Deck 2: Anatomy of an Organic Molecule
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/88
Play
Full screen (f)
Deck 2: Anatomy of an Organic Molecule
1
A molecule contains only carbon and hydrogen atoms,a single ring,and two ð bonds.Which of the following represents its possible formula?
A)C10H16
B)C12H18
C)C9H18
D)C11H16
A)C10H16
B)C12H18
C)C9H18
D)C11H16
C10H16
2
What is the IUPAC name of the following molecule? 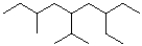
A)7-ethyl-3-methyl-5-isopropylnonane
B)3-ethyl-7-methyl-5-isopropylnonane
C)7-ethyl-5-isopropyl-3-methylnonane
D)3-ethyl-5-isopropyl-7-methylnonane

A)7-ethyl-3-methyl-5-isopropylnonane
B)3-ethyl-7-methyl-5-isopropylnonane
C)7-ethyl-5-isopropyl-3-methylnonane
D)3-ethyl-5-isopropyl-7-methylnonane
3-ethyl-5-isopropyl-7-methylnonane
3
What is the general formula for a saturated alkane?
A)CnH2n+2
B)CnH2n
C)CnH2n-2
D)CnHn
A)CnH2n+2
B)CnH2n
C)CnH2n-2
D)CnHn
CnH2n+2
4
Which of the following molecules contains both a hydrogen bond donor and a hydrogen bond acceptor?
A)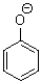
B)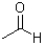
C)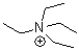
D)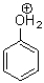
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
5
Which of the following molecules CANNOT act as a hydrogen bond acceptor?
A)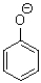
B)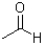
C)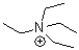
D)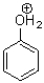
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
6
What is the IUPAC name of the following molecule? 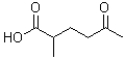
A)6-carboxy-5-methylhexan-2-one
B)1-carboxy-2-methylhexan-5-one
C)2-methyl-5-oxohexanoic acid
D)5-methyl-2-oxohexanoic acid

A)6-carboxy-5-methylhexan-2-one
B)1-carboxy-2-methylhexan-5-one
C)2-methyl-5-oxohexanoic acid
D)5-methyl-2-oxohexanoic acid

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
7
What describes the following functional group? 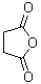
A)anhydride
B)ether
C)acetal
D)ester

A)anhydride
B)ether
C)acetal
D)ester

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
8
Which functional group is NOT present in the structure of heroin shown below? 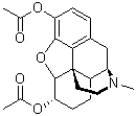
A)amine
B)ester
C)ether
D)acid anhydride

A)amine
B)ester
C)ether
D)acid anhydride

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
9
Which functional group is present in the structure of heroin shown below? 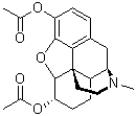
A)amide
B)acid anhydride
C)alkene
D)ether

A)amide
B)acid anhydride
C)alkene
D)ether

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
10
What describes the following functional group? 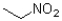
A)nitro
B)nitrile
C)amine
D)amide
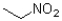
A)nitro
B)nitrile
C)amine
D)amide

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
11
Which of the following best describes the molecules shown below? 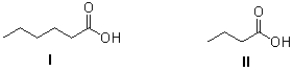
A)I is more soluble in water and has a higher melting point than II.
B)I is less soluble in water and has a higher melting point than II.
C)I is more soluble in water and has a lower melting point than II.
D)I is less soluble in water and has a lower melting point than II.
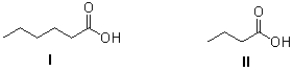
A)I is more soluble in water and has a higher melting point than II.
B)I is less soluble in water and has a higher melting point than II.
C)I is more soluble in water and has a lower melting point than II.
D)I is less soluble in water and has a lower melting point than II.

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
12
What describes the following functional group? 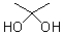
A)anhydride
B)hemiacetal
C)acetal
D)hydrate

A)anhydride
B)hemiacetal
C)acetal
D)hydrate

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
13
What describes the following functional group? 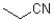
A)nitro
B)nitrile
C)amine
D)amide
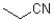
A)nitro
B)nitrile
C)amine
D)amide

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
14
A linear hydrocarbon contains two sp hybridized carbons and four sp2 hybridized carbons.Which of the following represents its possible formula?
A)C9H14
B)C9H16
C)C10H14
D)C10H12
A)C9H14
B)C9H16
C)C10H14
D)C10H12

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
15
Which of the following best describes the molecules shown below? 
A)I has stronger London dispersion forces and a higher boiling point than II.
B)I has weaker London dispersion forces and a higher boiling point than II.
C)I has stronger London dispersion forces and a lower boiling point than II.
D)I has weaker London dispersion forces and a lower boiling point than II.

