Deck 6: Chemical Bonding I
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/139
Play
Full screen (f)
Deck 6: Chemical Bonding I
1
Identify the number of bonding pairs and lone pairs of electrons in water.
A) 1 bonding pair and 1 lone pair
B) 1 bonding pair and 2 lone pairs
C) 2 bonding pairs and 2 lone pairs
D) 2 bonding pairs and 1 lone pair
E) 3 bonding pairs and 2 lone pairs
A) 1 bonding pair and 1 lone pair
B) 1 bonding pair and 2 lone pairs
C) 2 bonding pairs and 2 lone pairs
D) 2 bonding pairs and 1 lone pair
E) 3 bonding pairs and 2 lone pairs
2 bonding pairs and 2 lone pairs
2
Using periodic trends,place the following bonds in order of increasing ionic character. Si-P Si-Cl Si-S
A) Si-P < Si-Cl < Si-S
B) Si-P < Si-S < Si-Cl
C) Si-S < Si-Cl < Si-P
D) Si-Cl < Si-P < Si-S
E) Si-Cl < Si-S < Si-P
A) Si-P < Si-Cl < Si-S
B) Si-P < Si-S < Si-Cl
C) Si-S < Si-Cl < Si-P
D) Si-Cl < Si-P < Si-S
E) Si-Cl < Si-S < Si-P
Si-P < Si-S < Si-Cl
3
Give the number of valence electrons for XeI2.
A) 22
B) 20
C) 18
D) 24
E) 16
A) 22
B) 20
C) 18
D) 24
E) 16
22
4
Choose the best Lewis structure for SF4.
A)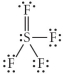
B)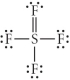
C)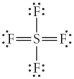
D)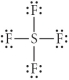
E)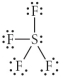
A)
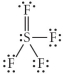
B)
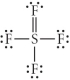
C)
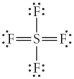
D)
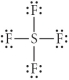
E)


Unlock Deck
Unlock for access to all 139 flashcards in this deck.
Unlock Deck
k this deck
5
Choose the bond below that is LEAST polar.
A) P-F
B) C-Br
C) C-F
D) C-I
E) C-Cl
A) P-F
B) C-Br
C) C-F
D) C-I
E) C-Cl

Unlock Deck
Unlock for access to all 139 flashcards in this deck.
Unlock Deck
k this deck
6
Identify the compound with the smallest dipole moment in the gas phase.
A) Cl2
B) ClF
C) HF
D) LiF
A) Cl2
B) ClF
C) HF
D) LiF

Unlock Deck
Unlock for access to all 139 flashcards in this deck.
Unlock Deck
k this deck
7
Give the number of valence electrons for CH2Cl2.
A) 16
B) 18
C) 20
D) 22
E) 12
A) 16
B) 18
C) 20
D) 22
E) 12

Unlock Deck
Unlock for access to all 139 flashcards in this deck.
Unlock Deck
k this deck
8
Choose the best Lewis structure for CH2Cl2.
A)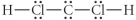
B)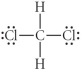
C)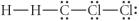
D)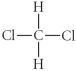
E)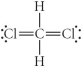
A)
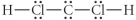
B)

C)
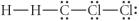
D)

E)


Unlock Deck
Unlock for access to all 139 flashcards in this deck.
Unlock Deck
k this deck
9
Identify the compound with the highest percent ionic character.
A) HF
B) IBr
C) HCl
D) LiF
A) HF
B) IBr
C) HCl
D) LiF

Unlock Deck
Unlock for access to all 139 flashcards in this deck.
Unlock Deck
k this deck
10
Identify the shortest bond.
A) single covalent bond
B) double covalent bond
C) triple covalent bond
D) All of the above bonds are the same length.
A) single covalent bond
B) double covalent bond
C) triple covalent bond
D) All of the above bonds are the same length.

Unlock Deck
Unlock for access to all 139 flashcards in this deck.
Unlock Deck
k this deck
11
Identify the compound with the largest dipole moment in the gas phase.
A) Cl2
B) ClF
C) HF
D) LiF
A) Cl2
B) ClF
C) HF
D) LiF

Unlock Deck
Unlock for access to all 139 flashcards in this deck.
Unlock Deck
k this deck
12
Choose the best Lewis structure for ICl5.
A)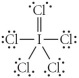
B)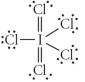
C)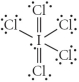
D)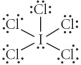
E)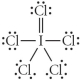
A)
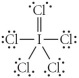
B)

C)

D)

E)


Unlock Deck
Unlock for access to all 139 flashcards in this deck.
Unlock Deck
k this deck
13
Identify the compound with the smallest percent ionic character.
A) HF
B) IBr
C) HCl
D) LiF
A) HF
B) IBr
C) HCl
D) LiF

Unlock Deck
Unlock for access to all 139 flashcards in this deck.
Unlock Deck
k this deck
14
Choose the best Lewis structure for BeF2.
A)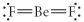
B)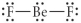
C)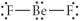
D)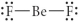
E)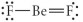
A)
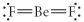
B)
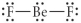
C)
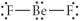
D)
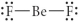
E)
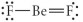

Unlock Deck
Unlock for access to all 139 flashcards in this deck.
Unlock Deck
k this deck
15
Choose the best Lewis structure for XeI2.
A)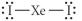
B)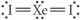
C)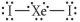
D)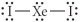
E)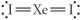
A)
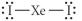
B)
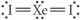
C)
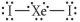
D)
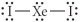
E)
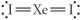

Unlock Deck
Unlock for access to all 139 flashcards in this deck.
Unlock Deck
k this deck
16
Give the number of valence electrons for ICl5.
A) 36
B) 40
C) 42
D) 44
E) 46
A) 36
B) 40
C) 42
D) 44
E) 46

Unlock Deck
Unlock for access to all 139 flashcards in this deck.
Unlock Deck
k this deck
17
Identify the weakest bond.
A) single covalent bond
B) double covalent bond
C) triple covalent bond
D) All of the above bonds are the same strength.
A) single covalent bond
B) double covalent bond
C) triple covalent bond
D) All of the above bonds are the same strength.

Unlock Deck
Unlock for access to all 139 flashcards in this deck.
Unlock Deck
k this deck
18
Identify the strongest bond.
A) single covalent bond
B) double covalent bond
C) triple covalent bond
D) All of the above bonds are the same strength.
A) single covalent bond
B) double covalent bond
C) triple covalent bond
D) All of the above bonds are the same strength.

Unlock Deck
Unlock for access to all 139 flashcards in this deck.
Unlock Deck
k this deck
19
Choose the best Lewis structure for OCl2.
A)
B)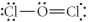
C)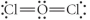
D)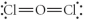
E)
A)

B)
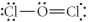
C)
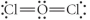
D)
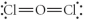
E)


Unlock Deck
Unlock for access to all 139 flashcards in this deck.
Unlock Deck
k this deck
20
Choose the bond below that is MOST polar.
A) H-I
B) H-Br
C) H-F
D) H-Cl
E) C-H
A) H-I
B) H-Br
C) H-F
D) H-Cl
E) C-H

Unlock Deck
Unlock for access to all 139 flashcards in this deck.
Unlock Deck
k this deck
21
Choose the best Lewis structure for PO43⁻.
A)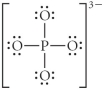
B)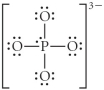
C)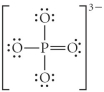
D)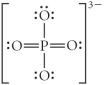
E)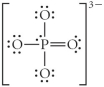
A)

B)

C)

D)

E)
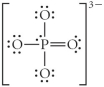

Unlock Deck
Unlock for access to all 139 flashcards in this deck.
Unlock Deck
k this deck
22
Draw the best Lewis structure for CH3+1.What is the formal charge on the C?
A) 0
B) 1
C) -1
D) 2
A) 0
B) 1
C) -1
D) 2

Unlock Deck
Unlock for access to all 139 flashcards in this deck.
Unlock Deck
k this deck
23
Draw the Lewis structure for NO2⁻ including any valid resonance structures.Which of the following statements is TRUE?
A) The nitrite ion contains one N-O single bond and one N O double bond.
O double bond.
B) The nitrite ion contains two N-O bonds that are equivalent to 1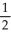 bonds.
bonds.
C) The nitrite ion contains two N O double bonds.
O double bonds.
D) The nitrite ion contains two N-O single bonds.
E) None of the above are true.
A) The nitrite ion contains one N-O single bond and one N
 O double bond.
O double bond.B) The nitrite ion contains two N-O bonds that are equivalent to 1
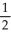 bonds.
bonds.C) The nitrite ion contains two N
 O double bonds.
O double bonds.D) The nitrite ion contains two N-O single bonds.
E) None of the above are true.

Unlock Deck
Unlock for access to all 139 flashcards in this deck.
Unlock Deck
k this deck
24
Give the number of valence electrons for SO42-.
A) 32
B) 30
C) 34
D) 28
E) 36
A) 32
B) 30
C) 34
D) 28
E) 36

Unlock Deck
Unlock for access to all 139 flashcards in this deck.
Unlock Deck
k this deck
25
Choose the best Lewis structure for SeO42⁻.
A)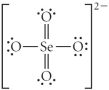
B)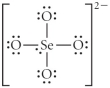
C)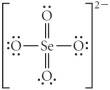
D)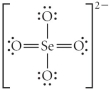
E)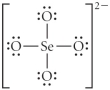
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 139 flashcards in this deck.
Unlock Deck
k this deck
26
Which of the following resonance structures for OCN⁻ will contribute most to the correct structure of OCN⁻?
A) O(2 lone pairs) C
C  N (2 lone pairs)
N (2 lone pairs)
B) O(1 lone pair) C-N(3 lone pairs)
C-N(3 lone pairs)
C) O(1 lone pair) C(2 lone pairs)
C(2 lone pairs)  N(1 lone pair)
N(1 lone pair)
D) O(3 lone pairs)-C N(with 1 lone pair)
N(with 1 lone pair)
E) They all contribute equally to the correct structure of OCN⁻.
A) O(2 lone pairs)
 C
C  N (2 lone pairs)
N (2 lone pairs)B) O(1 lone pair)
 C-N(3 lone pairs)
C-N(3 lone pairs)C) O(1 lone pair)
 C(2 lone pairs)
C(2 lone pairs)  N(1 lone pair)
N(1 lone pair)D) O(3 lone pairs)-C
 N(with 1 lone pair)
N(with 1 lone pair)E) They all contribute equally to the correct structure of OCN⁻.

Unlock Deck
Unlock for access to all 139 flashcards in this deck.
Unlock Deck
k this deck
27
Draw the Lewis structure for CO32- including any valid resonance structures.Which of the following statements is TRUE?
A) The CO32- ion contains one C-O single bond and two C O double bonds.
O double bonds.
B) The CO32- ion contains two C-O single bonds and one C O double bond.
O double bond.
C) The CO32- ion contains three C-O double bonds.
D) The CO32- ion contains two C-O single bonds and one C O triple bond.
O triple bond.
E) None of the above are true.
A) The CO32- ion contains one C-O single bond and two C
 O double bonds.
O double bonds.B) The CO32- ion contains two C-O single bonds and one C
 O double bond.
O double bond.C) The CO32- ion contains three C-O double bonds.
D) The CO32- ion contains two C-O single bonds and one C
 O triple bond.
O triple bond.E) None of the above are true.

Unlock Deck
Unlock for access to all 139 flashcards in this deck.
Unlock Deck
k this deck
28
Using Lewis structures and formal charge,which of the following ions is most stable?
OCN⁻ ONC⁻ NOC⁻
A) OCN⁻
B) ONC⁻
C) NOC⁻
D) None of these ions are stable according to Lewis theory.
E) All of these compounds are equally stable according to Lewis theory.
OCN⁻ ONC⁻ NOC⁻
A) OCN⁻
B) ONC⁻
C) NOC⁻
D) None of these ions are stable according to Lewis theory.
E) All of these compounds are equally stable according to Lewis theory.

Unlock Deck
Unlock for access to all 139 flashcards in this deck.
Unlock Deck
k this deck
29
Identify the compound with atoms that have an incomplete octet.
A) ICl5
B) CO2
C) BF3
D) Cl2
E) CO
A) ICl5
B) CO2
C) BF3
D) Cl2
E) CO

Unlock Deck
Unlock for access to all 139 flashcards in this deck.
Unlock Deck
k this deck
30
Choose the best Lewis structure for NH4⁺.
A)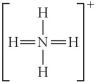
B)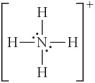
C)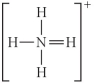
D)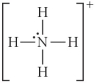
E)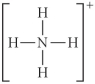
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 139 flashcards in this deck.
Unlock Deck
k this deck
31
Which compound has the longest carbon-carbon bond length?
A) CH3CH3
B) CH2CH2
C) HCCH
D) All bond lengths are the same.
A) CH3CH3
B) CH2CH2
C) HCCH
D) All bond lengths are the same.

Unlock Deck
Unlock for access to all 139 flashcards in this deck.
Unlock Deck
k this deck
32
Choose the best Lewis structure for NO3⁻.
A)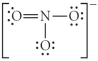
B)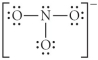
C)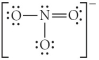
D)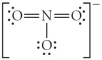
E)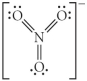
A)
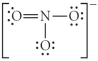
B)
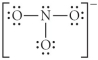
C)
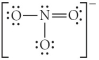
D)
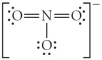
E)


Unlock Deck
Unlock for access to all 139 flashcards in this deck.
Unlock Deck
k this deck
33
Draw the best Lewis structure for the free radical,NO2.What is the formal charge on the N?
A) 0
B) +1
C) -1
D) +2
E) -2
A) 0
B) +1
C) -1
D) +2
E) -2

Unlock Deck
Unlock for access to all 139 flashcards in this deck.
Unlock Deck
k this deck
34
Which compound has the shortest carbon-carbon bond length?
A) CH3CH3
B) CH2CH2
C) HCCH
D) All bond lengths are the same.
A) CH3CH3
B) CH2CH2
C) HCCH
D) All bond lengths are the same.

Unlock Deck
Unlock for access to all 139 flashcards in this deck.
Unlock Deck
k this deck
35
Draw the best Lewis structure for BrO4⁻ and determine the formal charge on bromine.
A) -1
B) +1
C) 0
D) +2
E) +3
A) -1
B) +1
C) 0
D) +2
E) +3

Unlock Deck
Unlock for access to all 139 flashcards in this deck.
Unlock Deck
k this deck
36
Draw the best Lewis structure for Cl3⁻.What is the formal charge on the central Cl atom?
A) -1
B) 0
C) +1
D) +2
E) -2
A) -1
B) 0
C) +1
D) +2
E) -2

Unlock Deck
Unlock for access to all 139 flashcards in this deck.
Unlock Deck
k this deck
37
Draw the best Lewis structure for CH3-1.What is the formal charge on the C?
A) 0
B) 1
C) -1
D) 2
A) 0
B) 1
C) -1
D) 2

Unlock Deck
Unlock for access to all 139 flashcards in this deck.
Unlock Deck
k this deck
38
Draw the Lewis structure for SO42⁻.How many equivalent resonance structures can be drawn?
A) 6
B) 2
C) 4
D) 3
E) 8
A) 6
B) 2
C) 4
D) 3
E) 8

Unlock Deck
Unlock for access to all 139 flashcards in this deck.
Unlock Deck
k this deck
39
Choose the best Lewis structure for BF3.
A)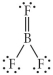
B)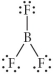
C)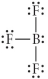
D)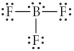
E)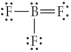
A)
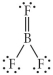
B)

C)
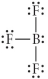
D)
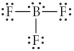
E)
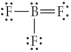

Unlock Deck
Unlock for access to all 139 flashcards in this deck.
Unlock Deck
k this deck
40
Choose the best Lewis structure for SO42⁻.
A)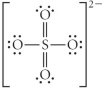
B)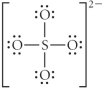
C)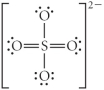
D)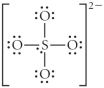
E)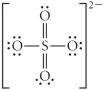
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 139 flashcards in this deck.
Unlock Deck
k this deck
41
Determine the electron geometry (eg)and molecular geometry (mg)of XeF2.
A) eg = trigonal bipyramidal, mg = bent
B) eg = linear, mg = linear
C) eg = tetrahedral, mg = linear
D) eg = trigonal bipyramidal, mg = linear
E) eg = tetrahedral, mg = bent
A) eg = trigonal bipyramidal, mg = bent
B) eg = linear, mg = linear
C) eg = tetrahedral, mg = linear
D) eg = trigonal bipyramidal, mg = linear
E) eg = tetrahedral, mg = bent

Unlock Deck
Unlock for access to all 139 flashcards in this deck.
Unlock Deck
k this deck
42
Determine the electron geometry (eg)and molecular geometry (mg)of CO32⁻.
A) eg = tetrahedral, mg = tetrahedral
B) eg = tetrahedral, mg = trigonal pyramidal
C) eg = trigonal planar, mg = bent
D) eg = trigonal planar, mg = trigonal planar
E) eg = tetrahedral, mg = trigonal planar
A) eg = tetrahedral, mg = tetrahedral
B) eg = tetrahedral, mg = trigonal pyramidal
C) eg = trigonal planar, mg = bent
D) eg = trigonal planar, mg = trigonal planar
E) eg = tetrahedral, mg = trigonal planar

Unlock Deck
Unlock for access to all 139 flashcards in this deck.
Unlock Deck
k this deck
43
Place the following in order of increasing bond length. C-F C-S C-Cl
A) C-S < C-Cl < C-F
B) C-Cl < C-F < C-S
C) C-F < C-S < C-Cl
D) C-F < C-Cl < C-S
E) C-S < C-F < C-Cl
A) C-S < C-Cl < C-F
B) C-Cl < C-F < C-S
C) C-F < C-S < C-Cl
D) C-F < C-Cl < C-S
E) C-S < C-F < C-Cl

Unlock Deck
Unlock for access to all 139 flashcards in this deck.
Unlock Deck
k this deck
44
Which compound has the highest carbon-carbon bond strength?
A) CH3CH3
B) CH2CH2
C) HCCH
D) All bond strengths are the same.
A) CH3CH3
B) CH2CH2
C) HCCH
D) All bond strengths are the same.

Unlock Deck
Unlock for access to all 139 flashcards in this deck.
Unlock Deck
k this deck
45
Determine the electron geometry (eg)and molecular geometry (mg)of ICl2⁻.
A) eg = tetrahedral, mg = bent
B) eg = tetrahedral, mg = trigonal pyramidal
C) eg = trigonal bipyramidal, mg = linear
D) eg = trigonal bipyramidal, mg = trigonal planar
E) eg = octahedral, mg = linear
A) eg = tetrahedral, mg = bent
B) eg = tetrahedral, mg = trigonal pyramidal
C) eg = trigonal bipyramidal, mg = linear
D) eg = trigonal bipyramidal, mg = trigonal planar
E) eg = octahedral, mg = linear

Unlock Deck
Unlock for access to all 139 flashcards in this deck.
Unlock Deck
k this deck
46
The bond angle in NH3 is
A) 107°
B) 104.5°
C) 120°
D) 109.5°
E) 95°
A) 107°
B) 104.5°
C) 120°
D) 109.5°
E) 95°

Unlock Deck
Unlock for access to all 139 flashcards in this deck.
Unlock Deck
k this deck
47
Place the following in order of decreasing XO bond length,where "X" represents the central atom in each of the following compounds or ions. SiO32⁻ CO2 CO32⁻
A) CO2 > SiO32⁻ > CO32⁻
B) CO2 > CO32⁻ > SiO32⁻
C) CO32⁻ > CO2 > SiO32⁻
D) CO32⁻ > SiO32⁻ > CO2
E) SiO32⁻ > CO32⁻ > CO2
A) CO2 > SiO32⁻ > CO32⁻
B) CO2 > CO32⁻ > SiO32⁻
C) CO32⁻ > CO2 > SiO32⁻
D) CO32⁻ > SiO32⁻ > CO2
E) SiO32⁻ > CO32⁻ > CO2

Unlock Deck
Unlock for access to all 139 flashcards in this deck.
Unlock Deck
k this deck
48
Determine the electron geometry (eg)and molecular geometry (mg)of PF5.
A) eg = trigonal bipyramidal, mg = trigonal bipyramidal
B) eg = octahedral, mg = octahedral
C) eg = trigonal bipyramidal, mg = tetrahedral
D) eg = tetrahedral, mg = trigonal pyramidal
E) eg = trigonal planar, mg = octahedral
A) eg = trigonal bipyramidal, mg = trigonal bipyramidal
B) eg = octahedral, mg = octahedral
C) eg = trigonal bipyramidal, mg = tetrahedral
D) eg = tetrahedral, mg = trigonal pyramidal
E) eg = trigonal planar, mg = octahedral

Unlock Deck
Unlock for access to all 139 flashcards in this deck.
Unlock Deck
k this deck
49
Determine the electron geometry (eg)and molecular geometry (mg)of CO2.
A) eg = tetrahedral, mg = tetrahedral
B) eg = linear, mg = trigonal planar
C) eg = trigonal planar, mg = bent
D) eg = linear, mg = linear
E) eg = trigonal planar, mg = trigonal planar
A) eg = tetrahedral, mg = tetrahedral
B) eg = linear, mg = trigonal planar
C) eg = trigonal planar, mg = bent
D) eg = linear, mg = linear
E) eg = trigonal planar, mg = trigonal planar

Unlock Deck
Unlock for access to all 139 flashcards in this deck.
Unlock Deck
k this deck
50
Determine the electron geometry (eg)and molecular geometry (mg)of NCl3.
A) eg = tetrahedral, mg = tetrahedral
B) eg = linear, mg = trigonal planar
C) eg = trigonal planar, mg = bent
D) eg = linear, mg = linear
E) eg = tetrahedral, mg = trigonal pyramidal
A) eg = tetrahedral, mg = tetrahedral
B) eg = linear, mg = trigonal planar
C) eg = trigonal planar, mg = bent
D) eg = linear, mg = linear
E) eg = tetrahedral, mg = trigonal pyramidal

Unlock Deck
Unlock for access to all 139 flashcards in this deck.
Unlock Deck
k this deck
51
Determine the electron geometry (eg)and molecular geometry (mg)of CH3+1.
A) eg = tetrahedral, mg = tetrahedral
B) eg = tetrahedral, mg = trigonal pyramidal
C) eg = trigonal planar, mg = bent
D) eg = trigonal planar, mg = trigonal planar
E) eg = tetrahedral, mg = trigonal planar
A) eg = tetrahedral, mg = tetrahedral
B) eg = tetrahedral, mg = trigonal pyramidal
C) eg = trigonal planar, mg = bent
D) eg = trigonal planar, mg = trigonal planar
E) eg = tetrahedral, mg = trigonal planar

Unlock Deck
Unlock for access to all 139 flashcards in this deck.
Unlock Deck
k this deck
52
Determine the electron geometry (eg)and molecular geometry (mg)of Br F3.
A) eg = trigonal planar, mg = trigonal planar
B) eg = trigonal bipyramidal, mg = T-shape
C) eg = trigonal planar, mg = bent
D) eg = trigonal bipyramidal, mg = see-saw
E) eg = tetrahedral, mg = trigonal pyramidal
A) eg = trigonal planar, mg = trigonal planar
B) eg = trigonal bipyramidal, mg = T-shape
C) eg = trigonal planar, mg = bent
D) eg = trigonal bipyramidal, mg = see-saw
E) eg = tetrahedral, mg = trigonal pyramidal

Unlock Deck
Unlock for access to all 139 flashcards in this deck.
Unlock Deck
k this deck
53
Identify the bond with the highest bond energy.
A) Si = O
B) N = N
C) C = C
D) C = N
E) O = O
A) Si = O
B) N = N
C) C = C
D) C = N
E) O = O

Unlock Deck
Unlock for access to all 139 flashcards in this deck.
Unlock Deck
k this deck
54
Place the following in order of increasing bond length. NO2⁻ NO3⁻ NO
A) NO < NO2⁻ < NO3⁻
B) NO2⁻ < NO3⁻ < NO
C) NO3⁻ < NO < NO2⁻
D) NO < NO3⁻ < NO2⁻
E) NO3⁻ < NO2⁻ < NO
A) NO < NO2⁻ < NO3⁻
B) NO2⁻ < NO3⁻ < NO
C) NO3⁻ < NO < NO2⁻
D) NO < NO3⁻ < NO2⁻
E) NO3⁻ < NO2⁻ < NO

Unlock Deck
Unlock for access to all 139 flashcards in this deck.
Unlock Deck
k this deck
55
Determine the electron geometry (eg)and molecular geometry (mg)of SiF4.
A) eg = tetrahedral, mg = trigonal pyramidal
B) eg = octahedral, mg = square planar
C) eg = trigonal bipyramidal, mg = trigonal pyramidal
D) eg = tetrahedral, mg = bent
E) eg = tetrahedral, mg = tetrahedral
A) eg = tetrahedral, mg = trigonal pyramidal
B) eg = octahedral, mg = square planar
C) eg = trigonal bipyramidal, mg = trigonal pyramidal
D) eg = tetrahedral, mg = bent
E) eg = tetrahedral, mg = tetrahedral

Unlock Deck
Unlock for access to all 139 flashcards in this deck.
Unlock Deck
k this deck
56
Determine the electron geometry (eg)and molecular geometry(mg)of BCl3.
A) eg = trigonal planar, mg = trigonal planar
B) eg = tetrahedral, mg = trigonal planar
C) eg = tetrahedral, mg = trigonal pyramidal
D) eg = trigonal planar, mg = bent
E) eg = trigonal bipyramidal, mg = trigonal bipyramidal
A) eg = trigonal planar, mg = trigonal planar
B) eg = tetrahedral, mg = trigonal planar
C) eg = tetrahedral, mg = trigonal pyramidal
D) eg = trigonal planar, mg = bent
E) eg = trigonal bipyramidal, mg = trigonal bipyramidal

Unlock Deck
Unlock for access to all 139 flashcards in this deck.
Unlock Deck
k this deck
57
Give the approximate bond angle for a molecule with an octahedral shape.
A) 109.5°
B) 180°
C) 120°
D) 105°
E) 90°
A) 109.5°
B) 180°
C) 120°
D) 105°
E) 90°

Unlock Deck
Unlock for access to all 139 flashcards in this deck.
Unlock Deck
k this deck
58
The bond angle in H2O is
A) 107°
B) 104.5°
C) 120°
D) 109.5°
E) 95°
A) 107°
B) 104.5°
C) 120°
D) 109.5°
E) 95°

Unlock Deck
Unlock for access to all 139 flashcards in this deck.
Unlock Deck
k this deck
59
Rank the following molecules in decreasing bond energy. Cl2 Br2 F2 I2
A) I2 > Br2 > Cl2 > F2
B) Cl2 > Br2 > F2 > I2
C) I2 > Cl2 > Br2 > F2
D) Cl2 > I2 > F2 > Br2
A) I2 > Br2 > Cl2 > F2
B) Cl2 > Br2 > F2 > I2
C) I2 > Cl2 > Br2 > F2
D) Cl2 > I2 > F2 > Br2

Unlock Deck
Unlock for access to all 139 flashcards in this deck.
Unlock Deck
k this deck
60
Place the following in order of decreasing bond length. H-F H-I H-Br
A) H-F > H-Br > H-I
B) H-I > H-F > H-Br
C) H-I > H-Br > H-F
D) H-Br > H-F > H-I
E) H-F > H-I > H-Br
A) H-F > H-Br > H-I
B) H-I > H-F > H-Br
C) H-I > H-Br > H-F
D) H-Br > H-F > H-I
E) H-F > H-I > H-Br

Unlock Deck
Unlock for access to all 139 flashcards in this deck.
Unlock Deck
k this deck
61
Determine the electron geometry (eg)and molecular geometry (mg)of the underlined atom CH3OCH3.
A) eg = tetrahedral, mg = tetrahedral
B) eg = linear, eg = linear
C) eg = tetrahedral, mg = bent
D) eg = trigonal bipyramidal, mg = tetrahedral
E) eg = octahedral, mg = square planar
A) eg = tetrahedral, mg = tetrahedral
B) eg = linear, eg = linear
C) eg = tetrahedral, mg = bent
D) eg = trigonal bipyramidal, mg = tetrahedral
E) eg = octahedral, mg = square planar

Unlock Deck
Unlock for access to all 139 flashcards in this deck.
Unlock Deck
k this deck
62
Determine the electron geometry (eg),molecular geometry (mg),and polarity of SO2.
A) eg = tetrahedral, mg = bent, polar
B) eg = trigonal planar, mg = bent, polar
C) eg = linear, mg = linear, nonpolar
D) eg = tetrahedral, mg = tetrahedral, nonpolar
E) eg = trigonal pyramidal, mg = trigonal pyramidal, polar
A) eg = tetrahedral, mg = bent, polar
B) eg = trigonal planar, mg = bent, polar
C) eg = linear, mg = linear, nonpolar
D) eg = tetrahedral, mg = tetrahedral, nonpolar
E) eg = trigonal pyramidal, mg = trigonal pyramidal, polar

Unlock Deck
Unlock for access to all 139 flashcards in this deck.
Unlock Deck
k this deck
63
Place the following in order of increasing X-A-X bond angle,where A represents the central atom and X represents the outer atoms in each molecule. HCN H2O H3O⁺
A) HCN < H2O < H3O⁺
B) H3O⁺ < H2O < HCN
C) HCN < H3O⁺ < H2O
D) H2O < HCN < H3O⁺
E) H2O < H3O⁺ < HCN
A) HCN < H2O < H3O⁺
B) H3O⁺ < H2O < HCN
C) HCN < H3O⁺ < H2O
D) H2O < HCN < H3O⁺
E) H2O < H3O⁺ < HCN

Unlock Deck
Unlock for access to all 139 flashcards in this deck.
Unlock Deck
k this deck
64
Determine the electron geometry (eg)and molecular geometry (mg)of XeF4.
A) eg = tetrahedral, mg = tetrahedral
B) eg = linear, eg = linear
C) eg = tetrahedral, mg = bent
D) eg = trigonal bipyramidal, mg = tetrahedral
E) eg = octahedral, mg = square planar
A) eg = tetrahedral, mg = tetrahedral
B) eg = linear, eg = linear
C) eg = tetrahedral, mg = bent
D) eg = trigonal bipyramidal, mg = tetrahedral
E) eg = octahedral, mg = square planar

Unlock Deck
Unlock for access to all 139 flashcards in this deck.
Unlock Deck
k this deck
65
Place the following in order of increasing dipole moment. I.BCl3 II.BIF2 III.BClF2
A) I < II = III
B) II < III < I
C) I < II < III
D) II < I < III
E) I < III < II
A) I < II = III
B) II < III < I
C) I < II < III
D) II < I < III
E) I < III < II

Unlock Deck
Unlock for access to all 139 flashcards in this deck.
Unlock Deck
k this deck
66
Determine the electron geometry,molecular geometry and polarity of SF6.
A) eg = trigonal bipyramidal, mg = trigonal bipyramidal, nonpolar
B) eg = tetrahedral, mg = tetrahedral, polar
C) eg = trigonal bipyramidal, mg = see-saw, polar
D) eg = octahedral, mg = trigonal bipyramidal, nonpolar
E) eg = octahedral, mg = octahedral, nonpolar
A) eg = trigonal bipyramidal, mg = trigonal bipyramidal, nonpolar
B) eg = tetrahedral, mg = tetrahedral, polar
C) eg = trigonal bipyramidal, mg = see-saw, polar
D) eg = octahedral, mg = trigonal bipyramidal, nonpolar
E) eg = octahedral, mg = octahedral, nonpolar

Unlock Deck
Unlock for access to all 139 flashcards in this deck.
Unlock Deck
k this deck
67
Determine the electron geometry (eg),molecular geometry (mg),and polarity of SO3.
A) eg = tetrahedral, mg = trigonal pyramidal, polar
B) eg = tetrahedral, mg = tetrahedral, nonpolar
C) eg = trigonal planar, mg = trigonal planar, nonpolar
D) eg = trigonal bipyramidal, mg = trigonal planar, polar
E) eg = trigonal pyramidal, mg = bent, nonpolar
A) eg = tetrahedral, mg = trigonal pyramidal, polar
B) eg = tetrahedral, mg = tetrahedral, nonpolar
C) eg = trigonal planar, mg = trigonal planar, nonpolar
D) eg = trigonal bipyramidal, mg = trigonal planar, polar
E) eg = trigonal pyramidal, mg = bent, nonpolar

Unlock Deck
Unlock for access to all 139 flashcards in this deck.
Unlock Deck
k this deck
68
Determine the electron geometry,molecular geometry and polarity of HBrO2.
A) eg = trigonal bipyramidal, mg = trigonal planar, nonpolar
B) eg = octahedral, mg = square planar, nonpolar
C) eg = tetrahedral, mg = bent, polar
D) eg = tetrahedral, mg = linear, nonpolar
E) eg = linear, mg = linear, polar
A) eg = trigonal bipyramidal, mg = trigonal planar, nonpolar
B) eg = octahedral, mg = square planar, nonpolar
C) eg = tetrahedral, mg = bent, polar
D) eg = tetrahedral, mg = linear, nonpolar
E) eg = linear, mg = linear, polar

Unlock Deck
Unlock for access to all 139 flashcards in this deck.
Unlock Deck
k this deck
69
Determine the electron geometry (eg),molecular geometry (mg),and polarity of PCl3.
A) eg = tetrahedral, mg = bent, polar
B) eg = trigonal planar, mg = trigonal planar, nonpolar
C) eg = linear, mg = linear, nonpolar
D) eg = tetrahedral, mg = trigonal pyramidal, polar
E) eg = trigonal pyramidal, mg = trigonal pyramidal, polar
A) eg = tetrahedral, mg = bent, polar
B) eg = trigonal planar, mg = trigonal planar, nonpolar
C) eg = linear, mg = linear, nonpolar
D) eg = tetrahedral, mg = trigonal pyramidal, polar
E) eg = trigonal pyramidal, mg = trigonal pyramidal, polar

Unlock Deck
Unlock for access to all 139 flashcards in this deck.
Unlock Deck
k this deck
70
Determine the electron geometry (eg)and molecular geometry (mg)of the underlined atom H2CO.
A) eg = tetrahedral, mg = tetrahedral
B) eg = trigonal planar, eg = trigonal planar
C) eg = tetrahedral, mg = bent
D) eg = trigonal bipyramidal, mg = tetrahedral
E) eg = octahedral, mg = square planar
A) eg = tetrahedral, mg = tetrahedral
B) eg = trigonal planar, eg = trigonal planar
C) eg = tetrahedral, mg = bent
D) eg = trigonal bipyramidal, mg = tetrahedral
E) eg = octahedral, mg = square planar

Unlock Deck
Unlock for access to all 139 flashcards in this deck.
Unlock Deck
k this deck
71
How many of the following molecules are polar?
PCl5 COS XeO3 SeBr2
A) 2
B) 0
C) 1
D) 3
E) 4
PCl5 COS XeO3 SeBr2
A) 2
B) 0
C) 1
D) 3
E) 4

Unlock Deck
Unlock for access to all 139 flashcards in this deck.
Unlock Deck
k this deck
72
Consider the molecule below.Determine the molecular geometry at each of the 3 labeled atoms. 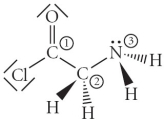
A) 1 = trigonal planar, 2 = tetrahedral, 3 = trigonal pyramidal
B) 1 = tetrahedral, 2 = tetrahedral, 3 = tetrahedral
C) 1 = trigonal planar, 2 = tetrahedral, 3 = tetrahedral
D) 1 = tetrahedral, 2 = tetrahedral, 3 = trigonal planar
E) 1 = trigonal planar, 2 = trigonal pyramidal, 3 = trigonal pyramidal

A) 1 = trigonal planar, 2 = tetrahedral, 3 = trigonal pyramidal
B) 1 = tetrahedral, 2 = tetrahedral, 3 = tetrahedral
C) 1 = trigonal planar, 2 = tetrahedral, 3 = tetrahedral
D) 1 = tetrahedral, 2 = tetrahedral, 3 = trigonal planar
E) 1 = trigonal planar, 2 = trigonal pyramidal, 3 = trigonal pyramidal

Unlock Deck
Unlock for access to all 139 flashcards in this deck.
Unlock Deck
k this deck
73
Consider the molecule below.Determine the molecular geometry at each of the 2 labeled carbons. 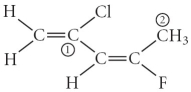
A) C1 = tetrahedral, C2 = linear
B) C1 = trigonal planar, C2 = bent
C) C1 = bent, C2 = trigonal planar
D) C1 = trigonal planar, C2 = tetrahedral
E) C1 = trigonal pyramidal, C2 = see-saw

A) C1 = tetrahedral, C2 = linear
B) C1 = trigonal planar, C2 = bent
C) C1 = bent, C2 = trigonal planar
D) C1 = trigonal planar, C2 = tetrahedral
E) C1 = trigonal pyramidal, C2 = see-saw

Unlock Deck
Unlock for access to all 139 flashcards in this deck.
Unlock Deck
k this deck
74
Place the following in order of decreasing X-A-X bond angle,where A represents the central atom and X represents the outer atoms in each molecule. CS2 CF4 SCl2
A) CS2 > SCl2 > CF4
B) SCl2 > CF4 > CS2
C) CF4 > SCl2 > CS2
D) CS2 > CF4 > SCl2
E) CF4 > CS2 > SCl2
A) CS2 > SCl2 > CF4
B) SCl2 > CF4 > CS2
C) CF4 > SCl2 > CS2
D) CS2 > CF4 > SCl2
E) CF4 > CS2 > SCl2

Unlock Deck
Unlock for access to all 139 flashcards in this deck.
Unlock Deck
k this deck
75
Place the following in order of decreasing X-A-X bond angle,where A represents the central atom and X represents the outer atoms in each molecule. N2O NCl3 NO2⁻
A) NCl3 > NO2⁻ > N2O
B) NO2⁻ > N2O > NCl3
C) N2O > NO2⁻ > NCl3
D) NCl3 > N2O > NO2⁻
E) N2O > NCl3 > NO2⁻
A) NCl3 > NO2⁻ > N2O
B) NO2⁻ > N2O > NCl3
C) N2O > NO2⁻ > NCl3
D) NCl3 > N2O > NO2⁻
E) N2O > NCl3 > NO2⁻

Unlock Deck
Unlock for access to all 139 flashcards in this deck.
Unlock Deck
k this deck
76
Place the following in order of increasing F-A-F bond angle,where A represents the central atom in each molecule. PF3 OF2 PF4⁺
A) PF3 < OF2 < PF4⁺
B) OF2 < PF3 < PF4⁺
C) OF2 < PF4⁺ < PF3
D) PF4⁺ < OF2 < PF3
E) PF4⁺ < PF3 < OF2
A) PF3 < OF2 < PF4⁺
B) OF2 < PF3 < PF4⁺
C) OF2 < PF4⁺ < PF3
D) PF4⁺ < OF2 < PF3
E) PF4⁺ < PF3 < OF2

Unlock Deck
Unlock for access to all 139 flashcards in this deck.
Unlock Deck
k this deck
77
Determine the electron geometry (eg)and molecular geometry (mg)of the underlined atom CH3OCH3.
A) eg = tetrahedral, mg = tetrahedral
B) eg = linear, eg = linear
C) eg = tetrahedral, mg = bent
D) eg = trigonal bipyramidal, mg = tetrahedral
E) eg = octahedral, mg = square planar
A) eg = tetrahedral, mg = tetrahedral
B) eg = linear, eg = linear
C) eg = tetrahedral, mg = bent
D) eg = trigonal bipyramidal, mg = tetrahedral
E) eg = octahedral, mg = square planar

Unlock Deck
Unlock for access to all 139 flashcards in this deck.
Unlock Deck
k this deck
78
Determine the electron geometry (eg),molecular geometry(mg)and polarity of XeO3.
A) eg = trigonal planar, mg = trigonal planar, nonpolar
B) eg = tetrahedral, mg = trigonal pyramidal, polar
C) eg = trigonal planar, mg = trigonal pyramidal, polar
D) eg = trigonal bipyramidal, mg = trigonal planar, nonpolar
E) eg = octahedral, mg = tetrahedral, nonpolar
A) eg = trigonal planar, mg = trigonal planar, nonpolar
B) eg = tetrahedral, mg = trigonal pyramidal, polar
C) eg = trigonal planar, mg = trigonal pyramidal, polar
D) eg = trigonal bipyramidal, mg = trigonal planar, nonpolar
E) eg = octahedral, mg = tetrahedral, nonpolar

Unlock Deck
Unlock for access to all 139 flashcards in this deck.
Unlock Deck
k this deck
79
Place the following in order of increasing X-Se-X bond angle,where X represents the outer atoms in each molecule. SeO2 SeCl6 SeF2
A) SeCl6 < SeF2 < SeO2
B) SeF2 < SeO2 < SeCl6
C) SeF2 < SeCl6 < SeO2
D) SeO2 < SeF2 < SeCl6
E) SeCl6 < SeO2 < SeF2
A) SeCl6 < SeF2 < SeO2
B) SeF2 < SeO2 < SeCl6
C) SeF2 < SeCl6 < SeO2
D) SeO2 < SeF2 < SeCl6
E) SeCl6 < SeO2 < SeF2

Unlock Deck
Unlock for access to all 139 flashcards in this deck.
Unlock Deck
k this deck
80
How many of the following molecules are polar?
BrCl3 CS2 SiF4 SO3
A) 1
B) 2
C) 3
D) 4
E) 0
BrCl3 CS2 SiF4 SO3
A) 1
B) 2
C) 3
D) 4
E) 0

Unlock Deck
Unlock for access to all 139 flashcards in this deck.
Unlock Deck
k this deck



