Deck 13: Crystalline Solids and Modern Materials
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/43
Play
Full screen (f)
Deck 13: Crystalline Solids and Modern Materials
1
In a semiconductor,the bonding molecular orbitals that contain electrons are referred to as the ________,while the antibonding orbitals that are completely empty are referred to as the ________.
A) single bond, antibond
B) conduction band, valence band
C) valence band, conduction band
D) n-type, p-type
E) p-type, n-type
A) single bond, antibond
B) conduction band, valence band
C) valence band, conduction band
D) n-type, p-type
E) p-type, n-type
valence band, conduction band
2
A common use of polyvinyl chloride is
A) plumbing.
B) paint.
C) plastic bottles.
D) styrofoam.
E) all of the above.
A) plumbing.
B) paint.
C) plastic bottles.
D) styrofoam.
E) all of the above.
plumbing.
3
When an X-ray beam with a wavelength of 180 pm strikes the surface of a crystal,it produces a maximum reflection at an angle of 54.0°.If n=1,what is the separation between layers of atoms in the crystal?
A) 151 nm
B) 38.9 mm
C) 111 nm
D) 55.3 nm
E) 83.5 nm
A) 151 nm
B) 38.9 mm
C) 111 nm
D) 55.3 nm
E) 83.5 nm
111 nm
4
Which of the following represent the addition polymer formed from the compound below?
CH2=CHCl
A)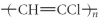
B)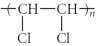
C)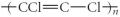
D)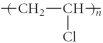
E)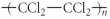
CH2=CHCl
A)
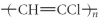
B)
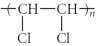
C)
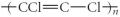
D)
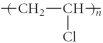
E)

Unlock Deck
Unlock for access to all 43 flashcards in this deck.
Unlock Deck
k this deck
5
All of the following are examples of allotropes of carbon EXCEPT
A) graphite
B) glass
C) diamond
D) graphine
E) all of the above
A) graphite
B) glass
C) diamond
D) graphine
E) all of the above

Unlock Deck
Unlock for access to all 43 flashcards in this deck.
Unlock Deck
k this deck
6
Identify the type of solid for ice.
A) metallic atomic solid
B) ionic solid
C) nonbonding atomic solid
D) molecular solid
E) networking atomic solid
A) metallic atomic solid
B) ionic solid
C) nonbonding atomic solid
D) molecular solid
E) networking atomic solid

Unlock Deck
Unlock for access to all 43 flashcards in this deck.
Unlock Deck
k this deck
7
Identify the type of solid for AgCl.
A) metallic atomic solid
B) ionic solid
C) nonbonding atomic solid
D) molecular solid
E) networking atomic solid
A) metallic atomic solid
B) ionic solid
C) nonbonding atomic solid
D) molecular solid
E) networking atomic solid

Unlock Deck
Unlock for access to all 43 flashcards in this deck.
Unlock Deck
k this deck
8
A metal crystallizes in a face centered cubic structure and has a density of 11.9 g/cm3.If the radius of the metal atom is 138 pm,what is the identity of the metal?
A) At
B) Pd
C) Mn
D) Fe
E) Cr
A) At
B) Pd
C) Mn
D) Fe
E) Cr

Unlock Deck
Unlock for access to all 43 flashcards in this deck.
Unlock Deck
k this deck
9
Which of the following represent the addition polymer formed from the compound below?
CH2=CH-CH3
A)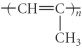
B)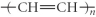
C)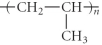
D)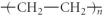
E)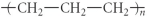
CH2=CH-CH3
A)
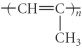
B)
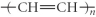
C)
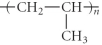
D)
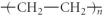
E)
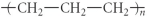

Unlock Deck
Unlock for access to all 43 flashcards in this deck.
Unlock Deck
k this deck
10
Diamond crystallizes in a cubic lattice with an edge length of 357 pm.If there are a total of 8 carbon atoms in the unit cell,what is the density of diamond?
A) 12.9 g/cm3
B) 3.54 g/cm3
C) 8.26 g/cm3
D) 2.26 g/cm3
E) 6.20 g/cm3
A) 12.9 g/cm3
B) 3.54 g/cm3
C) 8.26 g/cm3
D) 2.26 g/cm3
E) 6.20 g/cm3

