Deck 10: Synthesis Using Aromatic Materials: Electrophilic Aromatic Substitution and Directed Ortho Metalation
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/88
Play
Full screen (f)
Deck 10: Synthesis Using Aromatic Materials: Electrophilic Aromatic Substitution and Directed Ortho Metalation
1
Why is nitrobenzene NOT compatible with Friedel-Crafts alkylation?
A)because the nitro group is too electron withdrawing to undergo Friedel-Crafts alkylation
B)because the nitrogen acts as a Lewis base and reacts with AlCl3 instead
C)because the nitro group is too electron donating to undergo Friedel-Crafts alkylation
D)because the nitrogen group acts as a Lewis acid and reacts with AlCl3 instead
A)because the nitro group is too electron withdrawing to undergo Friedel-Crafts alkylation
B)because the nitrogen acts as a Lewis base and reacts with AlCl3 instead
C)because the nitro group is too electron donating to undergo Friedel-Crafts alkylation
D)because the nitrogen group acts as a Lewis acid and reacts with AlCl3 instead
because the nitro group is too electron withdrawing to undergo Friedel-Crafts alkylation
2
What set of reagents would most effectively produce ethylbenzene from benzene shown below? 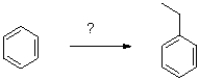
A)AlCl3,EtCl
B)1)AlCl3,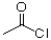 2)Zn/HCl
2)Zn/HCl
C)1)MgBrEt,ether 2)H3O+
D)1)EtBr

A)AlCl3,EtCl
B)1)AlCl3,
 2)Zn/HCl
2)Zn/HClC)1)MgBrEt,ether 2)H3O+
D)1)EtBr
1)AlCl3,  2)Zn/HCl
2)Zn/HCl
 2)Zn/HCl
2)Zn/HCl 3
What is the role of FeBr3 in the following reaction? 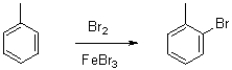
A)It activates bromine and makes it more nucleophilic.
B)It activates bromine and makes it more electrophilic.
C)It activates toluene and makes it more nucleophilic.
D)It activates toluene and makes it more electrophilic.
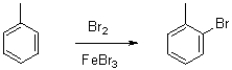
A)It activates bromine and makes it more nucleophilic.
B)It activates bromine and makes it more electrophilic.
C)It activates toluene and makes it more nucleophilic.
D)It activates toluene and makes it more electrophilic.
It activates bromine and makes it more electrophilic.
4
As substituents on aromatic rings,which of the following best describes halides?
A)meta directing and activating
B)meta directing and deactivating
C)ortho/para directing and activating
D)ortho/para directing and deactivating
A)meta directing and activating
B)meta directing and deactivating
C)ortho/para directing and activating
D)ortho/para directing and deactivating

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
5
Which of the following would be the product of the reaction shown below? 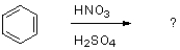
A)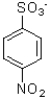
B)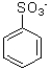
C)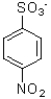
D)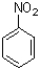
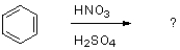
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
6
What is the role of sulfuric acid in the following reaction scheme? 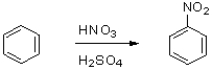
A)to protonate the aromatic ring and make it a better electrophile
B)to form dipole interactions with the developing positive charge in the aromatic ring
C)to protonate nitric acid to form the nitronium ion
D)to regenerate nitric acid

A)to protonate the aromatic ring and make it a better electrophile
B)to form dipole interactions with the developing positive charge in the aromatic ring
C)to protonate nitric acid to form the nitronium ion
D)to regenerate nitric acid

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
7
Which of the following would be the product of the reaction shown below? 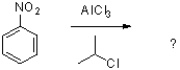
A)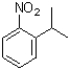
B)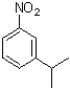
C)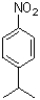
D)No reaction would occur.

A)

B)

C)

D)No reaction would occur.

