Deck 2: The Chemistry of Life
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/76
Play
Full screen (f)
Deck 2: The Chemistry of Life
1
The mass number of an atom is defined as:
A)The total number of protons, neutrons, and electrons of an atom
B)The total number of protons and electrons of an atom
C)The total number of protons and neutrons of an atom
D)The total number of neutrons and electrons of an atom
E)The total number of protons of an atom
A)The total number of protons, neutrons, and electrons of an atom
B)The total number of protons and electrons of an atom
C)The total number of protons and neutrons of an atom
D)The total number of neutrons and electrons of an atom
E)The total number of protons of an atom
C
2
A base:
A)Has a value of 7 on the pH scale
B)Is a chemical that adds hydrogen ions to a solution
C)Is a chemical that absorbs hydrogen ions from a solution
D)Has a value below 7 on the pH scale
A)Has a value of 7 on the pH scale
B)Is a chemical that adds hydrogen ions to a solution
C)Is a chemical that absorbs hydrogen ions from a solution
D)Has a value below 7 on the pH scale
C
3
In a chemical equation:
A)The reactants are on the right of the yields arrow
B)Reactants and products are on both sides of the yields arrow
C)The products are on the left of the yields arrow
D)The reactants are on the left of the yields arrow
E)The number of atoms of each element may be different on the two sides of the yields arrow
A)The reactants are on the right of the yields arrow
B)Reactants and products are on both sides of the yields arrow
C)The products are on the left of the yields arrow
D)The reactants are on the left of the yields arrow
E)The number of atoms of each element may be different on the two sides of the yields arrow
D
4
The second energy shell of an atom contains a maximum of:
A)Eight electrons
B)Two electrons
C)Four electrons
D)One electron
E)Sixteen electrons
A)Eight electrons
B)Two electrons
C)Four electrons
D)One electron
E)Sixteen electrons

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
5
Ice floats on water because:
A)The molecules are closer together in ice than in water
B)The molecules are farther apart in ice than in water
C)Ice is more dense than water
A)The molecules are closer together in ice than in water
B)The molecules are farther apart in ice than in water
C)Ice is more dense than water

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
6
Evaporation is:
A)The conversion of a liquid into a vapor
B)The conversion of a solid into a vapor
C)The conversion of a vapor into a liquid
D)The conversion of a vapor into a solid
E)All are correct
A)The conversion of a liquid into a vapor
B)The conversion of a solid into a vapor
C)The conversion of a vapor into a liquid
D)The conversion of a vapor into a solid
E)All are correct

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
7
If an atom has a valence shell that is full it:
A)Is highly reactive
B)Is not chemically stable
C)Is highly likely to combine with other atoms
D)Is not inert
E)Is chemically stable
A)Is highly reactive
B)Is not chemically stable
C)Is highly likely to combine with other atoms
D)Is not inert
E)Is chemically stable

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
8
The first energy shell of an atom contains a maximum of:
A)One electron
B)Two electrons
C)Four electrons
D)Eight electrons
E)Sixteen electrons
A)One electron
B)Two electrons
C)Four electrons
D)Eight electrons
E)Sixteen electrons

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
9
The atomic number of an atom or element is:
A)The number of neutrons in the nucleus
B)The number of electrons in the nucleus
C)The number of protons in the nucleus
D)The number of neutrons in the orbitals
E)The number of protons in the orbitals
A)The number of neutrons in the nucleus
B)The number of electrons in the nucleus
C)The number of protons in the nucleus
D)The number of neutrons in the orbitals
E)The number of protons in the orbitals

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
10
In the example of ionic bond formation between sodium and chlorine,which of the following is not a true statement?
A)Na is the chemical symbol for sodium
B)Chlorine donates an electron
C)Sodium donates an electron
D)Sodium becomes positively charged
E)The bond that is formed is a strong bond
A)Na is the chemical symbol for sodium
B)Chlorine donates an electron
C)Sodium donates an electron
D)Sodium becomes positively charged
E)The bond that is formed is a strong bond

