, so the enzymes could not interact with the stain.

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
51
An oil is a lipid that is ________ at room temperature.
A) liquid
B) saturated
C) supersaturated
D) solid
A) liquid
B) saturated
C) supersaturated
D) solid

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
52
When you place a piece of red meat on a hot barbeque,it slowly changes from soft to firm.Meat is primarily made of proteins.Which of the following might account for the change in texture during cooking?
A) The heat causes the cells in the meat to produce more protein.
B) The heat causes chemical bonds to form between the proteins and nucleic acids in the meat.
C) The heat from the barbeque converts proteins into lipids.
D) The addition of heat causes proteins to denature and link together.
A) The heat causes the cells in the meat to produce more protein.
B) The heat causes chemical bonds to form between the proteins and nucleic acids in the meat.
C) The heat from the barbeque converts proteins into lipids.
D) The addition of heat causes proteins to denature and link together.

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
53
Nucleotides
A) are the building blocks of proteins.
B) are involved in every chemical reaction in the cell.
C) form physical structures such as hair.
D) are the building blocks of nucleic acids.
A) are the building blocks of proteins.
B) are involved in every chemical reaction in the cell.
C) form physical structures such as hair.
D) are the building blocks of nucleic acids.

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
54
The following figure shows the structural and space-filling models for stearic acid and oleic acid. 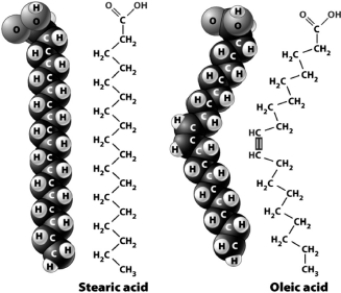 Although the two fatty acids have the same number of carbon atoms,they have different three-dimensional configurations; oleic acid has a slight bend near the middle.The result is that
Although the two fatty acids have the same number of carbon atoms,they have different three-dimensional configurations; oleic acid has a slight bend near the middle.The result is that
A) a pure sample of oleic acid would be more liquid than a pure sample of stearic acid.
B) stearic acid would be classified as an unsaturated fatty acid.
C) you would be more likely to find stearic acid in the form of an oil than in the form of a fat.
D) oleic acid would be classified a saturated fatty acid.
 Although the two fatty acids have the same number of carbon atoms,they have different three-dimensional configurations; oleic acid has a slight bend near the middle.The result is that
Although the two fatty acids have the same number of carbon atoms,they have different three-dimensional configurations; oleic acid has a slight bend near the middle.The result is thatA) a pure sample of oleic acid would be more liquid than a pure sample of stearic acid.
B) stearic acid would be classified as an unsaturated fatty acid.
C) you would be more likely to find stearic acid in the form of an oil than in the form of a fat.
D) oleic acid would be classified a saturated fatty acid.

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
55
We use soap to clean ourselves better than we could with water alone.Soaps contain phospholipids that are responsible for the cleansing action.Which of the following statements is the most likely explanation for how soaps work?
A) Phospholipids are ions and therefore mix with both the water and oily dirt.
B) Phospholipids are completely hydrophilic and, therefore, oily dirt takes the place of the phospholipid molecules that would be dissolved in the rinse water.
C) The phospholipid tail attaches to the oily dirt, while the phospholipid head interacts with the rinse water and carries the dirt (and soap) away with it.
D) The nonpolar fatty acid chains that make up the heads of the phospholipid are hydrophilic, and thus are repelled by the water.
A) Phospholipids are ions and therefore mix with both the water and oily dirt.
B) Phospholipids are completely hydrophilic and, therefore, oily dirt takes the place of the phospholipid molecules that would be dissolved in the rinse water.
C) The phospholipid tail attaches to the oily dirt, while the phospholipid head interacts with the rinse water and carries the dirt (and soap) away with it.
D) The nonpolar fatty acid chains that make up the heads of the phospholipid are hydrophilic, and thus are repelled by the water.