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
8
What route would work best for the synthesis of the following aromatic molecule from benzene shown below? 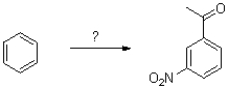
A)1)AlCl3,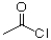 2)HNO3,H2SO4
2)HNO3,H2SO4
B)1)HNO3,H2SO4 2)AlCl3,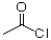
C)1)HNO3,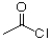
D)This product cannot be formed since Friedel-Crafts reactions are incompatible with nitrobenzene.

A)1)AlCl3,
 2)HNO3,H2SO4
2)HNO3,H2SO4B)1)HNO3,H2SO4 2)AlCl3,

C)1)HNO3,

D)This product cannot be formed since Friedel-Crafts reactions are incompatible with nitrobenzene.

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
9
What would be the expected product of the following reaction? 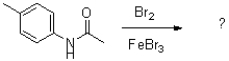
A)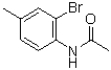
B)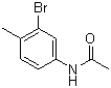
C)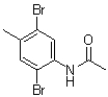
D)No product would form.

A)

B)

C)

D)No product would form.

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
10
Why is aniline NOT compatible with Friedel-Crafts alkylation?
A)because the amino group is too electron withdrawing to undergo Friedel-Crafts alkylation
B)because the nitrogen acts as a Lewis base and reacts with AlCl3 instead
C)because the amino group is too electron donating to undergo Friedel-Crafts alkylation
D)because the nitrogen group acts as a Lewis acid and reacts with AlCl3 instead
A)because the amino group is too electron withdrawing to undergo Friedel-Crafts alkylation
B)because the nitrogen acts as a Lewis base and reacts with AlCl3 instead
C)because the amino group is too electron donating to undergo Friedel-Crafts alkylation
D)because the nitrogen group acts as a Lewis acid and reacts with AlCl3 instead

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
11
Which of the following is an intermediate in the reaction shown below? 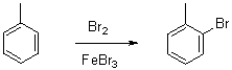
A)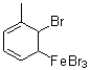
B)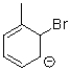
C)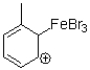
D)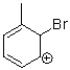
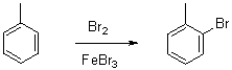
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
12
What sequence of reagents would best achieve the transformation shown below? 
A)1)Br2,FeBr3 2)HNO3,H2SO4 3)Fe,HCL
B)1)Br2,FeBr3 2)Fe,HCL 3)HNO3,H2SO4
C)1)HNO3,H2SO4 2)Br2,FeBr3 3)Fe,HCL
D)1)HNO3,H2SO4 2)Fe,HCL 3)Br2,FeBr3

A)1)Br2,FeBr3 2)HNO3,H2SO4 3)Fe,HCL
B)1)Br2,FeBr3 2)Fe,HCL 3)HNO3,H2SO4
C)1)HNO3,H2SO4 2)Br2,FeBr3 3)Fe,HCL
D)1)HNO3,H2SO4 2)Fe,HCL 3)Br2,FeBr3

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
13
Which of the following best describes the substituent on the molecule shown below? 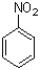
A)It is ortho/para directing by electron-delocalization.
B)It is meta directing by electron-delocalization.
C)It is ortho/para directing by induction.
D)It is meta directing by induction.

A)It is ortho/para directing by electron-delocalization.
B)It is meta directing by electron-delocalization.
C)It is ortho/para directing by induction.
D)It is meta directing by induction.

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
14
Which of the following statements best describes the set of aromatic molecules show below? 
A)A is the most activated and B is the least activated towards SEAr reactions.
B)C is the most activated and B is the least activated towards SEAr reactions.
C)A is the most activated and C is the least activated towards SEAr reactions.
D)B is the most activated and A is the least activated towards SEAr reactions.

A)A is the most activated and B is the least activated towards SEAr reactions.
B)C is the most activated and B is the least activated towards SEAr reactions.
C)A is the most activated and C is the least activated towards SEAr reactions.
D)B is the most activated and A is the least activated towards SEAr reactions.