A)I has stronger London dispersion forces and a higher boiling point than II.
B)I has weaker London dispersion forces and a higher boiling point than II.
C)I has stronger London dispersion forces and a lower boiling point than II.
D)I has weaker London dispersion forces and a lower boiling point than II.

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
16
What is the IUPAC name of the following molecule? 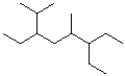
A)3,6-diethyl-2,5-dimethyloctane
B)3,6-diethyl-4,7-dimethyloctane
C)6-ethyl-3-isopropyl-5-dimethyloctane
D)3-ethyl-6-isopropyl-4-dimethyloctane

A)3,6-diethyl-2,5-dimethyloctane
B)3,6-diethyl-4,7-dimethyloctane
C)6-ethyl-3-isopropyl-5-dimethyloctane
D)3-ethyl-6-isopropyl-4-dimethyloctane

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
17
Which of the following formulas represents an unsaturated molecule.
A)C10H22O
B)C7H12F2Cl2
C)C5H10OBr2
D)C8H18N2
A)C10H22O
B)C7H12F2Cl2
C)C5H10OBr2
D)C8H18N2

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
18
Which of the following best describes a functional group?
A)It is a part of a molecule that contains ð bonds.
B)It is a part of a molecule that contains heteroatoms.
C)It is a part of a molecule that contains ð bonds and/or heteroatoms.
D)It is a part of a molecule that contains ð bonds and heteroatoms.
A)It is a part of a molecule that contains ð bonds.
B)It is a part of a molecule that contains heteroatoms.
C)It is a part of a molecule that contains ð bonds and/or heteroatoms.
D)It is a part of a molecule that contains ð bonds and heteroatoms.

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
19
What is the IUPAC name of the following molecule? 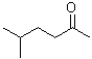
A)5-methylhexan-2-one
B)5-methylhexan-2-al
C)2-methylhexan-5-one
D)2-methylhexan-5-al

A)5-methylhexan-2-one
B)5-methylhexan-2-al
C)2-methylhexan-5-one
D)2-methylhexan-5-al

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
20
What is the IUPAC name of the following molecule? 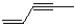
A)pent-1-en-3-yne
B)pent-3-yne-1-ene
C)pent-3-en-1-yne
D)pent-1-yne-3-ene
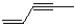
A)pent-1-en-3-yne
B)pent-3-yne-1-ene
C)pent-3-en-1-yne
D)pent-1-yne-3-ene

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
21
Which of the following best describes the solvent methanol?
A)polar and protic
B)polar and aprotic
C)non-polar and protic
D)non-polar and aprotic
A)polar and protic
B)polar and aprotic
C)non-polar and protic
D)non-polar and aprotic

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
22
Which of the following best describes the solvent dimethylforamide (DMF)shown below? 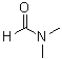
A)polar and protic
B)polar and aprotic
C)non-polar and protic
D)non-polar and aprotic

A)polar and protic
B)polar and aprotic
C)non-polar and protic
D)non-polar and aprotic

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
23
How many hydrogen bond accepting pairs of electrons does the molecule of penicillin (shown below)contain? 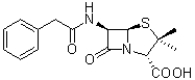
A)6
B)7
C)10
D)12

A)6
B)7
C)10
D)12

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
24
Which of the following best describes the molecule of heroin shown below? 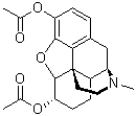
A)H-bond donor
B)H-bond acceptor
C)both H-bond donor and H-bond acceptor
D)neither a H-bond donor or a H-bond acceptor

A)H-bond donor
B)H-bond acceptor
C)both H-bond donor and H-bond acceptor
D)neither a H-bond donor or a H-bond acceptor

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
25
How many hydrogen bond donors does the molecule of penicillin (shown below)contain? 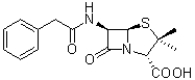
A)0
B)1
C)2
D)4

A)0
B)1
C)2
D)4

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
26
Which of the following best describes the solvent diethyl ether?
A)polar and protic
B)polar and aprotic
C)non-polar and protic
D)non-polar and aprotic
A)polar and protic
B)polar and aprotic
C)non-polar and protic
D)non-polar and aprotic

