Deck 5: Gases
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/121
Play
Full screen (f)
Deck 5: Gases
1
Which of the following describes Dalton's Law?
A)The pressure of a gas is proportional to its volume
B)The total pressure of a gas mixture is the sum of the partial pressures of each gas in the mixture
C)The temperature of a gas is proportional to its volume
D)Only one variable can be changed from an initial state to a final state for a gas
A)The pressure of a gas is proportional to its volume
B)The total pressure of a gas mixture is the sum of the partial pressures of each gas in the mixture
C)The temperature of a gas is proportional to its volume
D)Only one variable can be changed from an initial state to a final state for a gas
The total pressure of a gas mixture is the sum of the partial pressures of each gas in the mixture
2
The gas pressure in an aerosol can is 1.8 atm at 25°C.If the gas is an ideal gas, what pressure would develop in the can if it were heated to 475°C?
A)0.095 atm
B)0.72 atm
C)3.3 atm
D)4.5 atm
E)34 atm
A)0.095 atm
B)0.72 atm
C)3.3 atm
D)4.5 atm
E)34 atm
4.5 atm
3
A sample of oxygen gas has a volume of 545 mL at 35°C.The gas is heated to 151ºC at constant pressure in a container that can contract or expand.What is the final volume of the oxygen gas?
A)750.mL
B)396 mL
C)417 mL
D)267 mL
E)126 mL
A)750.mL
B)396 mL
C)417 mL
D)267 mL
E)126 mL
750.mL
4
Which of these properties is/are characteristic(s)of gases?
A)High compressibility
B)Relatively large distances between molecules
C)Formation of homogeneous mixtures regardless of the nature of gases
D)A and B.
E)A, B, and C.
A)High compressibility
B)Relatively large distances between molecules
C)Formation of homogeneous mixtures regardless of the nature of gases
D)A and B.
E)A, B, and C.

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
5
If the pressure on a gas sample is tripled and the absolute temperature is quadrupled, by what factor will the volume of the sample change?
A)12
B)4/3
C)3/4
D)1/3
E)4
A)12
B)4/3
C)3/4
D)1/3
E)4

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
6
The pressure of a gas sample was measured to be 654 mmHg.What is the pressure in kPa? (1 atm = 1.01325 × 105 Pa)
A)87.2 kPa
B)118 kPa
C)6.63 × 104 kPa
D)8.72 × 104 kPa
E)8.72 × 107 kPa
A)87.2 kPa
B)118 kPa
C)6.63 × 104 kPa
D)8.72 × 104 kPa
E)8.72 × 107 kPa

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
7
A sample of pure nitrogen has a temperature of 15 °C.What is the temperature of the nitrogen in units of Kelvin?
A)300 K
B)290 K
C)288 K
D)288.2 K
E)288.15 K
A)300 K
B)290 K
C)288 K
D)288.2 K
E)288.15 K

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
8
A small bubble rises from the bottom of a lake, where the temperature and pressure are 4°C and 3.0 atm, to the water's surface, where the temperature is 25°C and the pressure is 0.95 atm.Calculate the final volume of the bubble if its initial volume was 2.1 mL.
A)0.72 mL
B)6.2 mL
C)7.1 mL mL
D)22.4 mL
E)41.4 mL
A)0.72 mL
B)6.2 mL
C)7.1 mL mL
D)22.4 mL
E)41.4 mL

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
9
A sample of helium gas occupies 355mL at 23°C.If the container the He is in is expanded to 1.50 L at constant pressure, what is the final temperature for the He at this new volume?
A)1,250 °C
B)978 °C
C)70.1 °C
D)80.0 °C
E)1,520 °C
A)1,250 °C
B)978 °C
C)70.1 °C
D)80.0 °C
E)1,520 °C

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
10
A sample of pure oxygen gas has a pressure of 795 torr.What is the pressure of the oxygen in units of atmospheres?
A)0.795 atm
B)1.05 atm
C)0.604 atm
D)0.760 atm
E)1.01 atm
A)0.795 atm
B)1.05 atm
C)0.604 atm
D)0.760 atm
E)1.01 atm