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
15
Which of the following best describes the carboxylic acid substituent in benzoic acid shown below? 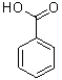
A)It is ortho/para directing and electron donating by electron-delocalization.
B)It is meta directing and electron withdrawing by electron-delocalization.
C)It is ortho/para directing and electron donating by electron-delocalization..
D)It is meta directing and electron withdrawing by electron-delocalization..

A)It is ortho/para directing and electron donating by electron-delocalization.
B)It is meta directing and electron withdrawing by electron-delocalization.
C)It is ortho/para directing and electron donating by electron-delocalization..
D)It is meta directing and electron withdrawing by electron-delocalization..

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
16
Which of the following best describes the substituent on the molecule shown below? 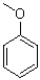
A)It is ortho/para directing and electron donating by electron-delocalization.
B)It is meta directing and electron withdrawing by electron-delocalization.
C)It is ortho/para directing and electron donating by electron-delocalization..
D)It is meta directing and electron withdrawing by electron-delocalization..

A)It is ortho/para directing and electron donating by electron-delocalization.
B)It is meta directing and electron withdrawing by electron-delocalization.
C)It is ortho/para directing and electron donating by electron-delocalization..
D)It is meta directing and electron withdrawing by electron-delocalization..

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
17
Which of the following compounds will the reaction below lead to? 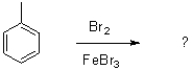
A)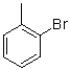
B)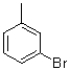
C)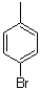
D)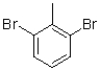
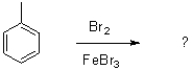
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
18
Compared to benzene,what best describes the reactivity of nitrobenzene (shown below)to SEAr reactions? 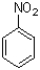
A)It is more reactive than benzene.
B)It is less reactive than benzene.
C)It is similarly reactive as benzene.
D)There is not enough information to determine reactivity.

A)It is more reactive than benzene.
B)It is less reactive than benzene.
C)It is similarly reactive as benzene.
D)There is not enough information to determine reactivity.

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
19
Which of the following best describes the two molecules shown below? 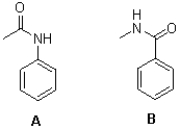
A)A is more reactive to SEAr than B and is meta directing.
B)A is less reactive to SEAr than B and is meta directing.
C)A is more reactive to SEAr than B and is para/ortho directing.
D)A is less reactive to SEAr than B and is para/ortho directing.

A)A is more reactive to SEAr than B and is meta directing.
B)A is less reactive to SEAr than B and is meta directing.
C)A is more reactive to SEAr than B and is para/ortho directing.
D)A is less reactive to SEAr than B and is para/ortho directing.

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
20
Which of the following statements best describes the set of aromatic molecules,show below? 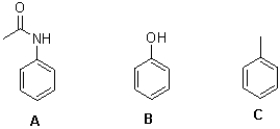
A)A is the most activated and B is the least activated towards SEAr reactions.
B)C is the most activated and B is the least activated towards SEAr reactions.
C)A is the most activated and C is the least activated towards SEAr reactions.
D)B is the most activated and C is the least activated towards SEAr reactions.

A)A is the most activated and B is the least activated towards SEAr reactions.
B)C is the most activated and B is the least activated towards SEAr reactions.
C)A is the most activated and C is the least activated towards SEAr reactions.
D)B is the most activated and C is the least activated towards SEAr reactions.

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
21
Which of the following aromatic molecules would NOT be suitable for directed ortho metalation?
A)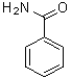
B)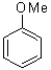
C)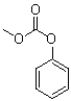
D)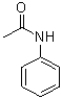
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
22
In an SEAr reaction,how would you describe an alkyl group on an aromatic ring?
A)strongly deactivating
B)weakly deactivating
C)weakly activating
D)strongly activating
A)strongly deactivating
B)weakly deactivating
C)weakly activating
D)strongly activating

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
23
Which of the following compounds will the reaction below lead to? 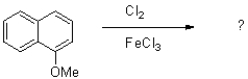
A)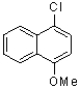
B)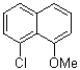
C)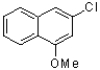
D)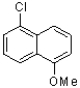

A)

B)

C)

D)


Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
24
Which of the following molecules would be required as a reacting to form the product in the reaction scheme shown below? 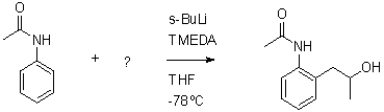
A)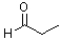
B)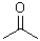
C)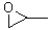
D)The transformation shown above is not possible.

