Deck 23: Organic and Biological Chemistry
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/285
Play
Full screen (f)
Deck 23: Organic and Biological Chemistry
1
Which is the condensed structure of a straight-chain hydrocarbon? 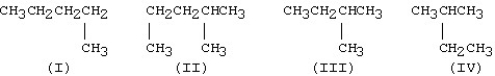
A)I
B)II
C)III
D)IV

A)I
B)II
C)III
D)IV
I
2
Which of the following condensed structures represent the same molecule? 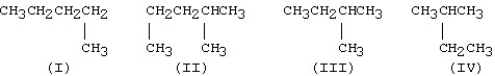
A)I and II
B)II and IV
C)III and IV
D)II and III

A)I and II
B)II and IV
C)III and IV
D)II and III
III and IV
3
Which one of the structures below is different from the other three? 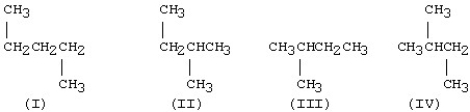
A)I
B)II
C)III
D)IV

A)I
B)II
C)III
D)IV
I
4
Some of the products from reaction of bromine and propane are shown below.(The hydrogen atoms are not shown in the structures. )How many are identical structures? 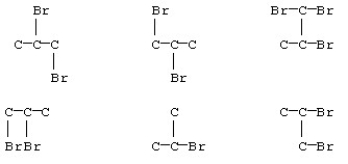
A)2
B)3
C)4
D)All are isomers of each other.
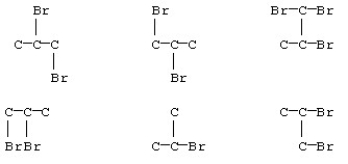
A)2
B)3
C)4
D)All are isomers of each other.

Unlock Deck
Unlock for access to all 285 flashcards in this deck.
Unlock Deck
k this deck
5
Compounds that have the same formula but different molecular structures are called
A)isoelectronic.
B)isomers.
C)isotones.
D)isotopes.
A)isoelectronic.
B)isomers.
C)isotones.
D)isotopes.

Unlock Deck
Unlock for access to all 285 flashcards in this deck.
Unlock Deck
k this deck
6
Which class of hydrocarbons has the general formula CnH2n+2?
A)alkanes
B)alkenes
C)alkynes
D)cylcloalkanes
A)alkanes
B)alkenes
C)alkynes
D)cylcloalkanes

Unlock Deck
Unlock for access to all 285 flashcards in this deck.
Unlock Deck
k this deck
7
Another term for alkanes is
A)alkenes.
B)alkynes.
C)saturated hydrocarbons.
D)unsaturated hydrocarbons.
A)alkenes.
B)alkynes.
C)saturated hydrocarbons.
D)unsaturated hydrocarbons.

Unlock Deck
Unlock for access to all 285 flashcards in this deck.
Unlock Deck
k this deck
8
Which functional group below represents a ketone?
A)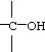
B)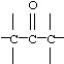
C)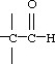
D)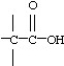
A)

B)

C)
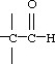
D)


Unlock Deck
Unlock for access to all 285 flashcards in this deck.
Unlock Deck
k this deck
9
Identify the functional group: 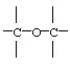
A)aldehyde
B)ketone
C)ester
D)ether

A)aldehyde
B)ketone
C)ester
D)ether

Unlock Deck
Unlock for access to all 285 flashcards in this deck.
Unlock Deck
k this deck
10
The most important characteristic of carbon atoms for forming organic molecules is
A)ability to bond together to form long chains.
B)ability to form multiple covalent bonds.
C)use of hybrid orbitals.
D)A and B
A)ability to bond together to form long chains.
B)ability to form multiple covalent bonds.
C)use of hybrid orbitals.
D)A and B

Unlock Deck
Unlock for access to all 285 flashcards in this deck.
Unlock Deck
k this deck
11
Identify the functional group: 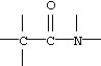
A)alcohol
B)amine
C)amide
D)ester

A)alcohol
B)amine
C)amide
D)ester

Unlock Deck
Unlock for access to all 285 flashcards in this deck.
Unlock Deck
k this deck
12
Which functional group below represents a carboxylic acid?
A)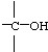
B)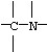
C)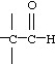
D)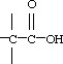
A)
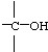
B)

C)
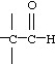
D)


Unlock Deck
Unlock for access to all 285 flashcards in this deck.
Unlock Deck
k this deck
13
Which of the following statements about isomers is false?
A)All alkanes have branched chain isomers.
B)As the number of carbon atoms increase in a compound,so do the number of possible isomers.
C)Isomers have different physical properties.
D)Isomers have the same formula but different molecular structures.
A)All alkanes have branched chain isomers.
B)As the number of carbon atoms increase in a compound,so do the number of possible isomers.
C)Isomers have different physical properties.
D)Isomers have the same formula but different molecular structures.

Unlock Deck
Unlock for access to all 285 flashcards in this deck.
Unlock Deck
k this deck
14
Which compound is a saturated hydrocarbon?
A)benzene
B)cyclohexene
C)2-methylpentane
D)propylene
A)benzene
B)cyclohexene
C)2-methylpentane
D)propylene

Unlock Deck
Unlock for access to all 285 flashcards in this deck.
Unlock Deck
k this deck
15
When alkanes react with chlorine in the presence of ultraviolet light,chlorine atoms substitute for one or more alkane hydrogen atoms.What is the number of different chloroalkane compounds that can be formed by the reaction of C2H6 with chlorine?
A)3
B)6
C)9
D)12
A)3
B)6
C)9
D)12

Unlock Deck
Unlock for access to all 285 flashcards in this deck.
Unlock Deck
k this deck
16
Which of the following statements about properties of organic compounds is not correct?
A)Most organic compounds are not soluble in water.
B)Most organic compounds do not conduct electricity.
C)Organic compounds have higher melting and boiling points than ionic compounds.
D)Organic compounds have weak intermolecular forces.
A)Most organic compounds are not soluble in water.
B)Most organic compounds do not conduct electricity.
C)Organic compounds have higher melting and boiling points than ionic compounds.
D)Organic compounds have weak intermolecular forces.

Unlock Deck
Unlock for access to all 285 flashcards in this deck.
Unlock Deck
k this deck
17
How many isomers are there for C5H12?
A)3
B)4
C)5
D)6
A)3
B)4
C)5
D)6

Unlock Deck
Unlock for access to all 285 flashcards in this deck.
Unlock Deck
k this deck
18
Which of the structures below are identical? 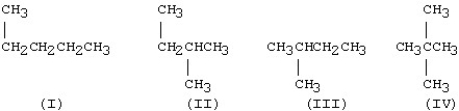
A)I and II
B)II and III
C)III and IV
D)I and IV

A)I and II
B)II and III
C)III and IV
D)I and IV

Unlock Deck
Unlock for access to all 285 flashcards in this deck.
Unlock Deck
k this deck
19
Which of the following statements is false regarding functional groups?
A)The chemical properties of the functional group dictate the chemistry of the larger molecule.
B)Each functional group has a characteristic chemical behavior.
C)A functional group consists of an atom or a group of atoms that is part of a larger molecule.
D)A functional group consists of only carbon and hydrogen atoms.
A)The chemical properties of the functional group dictate the chemistry of the larger molecule.
B)Each functional group has a characteristic chemical behavior.
C)A functional group consists of an atom or a group of atoms that is part of a larger molecule.
D)A functional group consists of only carbon and hydrogen atoms.

Unlock Deck
Unlock for access to all 285 flashcards in this deck.
Unlock Deck
k this deck
20
Which condensed structure below representing an alkane is not correct?
A)CH3CH2CH3
B)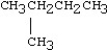
C)CH3CH2CH2CH3
D)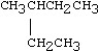
A)CH3CH2CH3
B)
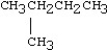
C)CH3CH2CH2CH3
D)
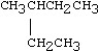

Unlock Deck
Unlock for access to all 285 flashcards in this deck.
Unlock Deck
k this deck
21
Which of the following compounds is 5-ethyl-4-isopropyl-2-methylnonane?
A)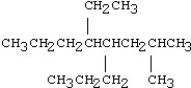
B)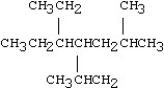
C)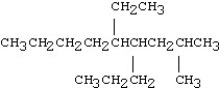
D)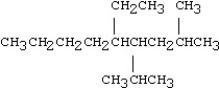
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 285 flashcards in this deck.
Unlock Deck
k this deck
22
(CH3)2CH- represents a butyl group.
A)normal
B)secondary
C)tertiary
D)quaternary
A)normal
B)secondary
C)tertiary
D)quaternary

Unlock Deck
Unlock for access to all 285 flashcards in this deck.
Unlock Deck
k this deck
23
Which name is not correct?
A)2,2-dimethylbutane
B)2,3-dimethylpentane
C)2,3,3-trimethylbutane
D)2,3,3-trimethylpentane
A)2,2-dimethylbutane
B)2,3-dimethylpentane
C)2,3,3-trimethylbutane
D)2,3,3-trimethylpentane

Unlock Deck
Unlock for access to all 285 flashcards in this deck.
Unlock Deck
k this deck
24
An alkyl group is named for the part of the alkane group that remains after a ________ is removed.
A)C atom
B)CH3 group
C)CH2 group
D)H atom
A)C atom
B)CH3 group
C)CH2 group
D)H atom

Unlock Deck
Unlock for access to all 285 flashcards in this deck.
Unlock Deck
k this deck
25
The names of compounds with carbon-carbon double bonds contain the suffix ________.
A)-ane
B)-ene
C)-yne
D)-one
A)-ane
B)-ene
C)-yne
D)-one

Unlock Deck
Unlock for access to all 285 flashcards in this deck.
Unlock Deck
k this deck
26
What class of hydrocarbons has the general formula CnH2n?
A)alkanes
B)alkenes and cycloalkanes
C)alkynes
D)aromatics
A)alkanes
B)alkenes and cycloalkanes
C)alkynes
D)aromatics

Unlock Deck
Unlock for access to all 285 flashcards in this deck.
Unlock Deck
k this deck
27
The functional groups in the molecule below are
O O
HCCH2COCH3
A)alkane,aldehyde,and ester.
B)alkane,aldehyde,ketone,and ether.
C)alkane,carboxylic acid,and ester.
D)alkane,ketone,and ester.
O O
HCCH2COCH3
A)alkane,aldehyde,and ester.
B)alkane,aldehyde,ketone,and ether.
C)alkane,carboxylic acid,and ester.
D)alkane,ketone,and ester.

Unlock Deck
Unlock for access to all 285 flashcards in this deck.
Unlock Deck
k this deck
28
None of the listed compounds contains a ring.Which has at least one triply-bonded carbon atom?
A)C2H6
B)C3H4
C)C4H8
D)All of these
A)C2H6
B)C3H4
C)C4H8
D)All of these

Unlock Deck
Unlock for access to all 285 flashcards in this deck.
Unlock Deck
k this deck
29
Which name is not correct?
A)1,2-dichloropentane
B)2,3-dimethylbutane
C)2-ethylbutane
D)4-propylheptane
A)1,2-dichloropentane
B)2,3-dimethylbutane
C)2-ethylbutane
D)4-propylheptane

Unlock Deck
Unlock for access to all 285 flashcards in this deck.
Unlock Deck
k this deck
30
None of the listed compounds contains a ring.Which has at least one doubly-bonded carbon atom?
A)C2H6
B)C3H4
C)C4H8
D)All of these
A)C2H6
B)C3H4
C)C4H8
D)All of these

Unlock Deck
Unlock for access to all 285 flashcards in this deck.
Unlock Deck
k this deck
31
Which functional group below represents an aldehyde?
A)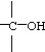
B)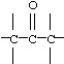
C)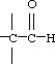
D)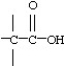
A)

B)

C)
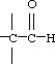
D)


Unlock Deck
Unlock for access to all 285 flashcards in this deck.
Unlock Deck
k this deck
32
Which statement below is false concerning nomenclature rules for alkanes?
A)Carbon atoms are numbered starting with the atom farthest from the branching point.
B)Each branching group is assigned a number referring to its point of attachment in the parent chain.
C)The name of the alkane is written as a single word.
D)The parent name is taken from the longest continuous chain of carbon atoms.
A)Carbon atoms are numbered starting with the atom farthest from the branching point.
B)Each branching group is assigned a number referring to its point of attachment in the parent chain.
C)The name of the alkane is written as a single word.
D)The parent name is taken from the longest continuous chain of carbon atoms.

Unlock Deck
Unlock for access to all 285 flashcards in this deck.
Unlock Deck
k this deck
33
What is the IUPAC name for the continuous carbon chain of C10H22?
A)decane
B)dipentane
C)tenalkane
D)tenane
A)decane
B)dipentane
C)tenalkane
D)tenane

Unlock Deck
Unlock for access to all 285 flashcards in this deck.
Unlock Deck
k this deck
34
What is the name for the alkyl group: 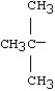
A)isobutyl
B)tert-butyl
C)tributyl
D)trimethyl
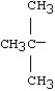
A)isobutyl
B)tert-butyl
C)tributyl
D)trimethyl

Unlock Deck
Unlock for access to all 285 flashcards in this deck.
Unlock Deck
k this deck
35
What is the IUPAC name for the following compound? 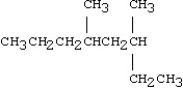
A)1,3-dimethyl-1-ethylhexane
B)4,6-dimethyl-6-ethylhexane
C)3,5-dimethyloctane
D)sec-hexylbutane

A)1,3-dimethyl-1-ethylhexane
B)4,6-dimethyl-6-ethylhexane
C)3,5-dimethyloctane
D)sec-hexylbutane

Unlock Deck
Unlock for access to all 285 flashcards in this deck.
Unlock Deck
k this deck
36
None of the listed compounds contains a ring.Which has at least one singly-bonded carbon atom?
A)C2H6
B)C3H4
C)C4H8
D)All of these
A)C2H6
B)C3H4
C)C4H8
D)All of these

Unlock Deck
Unlock for access to all 285 flashcards in this deck.
Unlock Deck
k this deck
37
The functional groups in the molecule below are
H2NCH2CO2H
A)alkane,amide,and carboxylic acid.
B)alkane,amine,and carboxylic acid.
C)alkane,amide,ketone,and alcohol.
D)alkane,amine,ketone,and alcohol.
H2NCH2CO2H
A)alkane,amide,and carboxylic acid.
B)alkane,amine,and carboxylic acid.
C)alkane,amide,ketone,and alcohol.
D)alkane,amine,ketone,and alcohol.

Unlock Deck
Unlock for access to all 285 flashcards in this deck.
Unlock Deck
k this deck
38
Which one of the following compounds is an alcohol?
A)CH3OCH3
B)CH3COCH3
C)(CH3)2CHOH
D)CH3CO2H
A)CH3OCH3
B)CH3COCH3
C)(CH3)2CHOH
D)CH3CO2H

Unlock Deck
Unlock for access to all 285 flashcards in this deck.
Unlock Deck
k this deck
39
What is the IUPAC name for the following compound? 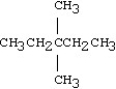
A)heptane
B)3,3-dimethylpentane
C)2,2-dimethyl-2-ethylpropane
D)2,2-diethylpropane

A)heptane
B)3,3-dimethylpentane
C)2,2-dimethyl-2-ethylpropane
D)2,2-diethylpropane

Unlock Deck
Unlock for access to all 285 flashcards in this deck.
Unlock Deck
k this deck
40
If the point of attachment to an alkane is by the middle carbon in a propyl group,the group is called
A)isopropyl.
B)n-propyl.
C)sec-propyl.
D)tert-propyl.
A)isopropyl.
B)n-propyl.
C)sec-propyl.
D)tert-propyl.

Unlock Deck
Unlock for access to all 285 flashcards in this deck.
Unlock Deck
k this deck
41
Which of the following is not a geometric (cis-trans)isomer?
A)1,1-dichloro-1-butene
B)1,2-dichloro-1-butene
C)2-butene
D)2-chloro-2-butene
A)1,1-dichloro-1-butene
B)1,2-dichloro-1-butene
C)2-butene
D)2-chloro-2-butene

Unlock Deck
Unlock for access to all 285 flashcards in this deck.
Unlock Deck
k this deck
42
In the addition reaction of water and propene in an acidic solution,the product is ________.
A)an alcohol
B)a carboxylic acid
C)an ether
D)a ketone
A)an alcohol
B)a carboxylic acid
C)an ether
D)a ketone

Unlock Deck
Unlock for access to all 285 flashcards in this deck.
Unlock Deck
k this deck
43
Name the product obtained from the addition of bromine to 3-methyl-1-butene.
A)1,1-dibromobutane
B)1,2-dibromo-3-methylbutane
C)1,2-dibromopentane
D)trans-1,2-dibromobutene
A)1,1-dibromobutane
B)1,2-dibromo-3-methylbutane
C)1,2-dibromopentane
D)trans-1,2-dibromobutene

Unlock Deck
Unlock for access to all 285 flashcards in this deck.
Unlock Deck
k this deck
44
The most important synthetic chemical reactions that alkenes and alkynes undergo are called
A)addition reactions.
B)combustion reactions.
C)photochemical halogenation reactions.
D)substitution reactions.
A)addition reactions.
B)combustion reactions.
C)photochemical halogenation reactions.
D)substitution reactions.

Unlock Deck
Unlock for access to all 285 flashcards in this deck.
Unlock Deck
k this deck
45
What is the hybridization of the carbon in (CH3)2C=CHCN indicated by the asterisk (*)?
A)sp
B)sp2
C)sp3
D)dsp3
A)sp
B)sp2
C)sp3
D)dsp3

Unlock Deck
Unlock for access to all 285 flashcards in this deck.
Unlock Deck
k this deck
46
What is the name of the following structure? 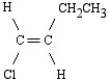
A)cis-1-ethyl-2-chloroethane
B)cis-1-chloro-1-butene
C)trans-1-chloro-1-butene
D)trans-1-chloro-2-ethylethane

A)cis-1-ethyl-2-chloroethane
B)cis-1-chloro-1-butene
C)trans-1-chloro-1-butene
D)trans-1-chloro-2-ethylethane

Unlock Deck
Unlock for access to all 285 flashcards in this deck.
Unlock Deck
k this deck
47
Which of the molecules shown below are unsaturated?
(1)CH3CH2CH2CH(CH3)2
(2)CH3CH=CHCH(CH3)2
(3)HCCCH2CH(CH3)2
A)All are unsaturated.
B)Only (2)and (3)are unsaturated.
C)Only (2)is unsaturated.
D)Only (3)is unsaturated.
(1)CH3CH2CH2CH(CH3)2
(2)CH3CH=CHCH(CH3)2
(3)HCCCH2CH(CH3)2
A)All are unsaturated.
B)Only (2)and (3)are unsaturated.
C)Only (2)is unsaturated.
D)Only (3)is unsaturated.

Unlock Deck
Unlock for access to all 285 flashcards in this deck.
Unlock Deck
k this deck
48
What is the molecular geometry around the carbon in (CH3)2C=CHCN indicated by the
Asterisk (*)?
A)linear
B)trigonal planar
C)trigonal pyramidal
D)tetrahedral
Asterisk (*)?
A)linear
B)trigonal planar
C)trigonal pyramidal
D)tetrahedral

Unlock Deck
Unlock for access to all 285 flashcards in this deck.
Unlock Deck
k this deck
49
Which of the following is a correct name for an alkyne?
A)3-methyl-2-pentyne
B)5-methyl-3-pentyne
C)2,2-dimethyl-4-pentyne
D)3,3-dimethyl-1-pentyne
A)3-methyl-2-pentyne
B)5-methyl-3-pentyne
C)2,2-dimethyl-4-pentyne
D)3,3-dimethyl-1-pentyne

Unlock Deck
Unlock for access to all 285 flashcards in this deck.
Unlock Deck
k this deck
50
Name the product of hydrogenation of trans-2-pentene.
A)pentane
B)cis-2-pentene
C)trans-2-pentane
D)none of these
A)pentane
B)cis-2-pentene
C)trans-2-pentane
D)none of these

Unlock Deck
Unlock for access to all 285 flashcards in this deck.
Unlock Deck
k this deck
51
Hydrogenation of vegetable oils converts them into what type of molecule?
A)esters
B)ethers
C)polymers
D)saturated fats
A)esters
B)ethers
C)polymers
D)saturated fats

Unlock Deck
Unlock for access to all 285 flashcards in this deck.
Unlock Deck
k this deck
52
Which one of the following molecules can rotate freely around its carbon-carbon bond?
A)acetylene
B)cyclopropane
C)ethane
D)ethylene
A)acetylene
B)cyclopropane
C)ethane
D)ethylene

Unlock Deck
Unlock for access to all 285 flashcards in this deck.
Unlock Deck
k this deck
53
What is the name of the following structure? 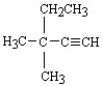
A)tert-butylethyne
B)3,3-dimethyl-1-pentyne
C)3-ethyl-3-methyl-1-butyne
D)trans-ethylmethylbutyne

A)tert-butylethyne
B)3,3-dimethyl-1-pentyne
C)3-ethyl-3-methyl-1-butyne
D)trans-ethylmethylbutyne

Unlock Deck
Unlock for access to all 285 flashcards in this deck.
Unlock Deck
k this deck
54
Which of the following statements about cycloalkanes is true?
A)The bonds in cyclopropane and cyclobutane are weaker than other cycloalkanes.
B)Cycloalkanes are also called "acyclic" compounds.
C)Cyclopropane has 109° bond angles.
D)Most cycloalkanes are planar molecules.
A)The bonds in cyclopropane and cyclobutane are weaker than other cycloalkanes.
B)Cycloalkanes are also called "acyclic" compounds.
C)Cyclopropane has 109° bond angles.
D)Most cycloalkanes are planar molecules.

Unlock Deck
Unlock for access to all 285 flashcards in this deck.
Unlock Deck
k this deck
55
Which of the following is trans-2-butene? (Only bonds are shown in the skeletal structures below;the carbon and hydrogen atoms are deleted. ) 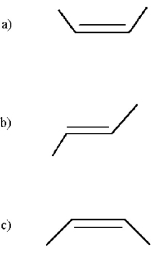
A)structure a
B)structure b
C)structure c
D)none of these
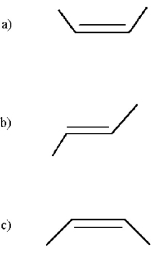
A)structure a
B)structure b
C)structure c
D)none of these

Unlock Deck
Unlock for access to all 285 flashcards in this deck.
Unlock Deck
k this deck
56
What is the product when pure water and ethene are mixed?
A)an alcohol
B)a carboxylic acid
C)an ether
D)no reaction occurs
A)an alcohol
B)a carboxylic acid
C)an ether
D)no reaction occurs

Unlock Deck
Unlock for access to all 285 flashcards in this deck.
Unlock Deck
k this deck
57
Which of the following statements about cis-trans isomers is not correct?
A)Conversion between cis- and trans-isomers occurs easily by rotation around the double bond.
B)In the trans- isomer,the groups of interest are on opposite sides across the double bond.
C)In the cis- isomer,the groups of interest are on the same side of the double bond.
D)There are no cis-trans isomers in alkynes.
A)Conversion between cis- and trans-isomers occurs easily by rotation around the double bond.
B)In the trans- isomer,the groups of interest are on opposite sides across the double bond.
C)In the cis- isomer,the groups of interest are on the same side of the double bond.
D)There are no cis-trans isomers in alkynes.

Unlock Deck
Unlock for access to all 285 flashcards in this deck.
Unlock Deck
k this deck
58
How many geometrical and structural isomers are there of butene?
A)3
B)4
C)5
D)6
A)3
B)4
C)5
D)6

Unlock Deck
Unlock for access to all 285 flashcards in this deck.
Unlock Deck
k this deck
59
Which compound will exhibit cis-trans isomerism?
A)1,2-dichloroethane
B)1,2-dichloroethene
C)dichloroethyne
D)ethylene
A)1,2-dichloroethane
B)1,2-dichloroethene
C)dichloroethyne
D)ethylene

Unlock Deck
Unlock for access to all 285 flashcards in this deck.
Unlock Deck
k this deck
60
Which compound will have the largest dipole moment?
A)cis-1,2-dichloroethylene
B)trans-1,2-dichloroethylene
C)tetrabromoethylene
D)tetrachloroethylene
A)cis-1,2-dichloroethylene
B)trans-1,2-dichloroethylene
C)tetrabromoethylene
D)tetrachloroethylene

Unlock Deck
Unlock for access to all 285 flashcards in this deck.
Unlock Deck
k this deck
61
Which name is not correct?
A)1,1 dimethylcyclobutane
B)1,2-dimethylcyclobutane
C)1,3-dimethylcyclohexane
D)1,4-dimethylcyclopentane
A)1,1 dimethylcyclobutane
B)1,2-dimethylcyclobutane
C)1,3-dimethylcyclohexane
D)1,4-dimethylcyclopentane

Unlock Deck
Unlock for access to all 285 flashcards in this deck.
Unlock Deck
k this deck
62
"Glycerol" is the common name for
A)ethanol.
B)2-propanol.
C)1,2-ethanediol.
D)1,2,3-propanetriol.
A)ethanol.
B)2-propanol.
C)1,2-ethanediol.
D)1,2,3-propanetriol.

Unlock Deck
Unlock for access to all 285 flashcards in this deck.
Unlock Deck
k this deck
63
What type of hybridization does a carbon atom have in a six-membered aromatic ring?
A)sp
B)sp2
C)sp3
D)sp3d
A)sp
B)sp2
C)sp3
D)sp3d

Unlock Deck
Unlock for access to all 285 flashcards in this deck.
Unlock Deck
k this deck
64
The C-C=O bond angle in acetic acid,shown below,is closest to
O
CH3C-OH
A)90°.
B)109.5°.
C)120°.
D)180°.
O
CH3C-OH
A)90°.
B)109.5°.
C)120°.
D)180°.

Unlock Deck
Unlock for access to all 285 flashcards in this deck.
Unlock Deck
k this deck
65
What type of compound contains the -NH2 functional group?
A)alcohol
B)amine
C)carboxylic acid
D)ether
A)alcohol
B)amine
C)carboxylic acid
D)ether

Unlock Deck
Unlock for access to all 285 flashcards in this deck.
Unlock Deck
k this deck
66
Which of the following compounds is most soluble in water?
A)decanol
B)dimethyl ether
C)ethane
D)ethanol
A)decanol
B)dimethyl ether
C)ethane
D)ethanol

Unlock Deck
Unlock for access to all 285 flashcards in this deck.
Unlock Deck
k this deck
67
Which structure is meta-dibromobenzene? 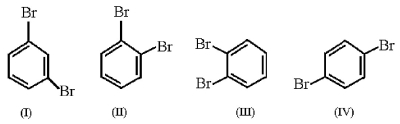
A)structure I
B)structure II
C)structure III
D)structure IV

A)structure I
B)structure II
C)structure III
D)structure IV

Unlock Deck
Unlock for access to all 285 flashcards in this deck.
Unlock Deck
k this deck
68
Which structures represent the same dibromobenzene compound? 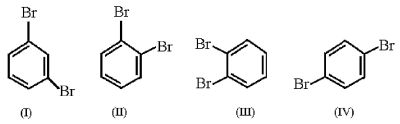
A)I and IV
B)II and III
C)I,II,and III
D)All structures are identical.

A)I and IV
B)II and III
C)I,II,and III
D)All structures are identical.

Unlock Deck
Unlock for access to all 285 flashcards in this deck.
Unlock Deck
k this deck
69
How many ether isomers have the formula C4H10O?
A)2
B)3
C)4
D)5
A)2
B)3
C)4
D)5

Unlock Deck
Unlock for access to all 285 flashcards in this deck.
Unlock Deck
k this deck
70
Which of the following statements about benzene is true? Benzene
A)contains 3 long bonds and 3 short bonds.
B)is a puckered,six-membered ring.
C)is unusually stable because of delocalized pi electrons.
D)readily undergoes addition reactions.
A)contains 3 long bonds and 3 short bonds.
B)is a puckered,six-membered ring.
C)is unusually stable because of delocalized pi electrons.
D)readily undergoes addition reactions.

Unlock Deck
Unlock for access to all 285 flashcards in this deck.
Unlock Deck
k this deck
71
Which of the following statements about amines is not correct? Amines are
A)bases (proton acceptors).
B)converted to ammonium salts by reaction with HCl.
C)organic derivatives of ammonia.
D)very water soluble.
A)bases (proton acceptors).
B)converted to ammonium salts by reaction with HCl.
C)organic derivatives of ammonia.
D)very water soluble.

Unlock Deck
Unlock for access to all 285 flashcards in this deck.
Unlock Deck
k this deck
72
Which reaction is not characteristic of aromatic compounds?
A)addition
B)halogenation
C)nitration
D)sulfonation
A)addition
B)halogenation
C)nitration
D)sulfonation

Unlock Deck
Unlock for access to all 285 flashcards in this deck.
Unlock Deck
k this deck
73
Which structure is para-dibromobenzene? 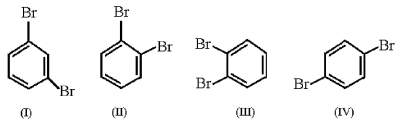
A)structure I
B)structure II
C)structure III
D)structure IV

A)structure I
B)structure II
C)structure III
D)structure IV

Unlock Deck
Unlock for access to all 285 flashcards in this deck.
Unlock Deck
k this deck
74
The cycloalkane represented by a square is called ________ and has the molecular formula ________.
A)cyclopropane,C3H6
B)cyclopropane,C3H8
C)cyclobutane,C4H4
D)cyclobutane,C4H8
A)cyclopropane,C3H6
B)cyclopropane,C3H8
C)cyclobutane,C4H4
D)cyclobutane,C4H8

Unlock Deck
Unlock for access to all 285 flashcards in this deck.
Unlock Deck
k this deck
75
What is the proper name for (CH3)2CHCH(OH)CH3?
A)isopropanol
B)isopentanol
C)2-methylpentanol
D)3-methyl-2-butanol
A)isopropanol
B)isopentanol
C)2-methylpentanol
D)3-methyl-2-butanol

Unlock Deck
Unlock for access to all 285 flashcards in this deck.
Unlock Deck
k this deck
76
"Rubbing alcohol" is the common name for
A)methanol.
B)ethanol.
C)2-propanol.
D)1,2-ethanediol.
A)methanol.
B)ethanol.
C)2-propanol.
D)1,2-ethanediol.

Unlock Deck
Unlock for access to all 285 flashcards in this deck.
Unlock Deck
k this deck
77
What are the C-C-C bond angles in cyclopropane and cyclohexane?
A)60° in both molecules
B)60° in cyclopropane and 109.5° in cyclohexane
C)60° in cyclopropane and 120° in cyclohexane
D)109.5° in both molecules
A)60° in both molecules
B)60° in cyclopropane and 109.5° in cyclohexane
C)60° in cyclopropane and 120° in cyclohexane
D)109.5° in both molecules

Unlock Deck
Unlock for access to all 285 flashcards in this deck.
Unlock Deck
k this deck
78
The boiling point of 1-propanol should be ________ than the boiling point of methyl ethyl ether.
A)higher than
B)lower than
C)the same as
D)cannot be determined
A)higher than
B)lower than
C)the same as
D)cannot be determined

Unlock Deck
Unlock for access to all 285 flashcards in this deck.
Unlock Deck
k this deck
79
Which compound is not an isomer of the other three?
A)cyclohexane
B)1,2-dimethylcyclobutane
C)1,2-dimethylcyclopropane
D)methylcyclopentane
A)cyclohexane
B)1,2-dimethylcyclobutane
C)1,2-dimethylcyclopropane
D)methylcyclopentane

Unlock Deck
Unlock for access to all 285 flashcards in this deck.
Unlock Deck
k this deck
80
"Wood alcohol" is the common name for
A)methanol.
B)ethanol.
C)2-propanol.
D)1,2-ethanediol.
A)methanol.
B)ethanol.
C)2-propanol.
D)1,2-ethanediol.

Unlock Deck
Unlock for access to all 285 flashcards in this deck.
Unlock Deck
k this deck