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
11
At constant temperature, the volume of the container that a sample of nitrogen gas is in is doubled.As a result the pressure of the nitrogen gas is halved.The amount of nitrogen gas is unchanged in this process.This is an example of:
A)Boyle's Law
B)Charles's Law
C)Gay-Lussac's Law
D)Avogadro's Law
E)Dalton's Law
A)Boyle's Law
B)Charles's Law
C)Gay-Lussac's Law
D)Avogadro's Law
E)Dalton's Law

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
12
A 45 mL sample of nitrogen gas is cooled from 135ºC to 15°C in a container that can contract or expand at constant pressure, what is the new volume of the nitrogen gas?
A)64 mL
B)5.0 mL
C)410 mL
D)32 mL
E)41 mL
A)64 mL
B)5.0 mL
C)410 mL
D)32 mL
E)41 mL

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
13
A pressure that will support a column of Hg to a height of 256 mm would support a column of water to what height? The density of mercury is 13.6 g/cm3; the density of water is 1.00 g/cm3.
A)1.00 × 102 ft
B)18.8 mm
C)33.8 ft
D)76.0 cm
E)348 cm
A)1.00 × 102 ft
B)18.8 mm
C)33.8 ft
D)76.0 cm
E)348 cm

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
14
If the pressure of a gas sample is quadrupled and the absolute temperature is doubled, by what factor does the volume of the sample change?
A)8
B)2
C)1/2
D)1/4
E)1/8
A)8
B)2
C)1/2
D)1/4
E)1/8

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
15
A sample of a gas occupies 1.40 × 103 mL at 25°C and 760 mmHg.What volume will it occupy at the same temperature and 380 mmHg?
A)2,800 mL
B)2,100 mL
C)1,400 mL
D)1,050 mL
E)700 mL
A)2,800 mL
B)2,100 mL
C)1,400 mL
D)1,050 mL
E)700 mL

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
16
At constant temperature and volume, a sample of oxygen gas is added to a sample of nitrogen gas.The pressure of the mixture is found by adding the pressures of the two individual gases.This is an example of:
A)Boyle's Law
B)Charles's Law
C)Gay-Lussac's Law
D)Avogadro's Law
E)Dalton's Law
A)Boyle's Law
B)Charles's Law
C)Gay-Lussac's Law
D)Avogadro's Law
E)Dalton's Law

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
17
What will happen to the height (h)of the column of mercury in the manometer shown below if the stopcock is opened? 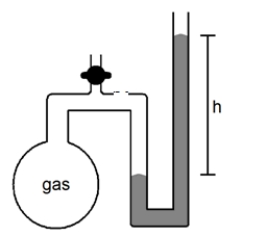
A)h will decrease
B)h will not change
C)h will increase
D)not enough information given to answer the question

A)h will decrease
B)h will not change
C)h will increase
D)not enough information given to answer the question

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
18
What will happen to the height (h)of the mercury column in the manometer shown below if the stopcock is opened, given that the atmospheric pressure is 755 mmHg? 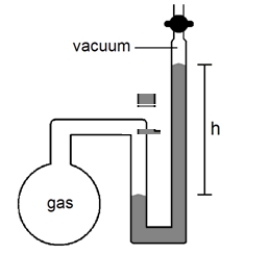
A)h will decrease
B)h will not change
C)h will increase
D)not enough information given to answer the question

A)h will decrease
B)h will not change
C)h will increase
D)not enough information given to answer the question

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
19
Which of the following statements is consistent with Boyle's Law concerning an ideal gas?
A)At constant temperature and moles, a plot of volume versus pressure is linear.
B)At constant pressure and volume, a plot of temperature versus moles is linear.
C)At constant pressure and moles, a plot of temperature versus volume is linear.
D)At constant temperature and moles, a plot of pressure versus the inverse of volume is linear.
E)At constant temperature and pressure, a plot of moles versus volume is linear.
A)At constant temperature and moles, a plot of volume versus pressure is linear.
B)At constant pressure and volume, a plot of temperature versus moles is linear.
C)At constant pressure and moles, a plot of temperature versus volume is linear.
D)At constant temperature and moles, a plot of pressure versus the inverse of volume is linear.
E)At constant temperature and pressure, a plot of moles versus volume is linear.