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
11
In a covalent bond:
A)Atoms share electrons
B)Atoms of opposite charges attract each other
C)Atoms share a proton
A)Atoms share electrons
B)Atoms of opposite charges attract each other
C)Atoms share a proton

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
12
The primary elements making up living organisms are:
A)Carbon, hydrogen, oxygen, calcium, iron, and iodine
B)Carbon, oxygen, iron, chlorine, sulfur, and phosphorous
C)Carbon, hydrogen, iron, sulfur, sodium, and calcium
D)Carbon, hydrogen, oxygen, sulfur, nitrogen, and phosphorous
E)Carbon, oxygen, sulfur, calcium, iron, and phosphorous
A)Carbon, hydrogen, oxygen, calcium, iron, and iodine
B)Carbon, oxygen, iron, chlorine, sulfur, and phosphorous
C)Carbon, hydrogen, iron, sulfur, sodium, and calcium
D)Carbon, hydrogen, oxygen, sulfur, nitrogen, and phosphorous
E)Carbon, oxygen, sulfur, calcium, iron, and phosphorous

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
13
If a covalent bond is polar:
A)Electrons are not shared by atoms
B)Protons are shared by atoms
C)The bond is not important to living cells
D)One of the atoms has a partial negative charge
E)The bond is not a strong bond
A)Electrons are not shared by atoms
B)Protons are shared by atoms
C)The bond is not important to living cells
D)One of the atoms has a partial negative charge
E)The bond is not a strong bond

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
14
Isotopes of the same element are different from one another in that:
A)They have a different number of protons
B)They have a different number of neutrons
C)They have a different number of electrons
A)They have a different number of protons
B)They have a different number of neutrons
C)They have a different number of electrons

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
15
In the example of ionic bond formation between sodium and chlorine:
A)Na is the chemical symbol for chlorine
B)Sodium accepts an electron
C)Chlorine accepts an electron
D)Chlorine becomes positively charged
E)Both sodium accepts an electron and chlorine becomes positively charged are correct
A)Na is the chemical symbol for chlorine
B)Sodium accepts an electron
C)Chlorine accepts an electron
D)Chlorine becomes positively charged
E)Both sodium accepts an electron and chlorine becomes positively charged are correct

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
16
An ionic bond is a bond in which:
A)Atoms share electrons
B)Atoms share a proton
C)Atoms of opposite charges attract each other
A)Atoms share electrons
B)Atoms share a proton
C)Atoms of opposite charges attract each other

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
17
A hydrogen bond:
A)Is a strong bond
B)Does not occur within a molecule
C)May occur between molecules
D)Is not important to living cells
E)Usually has a hydrogen atom with a partial negative charge
A)Is a strong bond
B)Does not occur within a molecule
C)May occur between molecules
D)Is not important to living cells
E)Usually has a hydrogen atom with a partial negative charge

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
18
An acid:
A)Has a value above seven on the pH scale
B)Is a chemical that takes hydrogen ions from a solution
C)Has a value of seven on the pH scale
D)Is a chemical that adds hydrogen ions to a solution
E)All are correct
A)Has a value above seven on the pH scale
B)Is a chemical that takes hydrogen ions from a solution
C)Has a value of seven on the pH scale
D)Is a chemical that adds hydrogen ions to a solution
E)All are correct

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
19
An ion is:
A)An atom that has gained electrons
B)An atom that has a positive charge
C)An atom that has lost electrons
D)An atom that has a negative charge
E)All are correct
A)An atom that has gained electrons
B)An atom that has a positive charge
C)An atom that has lost electrons
D)An atom that has a negative charge
E)All are correct

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
20
Which of the following is not a true statement?
A)Nitric oxide consists of one atom of nitrogen and one atom of oxygen
B)Nitric oxide is a gas
C)Nitric oxide passes freely into and out of cells
D)Nitric oxide is a harmful substance found in smog and acid rain
E)Nitric oxide has no true function in living organisms
A)Nitric oxide consists of one atom of nitrogen and one atom of oxygen
B)Nitric oxide is a gas
C)Nitric oxide passes freely into and out of cells
D)Nitric oxide is a harmful substance found in smog and acid rain
E)Nitric oxide has no true function in living organisms