A)

B)

C)

D)The transformation shown above is not possible.

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
25
Which of the following best describes FeBr3 in the reaction shown below? 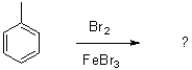
A)Lewis acid
B)Lewis base
C)solvent
D)nucleophile
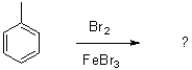
A)Lewis acid
B)Lewis base
C)solvent
D)nucleophile

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
26
Which of the following best describes the role of aluminum chloride in a Friedel-Crafts reaction?
A)It is a Lewis base and is used to activate the alkyl halide.
B)It is a Lewis acid and is used to activate the alkyl halide.
C)It is a Lewis base and is used to activate the aromatic ring.
D)It is a Lewis acid and is used to activate the aromatic ring.
A)It is a Lewis base and is used to activate the alkyl halide.
B)It is a Lewis acid and is used to activate the alkyl halide.
C)It is a Lewis base and is used to activate the aromatic ring.
D)It is a Lewis acid and is used to activate the aromatic ring.

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
27
Predict the product of the following reaction shown below. 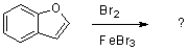
A)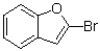
B)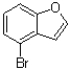
C)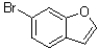
D)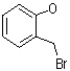
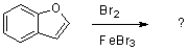
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
28
Which of the following compounds will the reaction below lead to? 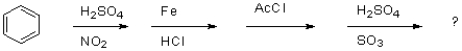
A)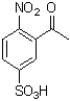
B)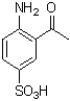
C)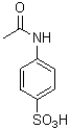
D)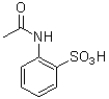

A)

B)

C)

D)


Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
29
Which of the following best describes an activating group in an SEAr reaction?
A)electron rich with a smaller ÄG of reaction relative to benzene
B)electron poor with a smaller ÄG of reaction relative to benzene
C)electron rich with a larger ÄG of reaction relative to benzene
D)electron poor with a larger ÄG of reaction relative to benzene
A)electron rich with a smaller ÄG of reaction relative to benzene
B)electron poor with a smaller ÄG of reaction relative to benzene
C)electron rich with a larger ÄG of reaction relative to benzene
D)electron poor with a larger ÄG of reaction relative to benzene

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
30
What is the IUPAC name for m-xylene?
A)1,2-dimethylbenzene
B)1,3-dimethylbenzene
C)2-methylphenol
D)3-methylphenol
A)1,2-dimethylbenzene
B)1,3-dimethylbenzene
C)2-methylphenol
D)3-methylphenol

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
31
Which of the following compounds will the reaction below lead to? 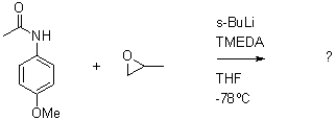
A)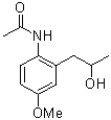
B)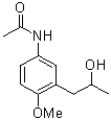
C)
D)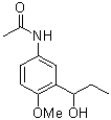

A)

B)

C)

D)


Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
32
Which of the following compounds will the reaction below lead to? 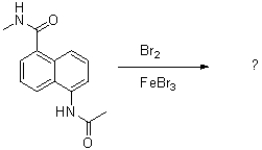
A)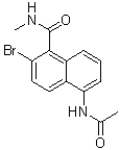
B)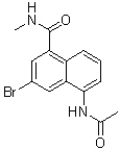
C)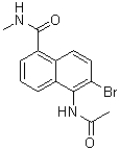
D)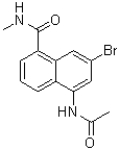

A)

B)

C)

D)


Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
33
What is the common name for methoxybenzene?
A)analine
B)anisole
C)phenol
D)toluene
A)analine
B)anisole
C)phenol
D)toluene

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
34
Which of the following statements best describes the reaction shown below? 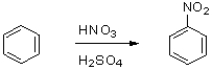
A)Benzene is the nucleophile;HNO3 is the electrophile.
B)Benzene is the electrophile;HNO3 is the nucleophile.
C)Benzene is the nucleophile;NO2 is the electrophile.
D)Benzene is the electrophile;NO2 is the nucleophile.