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
27
How many hydrogen bond accepting pairs of electrons does the molecule of heroin (shown below)contain? 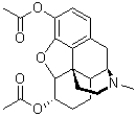
A)1
B)5
C)6
D)11

A)1
B)5
C)6
D)11

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
28
Which functional group is NOT present in the molecule of penicillin shown below? 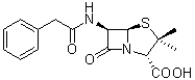
A)amide
B)benzene
C)carboxylic acid
D)ketone

A)amide
B)benzene
C)carboxylic acid
D)ketone

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
29
What is the strongest intermolecular force that the molecule shown below is capable of making? 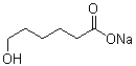
A)London dispersion
B)electrostatics
C)dipole-dipole
D)hydrogen bonding

A)London dispersion
B)electrostatics
C)dipole-dipole
D)hydrogen bonding

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
30
Rank the following in terms of increasing solubility in water (least to most). 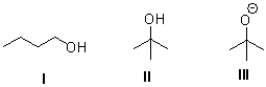
A)I,II,III
B)III,II,I
C)II,I,III
D)III,I,II

A)I,II,III
B)III,II,I
C)II,I,III
D)III,I,II

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
31
Which statement best describes the two molecules shown below? 
A)I has a higher melting point and higher solubility in water than II.
B)I has a higher melting point and lower solubility in water than II.
C)I has a lower melting point and higher solubility in water than II.
D)I has a lower melting point and lower solubility in water than II.

A)I has a higher melting point and higher solubility in water than II.
B)I has a higher melting point and lower solubility in water than II.
C)I has a lower melting point and higher solubility in water than II.
D)I has a lower melting point and lower solubility in water than II.

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
32
How many ð bonds can be found in the structure of penicillin shown below? 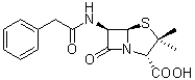
A)2
B)3
C)5
D)6

A)2
B)3
C)5
D)6

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
33
Which of the following best describes the solvent dimethylsulfoxide (DMSO)shown below? 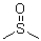
A)polar and protic
B)polar and aprotic
C)non-polar and protic
D)non-polar and aprotic

A)polar and protic
B)polar and aprotic
C)non-polar and protic
D)non-polar and aprotic

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
34
Which functional group is present in the molecule of penicillin shown below? 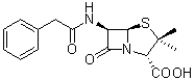
A)amine
B)ketone
C)carboxylic acid
D)alcohol

A)amine
B)ketone
C)carboxylic acid
D)alcohol

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
35
Which functional group has the highest priority using IUPAC naming?
A)ketone
B)alkene
C)ether
D)carboxylic acid
A)ketone
B)alkene
C)ether
D)carboxylic acid

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
36
How many hydrogen bond donor sites does the molecule of heroin (shown below)contain? 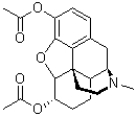
A)0
B)1
C)6
D)7

A)0
B)1
C)6
D)7

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
37
Which of the following represents the strongest intermolecular force?
A)London dispersion
B)electrostatics
C)dipole-dipole
D)hydrogen bonding
A)London dispersion
B)electrostatics
C)dipole-dipole
D)hydrogen bonding

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
38
What intermolecular force is possible in the following molecule of heroin,shown below? 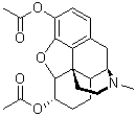
A)London dispersion
B)London forces and dipole-dipole
C)dipole-dipole and electrostatic
D)London dispersion and electrostatic

A)London dispersion
B)London forces and dipole-dipole
C)dipole-dipole and electrostatic
D)London dispersion and electrostatic

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
39
Which of the following would be least soluble in water?
A)
B)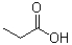
C)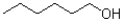
D)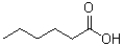
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
40
Rank the following in terms of increasing boiling point (least to most). 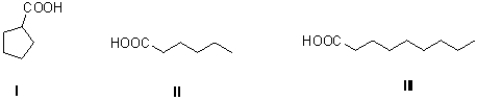
A)III,I,II
B)II,I,III
C)I,II,III
D)I,III,II

A)III,I,II
B)II,I,III
C)I,II,III
D)I,III,II

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
41
Acetonitrile is a polar protic solvent.

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
42
London dispersion is the principle intermolecular force among alkenes.

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
43
In order to be a hydrogen bond acceptor,an atom needs to have a lone pair of electrons.

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
44
Methanol is a polar protic solvent.

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
45
Ketones have a prefix of oxy- when naming using IUPAC rules.