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
21
The four major groups of organic compounds are:
A)Fats, waxes, carbohydrates, and amino acids
B)Carbohydrates, lipids, steroids, and monosaccharides
C)Lipids, fats, waxes, and steroids
D)Carbohydrates, lipids, proteins, and nucleic acids
E)Carbohydrates, proteins, amino acids, and nucleic acids
A)Fats, waxes, carbohydrates, and amino acids
B)Carbohydrates, lipids, steroids, and monosaccharides
C)Lipids, fats, waxes, and steroids
D)Carbohydrates, lipids, proteins, and nucleic acids
E)Carbohydrates, proteins, amino acids, and nucleic acids

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
22
Individual water molecules bind to each other through:
A)Covalent bonds
B)Ionic bonds
C)Hydrogen bonds
D)Hydrophobic bonds
E)Non-polar bonds
A)Covalent bonds
B)Ionic bonds
C)Hydrogen bonds
D)Hydrophobic bonds
E)Non-polar bonds

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
23
A substance having a pH of 13 would best be described as:
A)A weak acid
B)A weak base
C)Neutral
D)A strong acid
E)A strong base
A)A weak acid
B)A weak base
C)Neutral
D)A strong acid
E)A strong base

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
24
A substance having a pH of 6 would best be described as:
A)A weak acid
B)Neutral
C)A weak base
D)A strong acid
E)A strong base
A)A weak acid
B)Neutral
C)A weak base
D)A strong acid
E)A strong base

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
25
Water is best described as which of the following?
A)An ion
B)A non-polar molecule
C)An atom
D)A polar molecule
E)An element
A)An ion
B)A non-polar molecule
C)An atom
D)A polar molecule
E)An element

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
26
Organic molecules are defined as chemical compounds that contain:
A)Carbon
B)Carbon and oxygen
C)Carbon and nitrogen
D)Carbon, hydrogen, and nitrogen
E)Carbon and hydrogen
A)Carbon
B)Carbon and oxygen
C)Carbon and nitrogen
D)Carbon, hydrogen, and nitrogen
E)Carbon and hydrogen

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
27
A substance having a pH of 2 would best be described as:
A)Neutral
B)A weak acid
C)A weak base
D)A strong base
E)A strong acid
A)Neutral
B)A weak acid
C)A weak base
D)A strong base
E)A strong acid

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
28
A process by which cells break polymers down into smaller units is:
A)Hydrolysis
B)Condensation
C)Reproduction
D)All are correct
A)Hydrolysis
B)Condensation
C)Reproduction
D)All are correct

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
29
A substance having a pH of 8 would best be described as:
A)Neutral
B)A weak base
C)A weak acid
D)A strong acid
E)A strong base
A)Neutral
B)A weak base
C)A weak acid
D)A strong acid
E)A strong base

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
30
A peptide bond:
A)Is an ionic bond in proteins
B)Is a covalent bond in carbohydrates
C)Is a covalent bond in proteins
D)Is an ionic bond in carbohydrates
A)Is an ionic bond in proteins
B)Is a covalent bond in carbohydrates
C)Is a covalent bond in proteins
D)Is an ionic bond in carbohydrates

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
31
A process by which cells build large molecules from monomers is:
A)Hydrolysis
B)Reproduction
C)Condensation
D)All are correct
A)Hydrolysis
B)Reproduction
C)Condensation
D)All are correct

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
32
Which is not a lipid?
A)A triglyceride
B)A phospholipid
C)A wax
D)A sterol
E)A starch molecule
A)A triglyceride
B)A phospholipid
C)A wax
D)A sterol
E)A starch molecule

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
33
An amino acid contains:
A)Nitrogen
B)Nitrogen and carbon
C)Carbon
D)Phosphorous
E)Carbon and phosphorous
A)Nitrogen
B)Nitrogen and carbon
C)Carbon
D)Phosphorous
E)Carbon and phosphorous