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
56
Which of the following is NOT a function of cholesterol?
A) Cholesterol is converted into other important molecules like steroid hormones.
B) Cholesterol is converted into a vitamin important in the growth and maintenance of bone and muscle.
C) Cholesterol is a necessary component in the cell membranes of plants.
D) A derivative of cholesterol aids in the digestion of fats.
A) Cholesterol is converted into other important molecules like steroid hormones.
B) Cholesterol is converted into a vitamin important in the growth and maintenance of bone and muscle.
C) Cholesterol is a necessary component in the cell membranes of plants.
D) A derivative of cholesterol aids in the digestion of fats.

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
57
In water,phospholipids arrange themselves such that
A) their fatty acid head groups are facing the water.
B) their hydrophobic tails are kept away from the water.
C) saturated fatty acids face the water, while unsaturated fatty acids are separated from the water.
D) the charged atoms on their fatty acid chains can interact directly with the water molecules.
A) their fatty acid head groups are facing the water.
B) their hydrophobic tails are kept away from the water.
C) saturated fatty acids face the water, while unsaturated fatty acids are separated from the water.
D) the charged atoms on their fatty acid chains can interact directly with the water molecules.

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
58
The sum of an atom's protons and neutrons is its ________.

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
59
Oxygen has six electrons in its outer shell that can hold up to eight electrons.As a result,oxygen will commonly form ________ covalent bonds.

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
60
Which of the following is NOT a method used to tenderize meat?
A) marinades with high pH
B) lemon juice, vinegar, or wine
C) brining or soaking in a salt water bath for several hours
D) pounding or grinding meat
A) marinades with high pH
B) lemon juice, vinegar, or wine
C) brining or soaking in a salt water bath for several hours
D) pounding or grinding meat

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
61
Most lipids contain one or more of the long,hydrophobic hydrocarbon chains known as ________.

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
62
Most living organisms consist of more than 70 percent ________ by weight.

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
63
Referencing the image below,________ water molecules would be required for hydrolysis to completely separate the 6-monomer polymer into individual monomers.



Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
64
Because they are made of hydrocarbon chains that repel water,the most hydrophobic of the four classes of organic compounds is the ________.

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
65
The monomers that are linked together to form a DNA polymer are called ________.

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
66
A compound that maintains the pH of a solution by taking up or releasing hydrogen ions is called a ________.

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
67
A type of organic compound that plays a role in both heredity and in energy delivery in cells is a ________.

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
68
The most versatile atom in living systems is ________.

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
69
Molecules with an uneven distribution of charge are described as ________.

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
70
The types of proteins that speed up the rate of chemical reactions in the cell are called ________.

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
71
The monomers in proteins are ________.

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
72
Molecules that are nonpolar and repelled by water are called ________.

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
73
Lipids with a four-ring structure are called ________.

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
74
Marinades that contain vinegar,wine,or yogurt are able to tenderize meat by breaking down collagen into smaller polypeptides because of their ________.

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
75
All the isotopes of a particular element have the same number of protons.

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
76
The number that represents neutrality on the pH scale is ________.

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
77
An atom is in its most stable state when all its electron shells are filled to capacity.

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
78
The following figure shows the chemical structure and space-filling models for stearic acid and oleic acid.
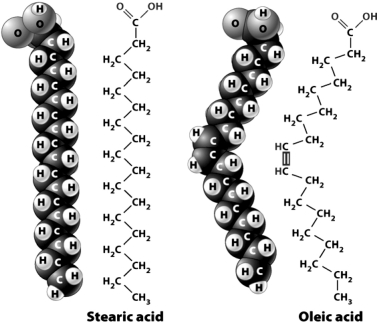 The reason oleic acid is slightly bent (as compared to stearic acid)is that it contains a ________ between two of its carbon atoms.
The reason oleic acid is slightly bent (as compared to stearic acid)is that it contains a ________ between two of its carbon atoms.
 The reason oleic acid is slightly bent (as compared to stearic acid)is that it contains a ________ between two of its carbon atoms.
The reason oleic acid is slightly bent (as compared to stearic acid)is that it contains a ________ between two of its carbon atoms.
Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
79
A group of monomers bonded together form a ________.

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
80
Covalent bonds contain ions.

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck