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
20
What is the pressure of the sample of gas trapped in the open-tube mercury manometer shown below if atmospheric pressure is 736 mmHg and h = 9.2 cm? 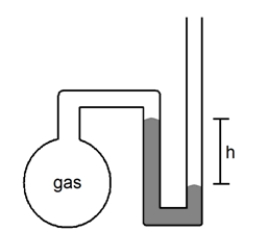
A)92 mmHg
B)644 mmHg
C)736 mmHg
D)828 mmHg

A)92 mmHg
B)644 mmHg
C)736 mmHg
D)828 mmHg

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
21
Calculate the density of Br2(g)at 59.0°C and 1.00 atm pressure.
A)3.45 g/L
B)5.83 g/L
C)22.4 g/L
D)27.2 g/L
E)769 g/L
A)3.45 g/L
B)5.83 g/L
C)22.4 g/L
D)27.2 g/L
E)769 g/L

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
22
Calculate the number of moles of gas contained in a 10.0 L tank at 22°C and 105 atm.(R = 0.08206 L.atm/K*mol)
A)1.71 × 10-3 mol
B)0.0231 mol
C)1.03 mol
D)43.4 mol
E)582 mol
A)1.71 × 10-3 mol
B)0.0231 mol
C)1.03 mol
D)43.4 mol
E)582 mol

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
23
Gases are sold in large cylinders for laboratory use.What pressure, in atmospheres, will be exerted by 2,500 g of oxygen gas (O2)when stored at 22°C in a 40.0 L cylinder?
A)3.6 atm
B)10.atm
C)47 atm
D)1,500 atm
E)7.6 × 104 atm
A)3.6 atm
B)10.atm
C)47 atm
D)1,500 atm
E)7.6 × 104 atm

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
24
Calculate the grams of SO2 gas present at STP in a 5.9 L container.
A)0.24 g
B)0.26 g
C)15 g
D)17 g
E)64 g
A)0.24 g
B)0.26 g
C)15 g
D)17 g
E)64 g

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
25
A gas evolved during the fermentation of sugar was collected.After purification its volume was found to be 25.0 L at 22.5°C and 702 mmHg.How many moles of gas were collected?
A)0.95 mol
B)1.05 mol
C)12.5 mol
D)22.4 mol
E)724 mol
A)0.95 mol
B)1.05 mol
C)12.5 mol
D)22.4 mol
E)724 mol

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
26
Calculate the density, in g/L, of SF6 gas at 27°C and 0.500 atm pressure.
A)3.38 × 10-3 g/L
B)2.96 g/L
C)22.4 g/L
D)32.9 g/L
E)3.38 kg/L
A)3.38 × 10-3 g/L
B)2.96 g/L
C)22.4 g/L
D)32.9 g/L
E)3.38 kg/L

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
27
Calculate the volume occupied by 25.2 g of CO2 at 0.84 atm and 25°C.R = 0.08206 L.atm/K.mol.
A)0.060 L
B)1.34 L
C)16.7 L
D)24.2 L
E)734 L
A)0.060 L
B)1.34 L
C)16.7 L
D)24.2 L
E)734 L

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
28
Calculate the density of CO2(g)at 120°C and 790 mmHg pressure.
A)0.032 g/L
B)1.4 g/L
C)1.8 g/L
D)3.4 g/L
E)8.0 g/L
A)0.032 g/L
B)1.4 g/L
C)1.8 g/L
D)3.4 g/L
E)8.0 g/L

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
29
The temperature of a sample of argon gas in a 365 mL container at 740.mmHg and 25°C is lowered to 12°C.Assuming the volume of the container and the amount of gas is unchanged, calculate the new pressure of the argon.
A)0.468 atm
B)0.931 atm
C)1.02 atm
D)1.54 atm
E)2.03 atm
A)0.468 atm
B)0.931 atm
C)1.02 atm
D)1.54 atm
E)2.03 atm

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
30
Calculate the density of Ar(g)at -11°C and 675 mmHg.
A)-39.3 g/L
B)1.52g/L
C)1.65 g/L
D)39.95 g/L
E)1254 g/L
A)-39.3 g/L
B)1.52g/L
C)1.65 g/L
D)39.95 g/L
E)1254 g/L