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
34
The four nitrogen bases found in RNA are:
A)Adenine, thymine, guanine, and uracil
B)Adenine, cytosine, guanine, and uracil
C)Adenine, thymine, cytosine, and uracil
D)Thymine, cytosine, guanine, and uracil
E)None of these are correct
A)Adenine, thymine, guanine, and uracil
B)Adenine, cytosine, guanine, and uracil
C)Adenine, thymine, cytosine, and uracil
D)Thymine, cytosine, guanine, and uracil
E)None of these are correct

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
35
The primary building block (monomer)of nucleic acids is:
A)A nucleotide
B)A glucose molecule
C)A fatty acid
D)An amino acid
E)Four interconnected rings
A)A nucleotide
B)A glucose molecule
C)A fatty acid
D)An amino acid
E)Four interconnected rings

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
36
Examples of monosaccharides are:
A)Glucose, maltose, and cellulose
B)Glucose, lactose, and maltose
C)Glucose, galactose, and fructose
D)Glucose, lactose, and cellulose
E)None of these are correct
A)Glucose, maltose, and cellulose
B)Glucose, lactose, and maltose
C)Glucose, galactose, and fructose
D)Glucose, lactose, and cellulose
E)None of these are correct

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
37
Within a single molecule of water,____ bonds are formed between oxygen and hydrogen?
A)Ionic bonds
B)Covalent bonds
C)Hydrogen bonds
D)Hydrophobic bonds
E)Nuclear bonds
A)Ionic bonds
B)Covalent bonds
C)Hydrogen bonds
D)Hydrophobic bonds
E)Nuclear bonds

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
38
A substance having a pH of 7 would best be described as:
A)A weak acid
B)A weak base
C)Neutral
D)A strong acid
E)A strong base
A)A weak acid
B)A weak base
C)Neutral
D)A strong acid
E)A strong base

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
39
The three major components in a nucleotide are:
A)Glucose, a nitrogen base, and a phosphate group
B)Glucose, a fatty acid, and glycerol
C)A nitrogen base, a six carbon sugar, and a phosphate group
D)A nitrogen base, a five carbon sugar, and a phosphate group
E)A carboxyl group, an R group, and an amino group
A)Glucose, a nitrogen base, and a phosphate group
B)Glucose, a fatty acid, and glycerol
C)A nitrogen base, a six carbon sugar, and a phosphate group
D)A nitrogen base, a five carbon sugar, and a phosphate group
E)A carboxyl group, an R group, and an amino group

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
40
The primary building block (monomer)of proteins is:
A)A glucose molecule
B)A fatty acid
C)A nucleotide
D)An amino acid
E)Four interconnected rings
A)A glucose molecule
B)A fatty acid
C)A nucleotide
D)An amino acid
E)Four interconnected rings

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
41
Many digestive enzymes are hydrolases.What do these enzymes have in common?
A)They use water to form bonds between monomers
B)They use water to break bonds in monomers
C)They use water to break bonds in polymers
D)They use water to form bonds between polymers
E)They release water in forming bonds between monomers
A)They use water to form bonds between monomers
B)They use water to break bonds in monomers
C)They use water to break bonds in polymers
D)They use water to form bonds between polymers
E)They release water in forming bonds between monomers

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
42
Saturated fats have long straight tails of fatty acids,while unsaturated fats have kinks in their tails due to double bonds.These kinks prevent the fats from packing together as tightly.Animals that are ectothermic (cold blooded)need to keep their membranes fluid at cooler temperature and thus use ______ in their membranes.
A)Mostly unsaturated fats
B)Mostly saturated fats
C)Equal amounts of saturated and unsaturated fats
D)Carbohydrates
E)Proteins
A)Mostly unsaturated fats
B)Mostly saturated fats
C)Equal amounts of saturated and unsaturated fats
D)Carbohydrates
E)Proteins

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
43
The term pH refers to:
A)[H+]2
B)[H+]
C)-log[H+]
D)-[H+]
E)log[H+]
A)[H+]2
B)[H+]
C)-log[H+]
D)-[H+]
E)log[H+]