A)Benzene is the nucleophile;HNO3 is the electrophile.
B)Benzene is the electrophile;HNO3 is the nucleophile.
C)Benzene is the nucleophile;NO2 is the electrophile.
D)Benzene is the electrophile;NO2 is the nucleophile.

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
35
Which of the following molecules would be required as a reacting to form the product in the reaction scheme shown below? 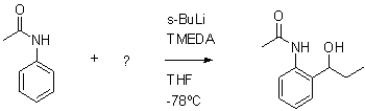
A)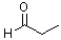
B)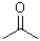
C)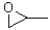
D)The transformation shown above is not possible.

A)

B)

C)

D)The transformation shown above is not possible.

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
36
Which molecule will react faster with Br2 in the presence of iron bromide and why? 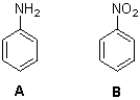
A)A because the amino group makes the ring more negative through charge delocalization
B)B because the nitro group makes the ring more negative through charge delocalization
C)A because the amino group makes the ring less negative through charge delocalization
D)B because the nitro group makes the ring less negative through charge delocalization

A)A because the amino group makes the ring more negative through charge delocalization
B)B because the nitro group makes the ring more negative through charge delocalization
C)A because the amino group makes the ring less negative through charge delocalization
D)B because the nitro group makes the ring less negative through charge delocalization

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
37
In an SEAr reaction,how would you describe a halogen group on an aromatic ring?
A)ortho/para directing and deactivating
B)ortho/para directing and activating
C)meta directing and deactivating
D)meta directing and activating
A)ortho/para directing and deactivating
B)ortho/para directing and activating
C)meta directing and deactivating
D)meta directing and activating

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
38
Which of the following best describes a deactivating group in an SEAr reaction?
A)electron rich with a smaller ÄG of reaction relative to benzene
B)electron poor with a smaller ÄG of reaction relative to benzene
C)electron rich with a larger ÄG of reaction relative to benzene
D)electron poor with a larger ÄG of reaction relative to benzene
A)electron rich with a smaller ÄG of reaction relative to benzene
B)electron poor with a smaller ÄG of reaction relative to benzene
C)electron rich with a larger ÄG of reaction relative to benzene
D)electron poor with a larger ÄG of reaction relative to benzene

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
39
Which of the following compounds will the reaction below lead to? 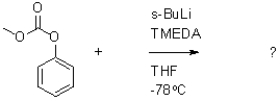
A)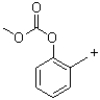
B)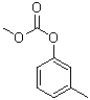
C)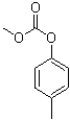
D)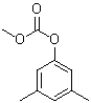

A)

B)

C)

D)


Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
40
In an SEAr reaction,how would you describe an amino group on an aromatic ring?
A)strongly deactivating
B)weakly deactivating
C)weakly activating
D)strongly activating
A)strongly deactivating
B)weakly deactivating
C)weakly activating
D)strongly activating

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
41
Carbocation rearrangements can involve either the movements of hydrides or alkyl groups.

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
42
In a Friedel-Crafts reaction,aluminum chloride acts as a Lewis acid.

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
43
Aromatic ð bonds are more nucleophilic then non-aromatic ð bonds.

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
44
Electron donating groups stabilize the arenium ion during SEAr reactions.

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
45
Sulfate groups are meta directing substituents in SEAr reactions.

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
46
Alkyl groups are meta directing substituents in SEAr reactions.

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
47
Amino groups are ortho/para directing substituents in SEAr reactions.