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
31
How many atoms of Ar gas are present in a 340 mL container at 55 °C and 720 mmHg?
A)0.012 Ar atoms
B)7.2 x 1021 Ar atoms
C)4.3 x 1022 Ar atoms
D)2.9 x 1023 Ar atoms
E)1.7 x 1024 Ar atoms
A)0.012 Ar atoms
B)7.2 x 1021 Ar atoms
C)4.3 x 1022 Ar atoms
D)2.9 x 1023 Ar atoms
E)1.7 x 1024 Ar atoms

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
32
At what temperature will a sample of nitrogen gas with a volume of 328 mL at 15°C and 748 mmHg occupy a volume of 0.898 L at a pressure of 642 mm Hg? Assume the amount of the nitrogen gas does not change.
A)676°C
B)404°C
C)396°C
D)274°C
E)123°C
A)676°C
B)404°C
C)396°C
D)274°C
E)123°C

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
33
At what temperature will a sample of oxygen gas with a volume of 0.110 L at 12°C and 822 mmHg occupy a volume of 345 mL at a pressure of 578 mm Hg? Assume the amount of the oxygen gas does not change.
A)129°C
B)355°C
C)402°C
D)629°C
E)903°C
A)129°C
B)355°C
C)402°C
D)629°C
E)903°C

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
34
Calculate the volume occupied by 56.5 g of argon gas at STP.
A)22.4 L
B)31.7 L
C)34.6 L
D)1,270 L
E)1,380 L
A)22.4 L
B)31.7 L
C)34.6 L
D)1,270 L
E)1,380 L

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
35
Calculate the mass, in grams, of 2.74 L of CO gas measured at 33°C and 945 mmHg.
A)0.263 g
B)2.46 g
C)3.80 g
D)35.2 g
E)206 g
A)0.263 g
B)2.46 g
C)3.80 g
D)35.2 g
E)206 g

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
36
Calculate the density, in g/L, of N2 gas at 35°C and 0.98 atm pressure.
A)0.039 g/L
B)0.34 g/L
C)0.54 g/L
D)1.1 g/L
E)9.6 g/L
A)0.039 g/L
B)0.34 g/L
C)0.54 g/L
D)1.1 g/L
E)9.6 g/L

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
37
A 250 mL flask contains 3.4 g of neon gas at 45°C.Calculate the pressure of the neon gas inside the flask.
A)0.050 atm
B)0.46 atm
C)18 atm
D)38 atm
E)3.5 x 102 atm
A)0.050 atm
B)0.46 atm
C)18 atm
D)38 atm
E)3.5 x 102 atm

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
38
Calculate the volume occupied by 35.2 g of methane gas (CH4)at 25°C and 1.0 atm.R = 0.08206 L.atm/K.mol.
A)0.0186 L
B)4.5 L
C)11.2 L
D)49.2 L
E)53.7 L
A)0.0186 L
B)4.5 L
C)11.2 L
D)49.2 L
E)53.7 L

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
39
Calculate the density, in g/L, of chlorine (Cl2)gas at STP.
A)2.13 × 10-2 g/L
B)1.58 g/L
C)3.16 g/L
D)46.9 g/L
E)0.316 kg/L
A)2.13 × 10-2 g/L
B)1.58 g/L
C)3.16 g/L
D)46.9 g/L
E)0.316 kg/L

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
40
How many atoms of He gas are present in a 450 mL container at 35°C and 740 mmHg?
A)0.017 He atoms
B)0.068 He atoms
C)1.2 x 105 He atoms
D)1.0 x 1022 He atoms
E)7.9 x 1024 He atoms
A)0.017 He atoms
B)0.068 He atoms
C)1.2 x 105 He atoms
D)1.0 x 1022 He atoms
E)7.9 x 1024 He atoms

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
41
A mixture of three gases has a total pressure of 1,380 mmHg at 298 K.The mixture is analyzed and is found to contain 1.27 mol CO2, 3.04 mol CO, and 1.50 mol Ar.What is the partial pressure of Ar?
A)0.258 atm
B)301 mmHg
C)356 mmHg
D)5,345 mmHg
E)8,020 mmHg
A)0.258 atm
B)301 mmHg
C)356 mmHg
D)5,345 mmHg
E)8,020 mmHg

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
42
What is the molar mass of Freon-11 gas if its density is 6.13 g/L at STP?
A)0.274 g/mol
B)3.64 g/mol
C)78.2 g/mol
D)137 g/mol
E)365 g/mol
A)0.274 g/mol
B)3.64 g/mol
C)78.2 g/mol
D)137 g/mol
E)365 g/mol