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
44
The most common isotope of carbon is 12C.14C will have ____ than 12C.
A)More protons
B)More neutrons
C)Fewer neutrons
D)Fewer protons
E)More electrons
A)More protons
B)More neutrons
C)Fewer neutrons
D)Fewer protons
E)More electrons

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
45
If a fossil has only 25% of its 14C remaining,how old is it?
A)5,730 years
B)2,865 years
C)1,432.5 years
D)22,920 years
E)11,460 years
A)5,730 years
B)2,865 years
C)1,432.5 years
D)22,920 years
E)11,460 years

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
46
How are the monomers in nucleic acids joined?
A)Peptide bonds between carbohydrates
B)Peptide bonds between amino acids
C)Phosphodiester bonds between amino acids
D)Peptide bonds between nucleotides
E)Phosphodiester bonds between nucleotides
A)Peptide bonds between carbohydrates
B)Peptide bonds between amino acids
C)Phosphodiester bonds between amino acids
D)Peptide bonds between nucleotides
E)Phosphodiester bonds between nucleotides

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
47
Blood pH is closely maintained at a pH of 7.4.A patient whose blood pH drops below 7.35 is suffering from metabolic acidosis and can go into a coma.What happens to the concentration of H+ ions in a patient with a blood pH of 6.4?
A)H+ concentration is decreased 10-fold
B)H+ concentration is decreased 2-fold
C)H+ concentration is increased 2-fold
D)H+ concentration is decreased 4-fold
E)H+ concentration is increased 10-fold
A)H+ concentration is decreased 10-fold
B)H+ concentration is decreased 2-fold
C)H+ concentration is increased 2-fold
D)H+ concentration is decreased 4-fold
E)H+ concentration is increased 10-fold

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
48
Which question can evolution not explain?
A)The diversity of species on Earth
B)The common ancestry of all species on Earth
C)How life started on Earth
D)The same types of molecules are found in all organisms
E)The origin of new species
A)The diversity of species on Earth
B)The common ancestry of all species on Earth
C)How life started on Earth
D)The same types of molecules are found in all organisms
E)The origin of new species

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
49
14C and 14N have the same:
A)Atomic number
B)Number of protons
C)Atomic mass
D)Number of neutrons
E)Number of electrons
A)Atomic number
B)Number of protons
C)Atomic mass
D)Number of neutrons
E)Number of electrons

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
50
In making beer,barley is partially fermented to produce malt.Barley is not sweet,but the malt is.What is the best explanation for this observation?
A)Fermentation allows the barley to make simple sugars from sunlight and carbon dioxide
B)Fermentation releases sugars stored in organelles in the barley
C)Fermentation causes the starch in the barley to taste sweet
D)The fermentation breaks starch in the barley down into simple sugars in the malt
E)Fermentation breaks down the simple sugars in the barley
A)Fermentation allows the barley to make simple sugars from sunlight and carbon dioxide
B)Fermentation releases sugars stored in organelles in the barley
C)Fermentation causes the starch in the barley to taste sweet
D)The fermentation breaks starch in the barley down into simple sugars in the malt
E)Fermentation breaks down the simple sugars in the barley

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
51
Saturated fats have long straight tails of fatty acids,while unsaturated fats from vegetables have kinks in their tails due to double bonds.These kinks prevent the fats from packing together as tightly.Hydrogenated vegetable oils have hydrogens added back to the double bonds and thus behave like ____.
A)Unsaturated fats
B)Waxes
C)Carbohydrates
D)Protein
E)Saturated fats
A)Unsaturated fats
B)Waxes
C)Carbohydrates
D)Protein
E)Saturated fats

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
52
How are the monomers in proteins joined?
A)Phosphodiester bonds between amino acids
B)Peptide bonds between amino acids
C)Peptide bonds between nucleotides
D)Phosphodiester bonds between nucleotides
E)Peptide bonds between carbohydrates
A)Phosphodiester bonds between amino acids
B)Peptide bonds between amino acids
C)Peptide bonds between nucleotides
D)Phosphodiester bonds between nucleotides
E)Peptide bonds between carbohydrates