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
48
What is the expected product of the reaction shown below? 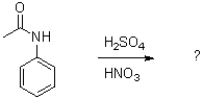
A)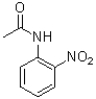
B)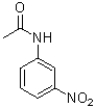
C)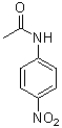
D)A mixture of

A)

B)

C)

D)A mixture of

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
49
Alcohol groups are activating substituents in SEAr reactions.

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
50
Nitro groups are activating substituents in SEAr reactions.

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
51
During an electrophilic aromatic substitution reaction,the aromatic ring becomes positively charged.

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
52
Sterics plays a role in whether a group adds ortho or para on an aromatic ring.

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
53
What is the driving force for the last step in an SEAr mechanism?
A)stabilization of charge
B)loss of an acidic proton
C)gain of a double bond
D)restoration of aromaticity
A)stabilization of charge
B)loss of an acidic proton
C)gain of a double bond
D)restoration of aromaticity

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
54
Meta directing groups tend to be deactivating groups in SEAr reactions.

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
55
The role of FeBr3 in the bromination of benzene is to make bromine more nucleophilic.

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
56
Friedel-Crafts acylation reactions run the risk of carbocation rearrangements.

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
57
A secondary carbocation will undergo rearrangement to become a primary carbocation.

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
58
What is the expected product of the reaction shown below? 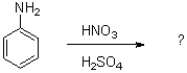
A)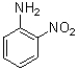
B)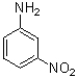
C)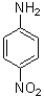
D)A mixture of

A)

B)

C)

D)A mixture of

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
59
The aromatic ð electrons act as the nucleophile in SEAr reactions.

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
60
Which of the following compounds will the reaction below lead to? 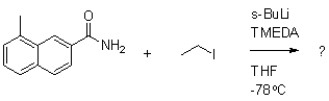
A)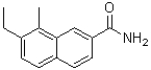
B)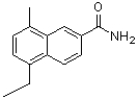
C)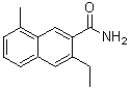
D)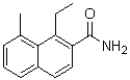
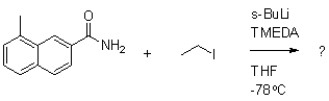
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
61
In an electrophilic aromatic substitution reaction,the aromatic group acts as a(n)_______________.

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
62
Design a reaction pathway to generate the following aromatic molecule from benzene.Show each intermediate. 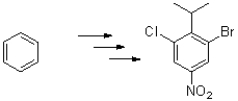


Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
63
Nitro groups on aromatic rings act as _______________ directors in SEAr reactions.

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
64
Acyl groups are meta directing substituents in SEAr reactions.

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
65
Fluorination of an aromatic ring through a SEAr mechanism requires no catalyst.

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
66
An alternative to electrophilic aromatic substitution reactions are DOM reactions,which stands for _______________.

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
67
Sulfate groups are considered para/ortho directors in SEAr reactions.

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
68
_______________ is a Lewis acid required for Friedel-Crafts reactions

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
69
Acylation of an amine to an amide increases its activation potential in SEAr reactions.

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
70
Nitro groups are strongly _______________ substituents in SEAr reactions.

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
71
Electron-donating substituents on aromatic rings act as _______________ directors in SEAr reactions.

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
72
Friedel-Crafts alkylations cannot be performed in the presence of a(n)________ or a(n)_______ group.

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
73
Protonation of an amino group plays no role in its directing influence in SEAr reactions.

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
74
SO3 is the nucleophile in the sulfation of aromatic rings.

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
75
Sulfate groups are considered deactivating groups in SEAr reactions.

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
76
Iron filings in the presence of hydrochloric acid can reduce an aromatic ketone into an alkyl group.

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
77
The common name for aminobenzene is _______________ .

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
78
The IUPAC name for anisole is _______________ .

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
79
Friedel-Crafts acylation often results in a(n)_______________ functional group.

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
80
Iron filings in the presence of hydrochloric acid can reduce a nitro group into an amino group.

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck