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
46
Ketones can act as hydrogen bond donors.

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
47
What is the IUPAC name of the compound shown below? 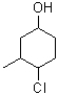
A)1-chloro-2-methylcyclohexan-2-ol
B)2-chloro-1-methylcyclohexan-5-ol
C)4-chloro-5-methylcyclohexanol
D)4-chloro-3-methylcyclohexanol

A)1-chloro-2-methylcyclohexan-2-ol
B)2-chloro-1-methylcyclohexan-5-ol
C)4-chloro-5-methylcyclohexanol
D)4-chloro-3-methylcyclohexanol

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
48
A nitrile bond contains two ð bonds.

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
49
The stronger the intermolecular forces,the higher the melting point.

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
50
Aldehydes have a suffix of -al when naming using IUPAC rules.

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
51
Diethyl ether is a non-polar solvent.

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
52
Which of the following molecules would be expected to have the largest melting point?
A)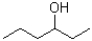
B)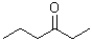
C)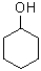
D)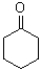
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
53
What is the IUPAC name of the compound shown below? 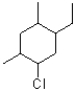
A)1-ethyl-2,4-dimethyl-5-chloro-cyclohexane
B)5-chloro-1-ethyl-2,4-dimethylcyclohexane
C)1-chloro-5-ethyl-2,4-dimethylcyclohexane
D)1-chloro-2,4-dimethyl-5-ethylcyclohexane

A)1-ethyl-2,4-dimethyl-5-chloro-cyclohexane
B)5-chloro-1-ethyl-2,4-dimethylcyclohexane
C)1-chloro-5-ethyl-2,4-dimethylcyclohexane
D)1-chloro-2,4-dimethyl-5-ethylcyclohexane

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
54
But- is a prefix that represents three carbons.

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
55
Branched hydrocarbons are capable of more London dispersion forces than linear hydrocarbons with the same number of carbons.

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
56
Branched hydrocarbons have higher boiling points than linear hydrocarbons with the same number of carbons.

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
57
Substituents on an organic molecule are named in alphabetical order using IUPAC rules.

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
58
Which of the following would be most soluble in water?
A)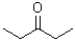
B)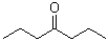
C)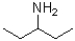
D)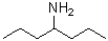
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
59
Branched hydrocarbons are less soluble in water than linear hydrocarbons of similar size.

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
60
What is the IUPAC name of the compound shown below? 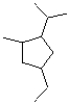
A)1-ethyl-3-isopropyl-4-methylcyclopentane
B)4-ethyl-1-isopropyl-2-methylcyclopentane
C)1-isopropyl-2-methyl-4-ethylcyclopentane
D)4-ethyl-2-methyl-1-isopropylcyclopentane

A)1-ethyl-3-isopropyl-4-methylcyclopentane
B)4-ethyl-1-isopropyl-2-methylcyclopentane
C)1-isopropyl-2-methyl-4-ethylcyclopentane
D)4-ethyl-2-methyl-1-isopropylcyclopentane

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
61
A molecule that dissolves in water is known as _______________ .

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
62
A molecule that is hydrophobic is expected to be insoluble in water.

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
63
Linear hydrocarbons are capable of stronger London dispersion than similarly sized branched hydrocarbons.

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
64
The prefix for a ketone is _______________ .

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
65
Solubility is governed by the type and strength of the intermolecular interactions between solvent and solute.

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
66
Dipole-dipole interactions involve the attraction of full formal charges.

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
67
Alcohols are more polar than carboxylic acids.

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
68
Secondary amines can act as hydrogen bond donors only.

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
69
Quaternary amines are capable of electrostatic interactions.

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
70
Esters are capable of acting as hydrogen bond _______________ .

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
71
The suffix for an aldehyde is _______________ .

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
72
A carbon-nitrogen triple bond is identified as a(n)_______________ functional group.

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
73
Electrostatic interactions are stronger than hydrogen bonds.

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
74
Ethers contain an oxygen atom with one ó bond and one ð bond.

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
75
Nitrobenzene has a chemical formula of _______________ .

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
76
DMF is known as a(n)_______________ solvent.

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
77
The term _______________ translates to water fearing and represents most alkanes.

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
78
Cyclic organic compounds are more soluble in water than linear organic compounds of similar size.

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
79
4-bromo-2-methylhexanoic acid has a formula of _______________ .

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
80
The general formula of a saturated hydrocarbon is_______________ .

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck