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
43
Gas A and gas B are combined in a flask at initial pressures of 1.0 atm each.The flask is sealed and over time they react to completion to give gas C according to the following chemical equation: 2A(g)+ B(g) C(g)
Assuming the temperature stays constant, what will be the total pressure in the flask after the reaction goes to completion?
A)0.33 atm
B)0.50 atm
C)0.67 atm
D)0.75 atm
E)1.0 atm
Assuming the temperature stays constant, what will be the total pressure in the flask after the reaction goes to completion?
A)0.33 atm
B)0.50 atm
C)0.67 atm
D)0.75 atm
E)1.0 atm

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
44
A 1.07 g sample of a Noble gas occupies a volume of 363 mL at 35°C and 678 mmHg.Identify the Noble gas in this sample? (R = 0.08206 L.atm/K.mol)
A)He
B)Ne
C)Ar
D)Kr
E)Xe
A)He
B)Ne
C)Ar
D)Kr
E)Xe

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
45
A sample of carbon monoxide gas was collected in a 2.0 L flask by displacing water at 28°C and 810 mmHg.Calculate the number of CO molecules in the flask.The vapor pressure of water at 28°C is 28.3 mmHg.
A)5.0 × 1022
B)5.2 × 1022
C)3.8 × 1023
D)5.4 × 1023
E)3.8 × 1025
A)5.0 × 1022
B)5.2 × 1022
C)3.8 × 1023
D)5.4 × 1023
E)3.8 × 1025

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
46
A gaseous compound is 30.4% nitrogen and 69.6% oxygen by mass.A 5.25-g sample of the gas occupies a volume of 1.00 L and exerts a pressure of 1.26 atm at -4.0°C.Which of these choices is its molecular formula?
A)NO
B)NO2
C)N3O6
D)N2O4
E)N2O5
A)NO
B)NO2
C)N3O6
D)N2O4
E)N2O5

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
47
Determine the molar mass of Freon-11 gas if a sample weighing 0.597 g occupies 100.cm3 at 95°C, and 1,000.mmHg.
A)0.19 g/mol
B)35.3 g/mol
C)70.9 g/mol
D)137 g/mol
E)384 g/mol
A)0.19 g/mol
B)35.3 g/mol
C)70.9 g/mol
D)137 g/mol
E)384 g/mol

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
48
What volume of sulfur dioxide gas at 45 °C and 723 mmHg will react completely with 0.870 L of oxygen gas at constant temperature and pressure? 2 SO2(g)+ O2(g) 2SO3(g)
A)0.0317 L
B)0.0634 L
C)0.870 L
D)1.74 L
E)3.48 L
A)0.0317 L
B)0.0634 L
C)0.870 L
D)1.74 L
E)3.48 L

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
49
Which one of these gases is "lighter-than-air"?
A)Cl2
B)SO2
C)PH3
D)NO2
E)Ne
A)Cl2
B)SO2
C)PH3
D)NO2
E)Ne

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
50
Which of these gases will have the greatest density at the same specified temperature and pressure?
A)H2
B)CClF3
C)CO2
D)C2H6
E)CF4
A)H2
B)CClF3
C)CO2
D)C2H6
E)CF4

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
51
How many liters of oxygen gas at 153°C and 0.820 atm can be produced by the decomposition of 22.4 g of solid KClO3? (The other decomposition product is solid potassium chloride.)
A)0.085 L
B)3.0 L
C)4.20 L
D)7.79 L
E)11.7 L
A)0.085 L
B)3.0 L
C)4.20 L
D)7.79 L
E)11.7 L

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
52
A 707 mg sample of a gas containing only carbon and oxygen occupies a volume of 452 mL at 63 °C and 745 mmHg.Identify the gas in the sample.(R = 0.08206 L.atm/K.mol)
A)CO
B)CO2
C)CO32-
D)C2O
E)C2O2
A)CO
B)CO2
C)CO32-
D)C2O
E)C2O2

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
53
Air contains 78% N2, 21% O2, and 1% Ar, by volume.What is the density of air at 1,000.torr and -10°C?
A)0.56 g/L
B)1.0 g/L
C)1.3 g/L
D)1.8 g/L
E)6.1 g/L
A)0.56 g/L
B)1.0 g/L
C)1.3 g/L
D)1.8 g/L
E)6.1 g/L