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
53
A nucleotide contains which of the following?
A)Amino acid
B)Sugar
C)Sugar and nitrogenous base
D)Nitrogenous base
E)Nitrogenous base and amino acid
A)Amino acid
B)Sugar
C)Sugar and nitrogenous base
D)Nitrogenous base
E)Nitrogenous base and amino acid

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
54
The polymers with the most complex and diverse three-dimensional structure are:
A)Saturated fats
B)Unsaturated fats
C)Proteins
D)Waxes
E)Carbohydrates
A)Saturated fats
B)Unsaturated fats
C)Proteins
D)Waxes
E)Carbohydrates

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
55
____ bonds are formed between monomers to form a polymer.
A)Ionic bonds
B)Covalent bonds
C)Hydrogen bonds
D)Hydrophobic bonds
E)Nuclear bonds
A)Ionic bonds
B)Covalent bonds
C)Hydrogen bonds
D)Hydrophobic bonds
E)Nuclear bonds

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
56
14C and 14N are both:
A)Atoms
B)Molecules
C)Compounds
D)Polymers
E)Ions
A)Atoms
B)Molecules
C)Compounds
D)Polymers
E)Ions

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
57
Trees take up water hundreds of feet away by:
A)Water's high density
B)Cohesion
C)Water's high boiling point
D)Adhesion
E)A neutral pH
A)Water's high density
B)Cohesion
C)Water's high boiling point
D)Adhesion
E)A neutral pH

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
58
Sugars (CH2O)n dissolve well in water because sugars form ____ bonds with water.
A)Covalent bonds
B)Ionic bonds
C)Hydrogen bonds
D)Hydrophobic bonds
E)Non-polar bonds
A)Covalent bonds
B)Ionic bonds
C)Hydrogen bonds
D)Hydrophobic bonds
E)Non-polar bonds

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
59
You can painlessly wade into a pool,but doing a belly flop off of the high diving board hurts because of ____ in water.
A)Water's high density
B)Adhesion
C)Water's high boiling point
D)A neutral pH
E)Cohesion
A)Water's high density
B)Adhesion
C)Water's high boiling point
D)A neutral pH
E)Cohesion

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
60
When 14C decays to 14N the number of protons ____ and the number of neutrons ___.
A)Decreases by 1; increases by 1
B)Stays the same; increases by 1
C)Increases by 1; stays the same
D)Increases by 1; decreases by 1
E)Decreases by 1; stays the same
A)Decreases by 1; increases by 1
B)Stays the same; increases by 1
C)Increases by 1; stays the same
D)Increases by 1; decreases by 1
E)Decreases by 1; stays the same

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
61
What essential function would lipids play in the origin of life?
A)The formation of membranes
B)Storage of information
C)Catalysis of reactions
D)Formation of a cytoskeleton
E)Anaerobic fermentation
A)The formation of membranes
B)Storage of information
C)Catalysis of reactions
D)Formation of a cytoskeleton
E)Anaerobic fermentation

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
62
If ice were more dense than water,then during the winter most organisms living in ponds and lakes in colder climates would become entrapped in ice and freeze.

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
63
Proteins store the genetic information of the cell and transmit it to the next generation.

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
64
Cohesion is a property of water in which water molecules tend to stick together.

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
65
How did the scientists determine if an organic molecule was extraterrestrial?
A)By looking for a decrease in 13C and 15N in the meteorite
B)By measuring the amount of amino acids in the meteorite
C)By measuring the amount of nucleotides in the meteorite
D)By looking for an increase in 13C and 15N in the meteorite
E)By measuring the total amount of carbon and nitrogen in the meteorite
A)By looking for a decrease in 13C and 15N in the meteorite
B)By measuring the amount of amino acids in the meteorite
C)By measuring the amount of nucleotides in the meteorite
D)By looking for an increase in 13C and 15N in the meteorite
E)By measuring the total amount of carbon and nitrogen in the meteorite