Unlock Deck
Unlock for access to all 43 flashcards in this deck.
Unlock Deck
k this deck
11
Identify the type of solid for argon.
A) metallic atomic solid
B) ionic solid
C) nonbonding atomic solid
D) molecular solid
E) networking atomic solid
A) metallic atomic solid
B) ionic solid
C) nonbonding atomic solid
D) molecular solid
E) networking atomic solid

Unlock Deck
Unlock for access to all 43 flashcards in this deck.
Unlock Deck
k this deck
12
Which of the following is an example of a polymer?
A) polyvinyl chloride
B) polystyrene
C) nylon
D) polyurethane
E) all of the above
A) polyvinyl chloride
B) polystyrene
C) nylon
D) polyurethane
E) all of the above

Unlock Deck
Unlock for access to all 43 flashcards in this deck.
Unlock Deck
k this deck
13
When an X-ray beam strikes the surface of a crystal with a lattice spacing of 145 nm,it produces a maximum reflection at an angle of 41.0°.What is the wavelength of the X-ray with a value of n=1?
A) 130°
B) 164°
C) 158°
D) 190°
E) 167°
A) 130°
B) 164°
C) 158°
D) 190°
E) 167°

Unlock Deck
Unlock for access to all 43 flashcards in this deck.
Unlock Deck
k this deck
14
Identify the type of solid for diamond.
A) metallic atomic solid
B) ionic solid
C) nonbonding atomic solid
D) molecular solid
E) networking atomic solid
A) metallic atomic solid
B) ionic solid
C) nonbonding atomic solid
D) molecular solid
E) networking atomic solid

Unlock Deck
Unlock for access to all 43 flashcards in this deck.
Unlock Deck
k this deck
15
Determine the radius of an Al atom (in pm)if the density of aluminum is 2.71 g/cm3.Aluminum crystallizes in a face centered cubic structure with an edge length of 2 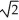 r.
r.
A) 143 pm
B) 227 pm
C) 96 pm
D) 172 pm
E) 193 pm
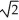 r.
r.A) 143 pm
B) 227 pm
C) 96 pm
D) 172 pm
E) 193 pm

Unlock Deck
Unlock for access to all 43 flashcards in this deck.
Unlock Deck
k this deck
16
A common use of polyurethane is
A) spray-on insulation.
B) water-protective coating.
C) foam furniture stuffing.
D) automotive parts.
E) all of the above.
A) spray-on insulation.
B) water-protective coating.
C) foam furniture stuffing.
D) automotive parts.
E) all of the above.

Unlock Deck
Unlock for access to all 43 flashcards in this deck.
Unlock Deck
k this deck
17
When an X-ray beam with a wavelength of 120 pm strikes the surface of a crystal with a lattice spacing of 165 nm,what is the maximum reflection angle with a value of n=1?
A) 54.0°
B) 21.3°
C) 32.6°
D) 18.4°
E) 38.3°
A) 54.0°
B) 21.3°
C) 32.6°
D) 18.4°
E) 38.3°

Unlock Deck
Unlock for access to all 43 flashcards in this deck.
Unlock Deck
k this deck
18
Vanadium crystallizes in a body centered cubic structure and has an atomic radius of 131 pm.Determine the density of vanadium,if the edge length of a bcc structure is 4r/ 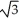 .
.
A) 3.06 g/cm3
B) 12.2 g/cm3
C) 6.11 g/cm3
D) 2.77 g/cm3
E) 8.46 g/cm3
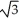 .
.A) 3.06 g/cm3
B) 12.2 g/cm3
C) 6.11 g/cm3
D) 2.77 g/cm3
E) 8.46 g/cm3

Unlock Deck
Unlock for access to all 43 flashcards in this deck.
Unlock Deck
k this deck
19
Identify the type of solid for gold.
A) metallic atomic solid
B) ionic solid
C) nonbonding atomic solid
D) molecular solid
E) networking atomic solid
A) metallic atomic solid
B) ionic solid
C) nonbonding atomic solid
D) molecular solid
E) networking atomic solid

Unlock Deck
Unlock for access to all 43 flashcards in this deck.
Unlock Deck
k this deck
20
Vanadium crystallizes in a body centered cubic structure.If the edge length of the unit cell is 301 pm,what is the radius?
A) 238 pm
B) 301 pm
C) 130 pm
D) 89.0 pm
E) 157 pm
A) 238 pm
B) 301 pm
C) 130 pm
D) 89.0 pm
E) 157 pm

Unlock Deck
Unlock for access to all 43 flashcards in this deck.
Unlock Deck
k this deck
21
Lithium crystallizes in a body-centered cubic structure.What is the coordination number of each atom?
A) 4
B) 6
C) 8
D) 12
A) 4
B) 6
C) 8
D) 12

Unlock Deck
Unlock for access to all 43 flashcards in this deck.
Unlock Deck
k this deck
22
Give the coordination number for a body-centered cubic cell.
A) 0
B) 6
C) 8
D) 10
E) 12
A) 0
B) 6
C) 8
D) 10
E) 12

Unlock Deck
Unlock for access to all 43 flashcards in this deck.
Unlock Deck
k this deck
23
Nickel has a face-centered cubic structure and has a density of 8.90 g/cm3.What is its atomic radius?
A) 125 pm
B) 249 pm
C) 353 pm
D) 997 pm
A) 125 pm
B) 249 pm
C) 353 pm
D) 997 pm

Unlock Deck
Unlock for access to all 43 flashcards in this deck.
Unlock Deck
k this deck
24
Which of the following is considered a molecular solid?
A) Au
B) NH4NO3
C) I2
D) Rn
E) None of these is a molecular solid.
A) Au
B) NH4NO3
C) I2
D) Rn
E) None of these is a molecular solid.

Unlock Deck
Unlock for access to all 43 flashcards in this deck.
Unlock Deck
k this deck
25
Which of the following is considered a nonbonding atomic solid?
A) Ne
B) Cu
C) I2
D) Ca
E) K
A) Ne
B) Cu
C) I2
D) Ca
E) K

Unlock Deck
Unlock for access to all 43 flashcards in this deck.
Unlock Deck
k this deck
26
Which type of bonding does Sr form upon solidification?
A) covalent network
B) ionic
C) metallic
D) molecular
A) covalent network
B) ionic
C) metallic
D) molecular

Unlock Deck
Unlock for access to all 43 flashcards in this deck.
Unlock Deck
k this deck
27
Which of the following is considered an atomic solid?
A) F2
B) CsBr
C) N2
D) Nb
E) None of these is an atomic solid.
A) F2
B) CsBr
C) N2
D) Nb
E) None of these is an atomic solid.

Unlock Deck
Unlock for access to all 43 flashcards in this deck.
Unlock Deck
k this deck
28
Which of the following forms a molecular solid?
A) CaO
B) C10H22
C) C, graphite
D) gold
A) CaO
B) C10H22
C) C, graphite
D) gold

Unlock Deck
Unlock for access to all 43 flashcards in this deck.
Unlock Deck
k this deck
29
What is the edge length of a face-centered cubic unit cell made up of atoms having a radius of 128 pm?
A) 181 pm
B) 362 pm
C) 512 pm
D) 1020 pm
A) 181 pm
B) 362 pm
C) 512 pm
D) 1020 pm

Unlock Deck
Unlock for access to all 43 flashcards in this deck.
Unlock Deck
k this deck
30
Gold crystallizes in a face-centered cubic structure.What is the edge length of the unit cell if the atomic radius of gold is 144 pm?
A) 204 pm
B) 288 pm
C) 333 pm
D) 407 pm
A) 204 pm
B) 288 pm
C) 333 pm
D) 407 pm

Unlock Deck
Unlock for access to all 43 flashcards in this deck.
Unlock Deck
k this deck
31
How many H- ions are around each Na+ ion in NaH,which has a cubic unit cell with H- ions on each corner and each face?
A) 1
B) 4
C) 6
D) 8
A) 1
B) 4
C) 6
D) 8

Unlock Deck
Unlock for access to all 43 flashcards in this deck.
Unlock Deck
k this deck
32
A polymer that eliminates an atom or small group of atoms is known as a(n)
A) addition polymer
B) condensation polymer
C) dimer
D) copolymer
E) substituted polymer
A) addition polymer
B) condensation polymer
C) dimer
D) copolymer
E) substituted polymer

Unlock Deck
Unlock for access to all 43 flashcards in this deck.
Unlock Deck
k this deck
33
Which of the following substances should have the highest melting point?
A) CO2
B) SrS
C) Kr
D) F2
E) MgO
A) CO2
B) SrS
C) Kr
D) F2
E) MgO

Unlock Deck
Unlock for access to all 43 flashcards in this deck.
Unlock Deck
k this deck
34
Which of the following forms an ionic solid?
A) Ag
B) C7H15NH2
C) Rb I
D) S O3
A) Ag
B) C7H15NH2
C) Rb I
D) S O3

Unlock Deck
Unlock for access to all 43 flashcards in this deck.
Unlock Deck
k this deck
35
Which of the following is considered an ionic solid?
A) (NH4)2CO3
B) CBr4
C) SeBr2
D) XeF4
E) None of these is an ionic solid.
A) (NH4)2CO3
B) CBr4
C) SeBr2
D) XeF4
E) None of these is an ionic solid.

Unlock Deck
Unlock for access to all 43 flashcards in this deck.
Unlock Deck
k this deck
36
Which of the following substances should have the highest melting point?
A) Fe
B) Ne
C) Xe
D) N2
E) CO
A) Fe
B) Ne
C) Xe
D) N2
E) CO

Unlock Deck
Unlock for access to all 43 flashcards in this deck.
Unlock Deck
k this deck
37
Cesium has a radius of 272 pm and crystallizes in a body-centered cubic structure.What is the edge length of the unit cell?
A) 314 pm
B) 385 pm
C) 544 pm
D) 628 pm
A) 314 pm
B) 385 pm
C) 544 pm
D) 628 pm

Unlock Deck
Unlock for access to all 43 flashcards in this deck.
Unlock Deck
k this deck
38
Na Cl crystallizes in a cubic unit cell with Cl- ions on each corner and each face.How many Na+ and Cl- ions are in each unit cell of Na Cl?
A) 1 Na+ ion and 1 Cl- ion
B) 2 Na+ ions and 2 Cl- ions
C) 4 Na+ ions and 4 Cl- ions
D) 8 Na+ ions and 8 Cl- ions
A) 1 Na+ ion and 1 Cl- ion
B) 2 Na+ ions and 2 Cl- ions
C) 4 Na+ ions and 4 Cl- ions
D) 8 Na+ ions and 8 Cl- ions

Unlock Deck
Unlock for access to all 43 flashcards in this deck.
Unlock Deck
k this deck
39
A common use of polyethylene is
A) insulation.
B) bottles.
C) pipe fitting.
D) film for meat packaging.
E) carpet fibers.
A) insulation.
B) bottles.
C) pipe fitting.
D) film for meat packaging.
E) carpet fibers.

Unlock Deck
Unlock for access to all 43 flashcards in this deck.
Unlock Deck
k this deck
40
Identify the copolymer.
A) nylon 6,6
B) polyvinyl chloride
C) polyethylene
D) polypropylene
E) polystyrene
A) nylon 6,6
B) polyvinyl chloride
C) polyethylene
D) polypropylene
E) polystyrene

Unlock Deck
Unlock for access to all 43 flashcards in this deck.
Unlock Deck
k this deck
41
Give the edge length in terms of r for a simple cubic cell.

Unlock Deck
Unlock for access to all 43 flashcards in this deck.
Unlock Deck
k this deck
42
What material needs to be added to portland cement in order to form concrete?
A) neon
B) nitrogen
C) lead
D) sand
E) soda ash
A) neon
B) nitrogen
C) lead
D) sand
E) soda ash

Unlock Deck
Unlock for access to all 43 flashcards in this deck.
Unlock Deck
k this deck
43
Which of the following can act as a p-type semiconductor for silicon?
A) fluorine
B) gallium
C) oxygen
D) phosphorus
E) arsenic
A) fluorine
B) gallium
C) oxygen
D) phosphorus
E) arsenic

Unlock Deck
Unlock for access to all 43 flashcards in this deck.
Unlock Deck
k this deck