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
54
A 0.271 g sample of an unknown vapor occupies 294 mL at 140.°C and 847 mmHg.The empirical formula of the compound is CH2.What is the molecular formula of the compound?
A)CH2
B)C2H4
C)C3H6
D)C4H8
E)C6H12
A)CH2
B)C2H4
C)C3H6
D)C4H8
E)C6H12

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
55
Determine the molar mass of chloroform gas if a sample weighing 0.389 g is collected in a flask with a volume of 102 cm3 at 97°C.The pressure of the chloroform is 728 mmHg.
A)8.28 × 10-3 g/mol
B)31.6 g/mol
C)112 g/mol
D)121g/mol
E)187g/mol
A)8.28 × 10-3 g/mol
B)31.6 g/mol
C)112 g/mol
D)121g/mol
E)187g/mol

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
56
A 792 mg sample of a gas containing only sulfur and oxygen occupies a volume of 372 mL at 54°C and 678 mmHg.Identify the gas in the sample.(R = 0.08206 L.atm/K.mol)
A)SO
B)SO2
C)SO3
D)S2O
E)S2O2
A)SO
B)SO2
C)SO3
D)S2O
E)S2O2

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
57
What volume of O2(g)at 810.mmHg pressure is required to react completely with a 4.50g sample of C(s)at 48°C? 2 C(s)+ O2(g) 2 CO(g)
A)1.22 L
B)3.47 L
C)4.63 L
D)9.26 L
E)111 L
A)1.22 L
B)3.47 L
C)4.63 L
D)9.26 L
E)111 L

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
58
A 1.17 g sample of an alkane hydrocarbon gas occupies a volume of 674 mL at 28°C and 741 mmHg.Alkanes are known to have the general formula CnH2n+2.What is the molecular formula of the gas in this sample? (R = 0.08206 L.atm/K.mol)
A)CH4
B)C2H6
C)C3H8
D)C4H10
E)C5H12
A)CH4
B)C2H6
C)C3H8
D)C4H10
E)C5H12

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
59
How many liters of chlorine gas at 25°C and 0.950 atm can be produced by the reaction of 12.0 g of MnO2 with excess HCl(aq)according to the following chemical equation? MnO2(s)+ 4HCl(aq) MnCl2(aq)+ 2H2O(l)+ Cl2(g)
A)5.36 × 10-3 L
B)0.138 L
C)0.282 L
D)3.09 L
E)3.55 L
A)5.36 × 10-3 L
B)0.138 L
C)0.282 L
D)3.09 L
E)3.55 L

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
60
A sample of hydrogen gas was collected over water at 21°C and 685 mmHg.The volume of the container was 7.80 L.Calculate the mass of H2(g)collected.(Vapor pressure of water = 18.6 mmHg at 21°C.)
A)0.283 g
B)0.572 g
C)0.589 g
D)7.14 g
E)435 g
A)0.283 g
B)0.572 g
C)0.589 g
D)7.14 g
E)435 g

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
61
If equal masses of O2(g)and HBr(g)are in separate containers of equal volume and temperature, which one of these statements is true?
A)The pressure in the O2 container is greater than that in the HBr container.
B)There are more HBr molecules than O2 molecules.
C)The average velocity of the O2 molecules is less than that of the HBr molecules.
D)The average kinetic energy of HBr molecules is greater than that of O2 molecules.
E)The pressures of both gases are the same.
A)The pressure in the O2 container is greater than that in the HBr container.
B)There are more HBr molecules than O2 molecules.
C)The average velocity of the O2 molecules is less than that of the HBr molecules.
D)The average kinetic energy of HBr molecules is greater than that of O2 molecules.
E)The pressures of both gases are the same.