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
66
In Table 2.7 why were amino acids analyzed for 15N? 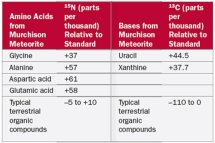
A)Nitrogen is not present in nucleotides or carbohydrates
B)Nitrogen is not present in nucleotides or lipids
C)Nitrogen is not present in carbohydrates or lipids
D)Nitrogen is not present in amino acids or carbohydrates
E)Nitrogen is not present in nucleotides or amino acids

A)Nitrogen is not present in nucleotides or carbohydrates
B)Nitrogen is not present in nucleotides or lipids
C)Nitrogen is not present in carbohydrates or lipids
D)Nitrogen is not present in amino acids or carbohydrates
E)Nitrogen is not present in nucleotides or amino acids

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
67
What hypothesis were the scientists testing?
A)Can organic molecules be made on Earth?
B)Can extraterrestrial life be detected on meteorites?
C)Can life be created from a mixture of organic molecules?
D)What conditions existed on Earth when life first began?
E)Were organic molecules found on meteorites extraterrestrial?
A)Can organic molecules be made on Earth?
B)Can extraterrestrial life be detected on meteorites?
C)Can life be created from a mixture of organic molecules?
D)What conditions existed on Earth when life first began?
E)Were organic molecules found on meteorites extraterrestrial?

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
68
The primary function of hemoglobin is to regulate the level of sugar in the blood.

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
69
How are 13C and 15N different from the more abundant isotopes 12C and 14N?
A)13C and 15N each have one more neutron than 12C and 14N
B)13C and 15N each have one more proton than 12C and 14N
C)13C and 15N each have one less neutron than 12C and 14N
D)13C and 15N each have one less proton than 12C and 14N
E)13C and 15N each have one less electron than 12C and 14N
A)13C and 15N each have one more neutron than 12C and 14N
B)13C and 15N each have one more proton than 12C and 14N
C)13C and 15N each have one less neutron than 12C and 14N
D)13C and 15N each have one less proton than 12C and 14N
E)13C and 15N each have one less electron than 12C and 14N

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
70
An essential amino acid is an amino acid that is found in all types of foods.

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
71
A substance in which other substances dissolve is called a solute.

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
72
If a protein is denatured,its structure has been changed enough to make the protein nonfunctional.

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
73
Which of the following conclusions can be made from Table 2.7? 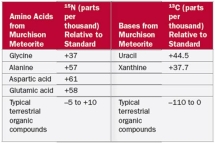
A)Glycine is a more abundant amino acid in the meteorite than in terrestrial samples
B)15N is more abundant in amino acids from the meteorite than from terrestrial samples
C)Amino acids in the meteorite contain more nitrogen than amino acids from terrestrial samples
D)13C is more abundant in amino acids from the meteorite than from terrestrial samples
E)Uracil is a more abundant amino acid in the meteorite than in terrestrial samples

A)Glycine is a more abundant amino acid in the meteorite than in terrestrial samples
B)15N is more abundant in amino acids from the meteorite than from terrestrial samples
C)Amino acids in the meteorite contain more nitrogen than amino acids from terrestrial samples
D)13C is more abundant in amino acids from the meteorite than from terrestrial samples
E)Uracil is a more abundant amino acid in the meteorite than in terrestrial samples

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
74
A fatty acid is unsaturated if there is at least one double bond between the carbon atoms in that fatty acid.

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
75
A peptide bond is a covalent bond formed between the amino group of one amino acid and the R group of another amino acid.

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
76
What is significant about detecting uracil in meteorites?
A)Uracil is found in DNA which is thought to be the original genetic material
B)Uracil is a precursor for many other biological molecules including DNA and proteins
C)Uracil is a very complex molecule that can only be made in living cells, thus confirming that extraterrestrial life exists
D)Uracil is found in RNA which is thought to be the original genetic material
E)Uracil can catalyze reactions
A)Uracil is found in DNA which is thought to be the original genetic material
B)Uracil is a precursor for many other biological molecules including DNA and proteins
C)Uracil is a very complex molecule that can only be made in living cells, thus confirming that extraterrestrial life exists
D)Uracil is found in RNA which is thought to be the original genetic material
E)Uracil can catalyze reactions

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck