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
62
Determine the pressure of the gas trapped in the apparatus shown below when the atmospheric pressure is 695 mmHg. 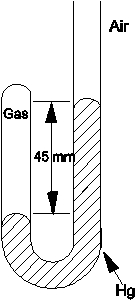
A)45 mmHg
B)650 mmHg
C)695 mmHg
D)740 mmHg
E)760 mmHg

A)45 mmHg
B)650 mmHg
C)695 mmHg
D)740 mmHg
E)760 mmHg

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
63
What mass of KClO3 must be decomposed to produce 126 L of oxygen gas at 133°C and 0.880 atm? (The other reaction product is solid KCl.)
A)24.6 g
B)70.8 g
C)272 g
D)408 g
E)612 g
A)24.6 g
B)70.8 g
C)272 g
D)408 g
E)612 g

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
64
Which statement is false?
A)The average kinetic energies of molecules from samples of different "ideal" gases is the same at the same temperature.
B)The molecules of an ideal gas are relatively far apart.
C)All molecules of an ideal gas have the same kinetic energy at constant temperature.
D)Molecules of a gas undergo many collisions with each other and the container walls.
E)Molecules of greater mass have a lower average speed than those of less mass at the same temperature.
A)The average kinetic energies of molecules from samples of different "ideal" gases is the same at the same temperature.
B)The molecules of an ideal gas are relatively far apart.
C)All molecules of an ideal gas have the same kinetic energy at constant temperature.
D)Molecules of a gas undergo many collisions with each other and the container walls.
E)Molecules of greater mass have a lower average speed than those of less mass at the same temperature.

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
65
A spacecraft is filled with 0.500 atm of N2 and 0.500 atm of O2.Suppose a micrometeor strikes this spacecraft and puts a very small hole in it's side.Under these circumstances,
A)O2 is lost from the craft 6.9% faster than N2 is lost.
B)O2 is lost from the craft 14% faster than N2 is lost.
C)N2 is lost from the craft 6.9% faster than O2 is lost.
D)N2 is lost from the craft 14% faster than O2 is lost.
E)N2 and O2 are lost from the craft at the same rate.
A)O2 is lost from the craft 6.9% faster than N2 is lost.
B)O2 is lost from the craft 14% faster than N2 is lost.
C)N2 is lost from the craft 6.9% faster than O2 is lost.
D)N2 is lost from the craft 14% faster than O2 is lost.
E)N2 and O2 are lost from the craft at the same rate.

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
66
A spacecraft is filled with 0.500 atm of O2 and 0.500 atm of He.If there is a very small hole in the side of this craft such that gas is lost slowly into outer space,
A)He is lost 2.8 times faster than O2 is lost.
B)He is lost 8 times faster than O2 is lost.
C)He is lost twice as fast as O2 is lost.
D)O2 is lost 2.8 times faster than He is lost.
E)O2 is lost 8 times faster than He is lost.
A)He is lost 2.8 times faster than O2 is lost.
B)He is lost 8 times faster than O2 is lost.
C)He is lost twice as fast as O2 is lost.
D)O2 is lost 2.8 times faster than He is lost.
E)O2 is lost 8 times faster than He is lost.

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
67
Liquid nitrogen has a density of 0.807 g/mL at -195.8 °C.If 1.00 L of N2(l)is allowed to warm to 25°C at a pressure of 1.00 atm, what volume will the gas occupy? (R = 0.08206 L.atm/K.mol)
A)59.1 L
B)182 L
C)705 L
D)1.41 × 103 L
E)1.97 × 104 L
A)59.1 L
B)182 L
C)705 L
D)1.41 × 103 L
E)1.97 × 104 L

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
68
What is the pressure of the gas trapped in the apparatus shown below when the atmospheric pressure is 720 mmHg? 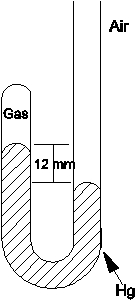
A)12 mmHg
B)708 mmHg
C)720 mmHg
D)732 mmHg
E)760 mmHg

A)12 mmHg
B)708 mmHg
C)720 mmHg
D)732 mmHg
E)760 mmHg

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
69
The molecules of different samples of an ideal gas have the same average kinetic energies, at the same
A)pressure.
B)temperature.
C)volume.
D)density.
A)pressure.
B)temperature.
C)volume.
D)density.

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
70
For a substance that remains a gas under the conditions listed, deviation from the ideal gas law would be most pronounced at
A)100°C and 2.0 atm.
B)0°C and 2.0 atm.
C)-100°C and 2.0 atm.
D)-100°C and 4.0 atm.
E)100°C and 4.0 atm.
A)100°C and 2.0 atm.
B)0°C and 2.0 atm.
C)-100°C and 2.0 atm.
D)-100°C and 4.0 atm.
E)100°C and 4.0 atm.

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
71
A sample of mercury(II)oxide is placed in a 5.00 L evacuated container and heated until it decomposes entirely to mercury metal and oxygen gas.The container is then cooled to 25°C.One now finds that the gas pressure inside the container is 1.73 atm.What mass of mercury(II)oxide was originally placed into the container?
A)1.51 g
B)45.6g
C)76.6 g
D)913 g
E)153 g
A)1.51 g
B)45.6g
C)76.6 g
D)913 g
E)153 g

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
72
Deviations from the ideal gas law are greater at
A)low temperatures and low pressures.
B)low temperatures and high pressures.
C)high temperatures and high pressures.
D)high temperatures and low pressures.
A)low temperatures and low pressures.
B)low temperatures and high pressures.
C)high temperatures and high pressures.
D)high temperatures and low pressures.

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
73
A method of removing CO2 from a spacecraft is to allow the CO2 to react with sodium hydroxide.(The products of the reaction are sodium carbonate and water.)What volume of carbon dioxide at 25°C and 749 mmHg can be removed per kilogram of sodium hydroxide that reacts?
A)276 L
B)284 L
C)301 L
D)310 L
E)620 L
A)276 L
B)284 L
C)301 L
D)310 L
E)620 L

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
74
When active metals such as magnesium are immersed in acid solution, hydrogen gas is evolved.Calculate the volume of H2(g)at 30.1°C and 0.85 atm that can be formed when 275 mL of 0.725 M HCl solution reacts with excess Mg to give hydrogen gas and aqueous magnesium chloride.
A)3.4 × 10-3 L
B)2.2 L
C)2.9 L
D)5.8 L
E)11.7 L
A)3.4 × 10-3 L
B)2.2 L
C)2.9 L
D)5.8 L
E)11.7 L

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
75
Which gas has molecules with the greatest average molecular speed at 25°C?
A)CH4
B)Kr
C)N2
D)CO2
E)Ar
A)CH4
B)Kr
C)N2
D)CO2
E)Ar

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
76
Samples of the following volatile liquids are opened simultaneously at one end of a room.If you are standing at the opposite end of this room, which species would you smell first? (Assume that your nose is equally sensitive to all these species.)
A)ethyl acetate (CH3COOC2H5)
B)camphor (C10H16O)
C)diethyl ether (C2H5OC2H5)
D)naphthalene (C10H8)
E)pentanethiol (C5H11SH)
A)ethyl acetate (CH3COOC2H5)
B)camphor (C10H16O)
C)diethyl ether (C2H5OC2H5)
D)naphthalene (C10H8)
E)pentanethiol (C5H11SH)

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
77
Which of these gas molecules have the highest average kinetic energy at 25°C?
A)H2
B)O2
C)N2
D)Cl2
E)All the gases have the same average kinetic energy.
A)H2
B)O2
C)N2
D)Cl2
E)All the gases have the same average kinetic energy.

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
78
The mole fraction of oxygen molecules in dry air is 0.2095.What volume of dry air at 1.00 atm and 25°C is required for burning 1.00 L of octane (C8H18, density = 0.7025 g/mL)completely, yielding carbon dioxide and water?
A)150 L
B)367 L
C)718 L
D)1880 L
E)8970 L
A)150 L
B)367 L
C)718 L
D)1880 L
E)8970 L

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
79
The mole fraction of oxygen molecules in dry air is 0.2095.What volume of dry air at 1.00 atm and 25°C is required for burning 1.00 L of hexane (C6H14, density = 0.660 g/mL)completely, yielding carbon dioxide and water?
A)187 L
B)712 L
C)894 L
D)1780 L
E)8490 L
A)187 L
B)712 L
C)894 L
D)1780 L
E)8490 L

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
80
Calculate the volume of H2(g)at 273 K and 2.00 atm that will be formed when 275 mL of 0.725 M HCl solution reacts with excess Mg to give hydrogen gas and aqueous magnesium chloride.
A)0.56 L
B)1.12 L
C)2.23 L
D)3.54 L
E)4.47 L
A)0.56 L
B)1.12 L
C)2.23 L
D)3.54 L
E)4.47 L

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck



