Deck 19: Nuclear Chemistry
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/133
Play
Full screen (f)
Deck 19: Nuclear Chemistry
1
Consider the following decay series: 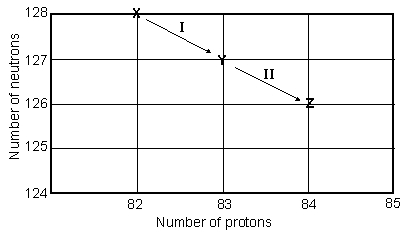 What type of nuclear process occurs at the transformation labeled I?
What type of nuclear process occurs at the transformation labeled I?
A)( emission)
B)( emission)
C)positron emission
D)electron capture
E)gamma radiation
 What type of nuclear process occurs at the transformation labeled I?
What type of nuclear process occurs at the transformation labeled I?A)( emission)
B)( emission)
C)positron emission
D)electron capture
E)gamma radiation
( emission)
2
Consider the following decay series: 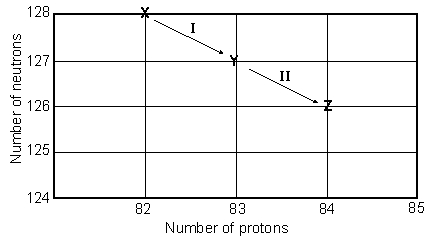
What type of nuclear process occurs at the transformation labeled II?
A)( emission)
B)( emission)
C)positron emission
D)electron capture
E)gamma radiation

What type of nuclear process occurs at the transformation labeled II?
A)( emission)
B)( emission)
C)positron emission
D)electron capture
E)gamma radiation
( emission)
3
List the number of protons, neutrons, and nucleons (protons + neutrons), in that order, for an isotope with the symbol: 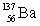
A)56, 81, 137
B)81, 56, 137
C)56, 137, 193
D)137, 56, 193
E)81, 137, 218
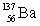
A)56, 81, 137
B)81, 56, 137
C)56, 137, 193
D)137, 56, 193
E)81, 137, 218
56, 81, 137
4
List the number of protons, neutrons, and nucleons (protons + neutrons), in that order, for an isotope with the symbol: 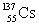
A)137, 55, 192
B)55, 137, 192
C)55, 82, 137
D)82, 55, 137
E)82, 137, 219
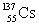
A)137, 55, 192
B)55, 137, 192
C)55, 82, 137
D)82, 55, 137
E)82, 137, 219

Unlock Deck
Unlock for access to all 133 flashcards in this deck.
Unlock Deck
k this deck
5
Which of the following is characteristic of a nuclear reaction? I.Electrons in atomic orbitals are involved in the breaking and forming of bonds
II.Elements are converted from one to another
III.Reaction rates are typically not affected by catalysts
IV.Atoms are rearranged by the breaking and forming of chemical bonds
A)I and II
B)I, II, and III
C)III and IV
D)II and III
E)I, II, III, and IV
II.Elements are converted from one to another
III.Reaction rates are typically not affected by catalysts
IV.Atoms are rearranged by the breaking and forming of chemical bonds
A)I and II
B)I, II, and III
C)III and IV
D)II and III
E)I, II, III, and IV

Unlock Deck
Unlock for access to all 133 flashcards in this deck.
Unlock Deck
k this deck
6
Consider the following decay series: 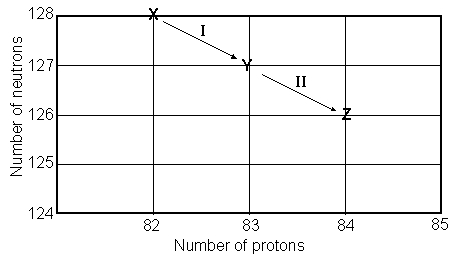 What is the complete element symbol for the product labeled Y?
What is the complete element symbol for the product labeled Y?
A)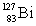
B)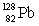
C)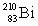
D)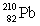
E)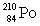
 What is the complete element symbol for the product labeled Y?
What is the complete element symbol for the product labeled Y?A)
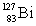
B)
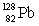
C)
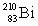
D)
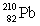
E)
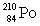

Unlock Deck
Unlock for access to all 133 flashcards in this deck.
Unlock Deck
k this deck
7
Alpha particles are identical to
A)protons.
B)helium atoms.
C)hydrogen atoms.
D)helium nuclei.
E)electrons.
A)protons.
B)helium atoms.
C)hydrogen atoms.
D)helium nuclei.
E)electrons.

Unlock Deck
Unlock for access to all 133 flashcards in this deck.
Unlock Deck
k this deck
8
Consider the following decay series: 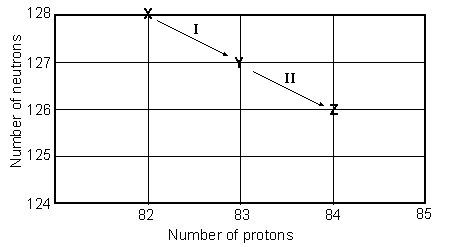 What is the symbol for the product labeled as Z?
What is the symbol for the product labeled as Z?
A)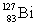
B)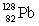
C)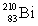
D)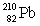
E)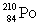
 What is the symbol for the product labeled as Z?
What is the symbol for the product labeled as Z?A)
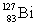
B)
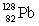
C)
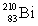
D)
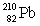
E)
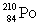

Unlock Deck
Unlock for access to all 133 flashcards in this deck.
Unlock Deck
k this deck
9
As a result of beta decay, the product nucleus is
A)one atomic number lower than the original element.
B)two atomic numbers higher than the original element.
C)one atomic number higher than the original element.
D)two atomic numbers lower than the original element.
E)four atomic numbers lower than the original element.
A)one atomic number lower than the original element.
B)two atomic numbers higher than the original element.
C)one atomic number higher than the original element.
D)two atomic numbers lower than the original element.
E)four atomic numbers lower than the original element.

Unlock Deck
Unlock for access to all 133 flashcards in this deck.
Unlock Deck
k this deck
10
When atoms of aluminum-27 are bombarded with alpha particles, a neutron and an element are produced.The particular isotope formed is 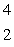 He +
He +  Al
Al 
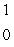 n + ____
n + ____
A)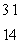 Si
Si
B)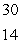 Si
Si
C)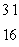 S
S
D)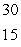 P
P
E)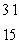 P
P
 He +
He + 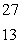 Al
Al 
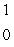 n + ____
n + ____A)
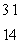 Si
SiB)
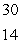 Si
SiC)
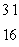 S
SD)
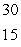 P
PE)
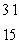 P
P
Unlock Deck
Unlock for access to all 133 flashcards in this deck.
Unlock Deck
k this deck
11
Consider the following decay series: 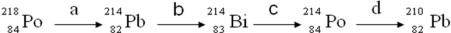 Which type of nuclear process occurs in steps a and b, respectively?
Which type of nuclear process occurs in steps a and b, respectively?
A)( emission, emission)
B)( emission, emission)
C)( emission, positron emission)
D)( emission, emission)
E)(positron emission, emission)
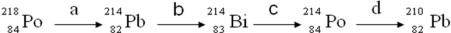 Which type of nuclear process occurs in steps a and b, respectively?
Which type of nuclear process occurs in steps a and b, respectively?A)( emission, emission)
B)( emission, emission)
C)( emission, positron emission)
D)( emission, emission)
E)(positron emission, emission)

Unlock Deck
Unlock for access to all 133 flashcards in this deck.
Unlock Deck
k this deck
12
What is the missing symbol in this plutonium fission reaction? 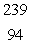 Pu +
Pu +  n
n  ______ +
______ + 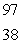 Sr + 3
Sr + 3 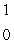 n
n
A)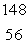 Ba
Ba
B)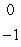
C)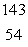 Xe
Xe
D)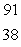 Sr
Sr
E)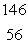 Ba
Ba
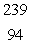 Pu +
Pu + 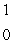 n
n  ______ +
______ + 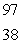 Sr + 3
Sr + 3  n
nA)
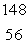 Ba
BaB)
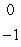
C)
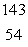 Xe
XeD)
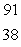 Sr
SrE)
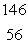 Ba
Ba
Unlock Deck
Unlock for access to all 133 flashcards in this deck.
Unlock Deck
k this deck
13
Predict the other product of the following nuclear transformation. 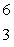 Li +
Li + 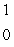 n
n  ____ +
____ + 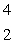 He
He
A)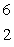 He
He
B)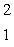 H
H
C)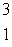 H
H
D)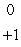
E)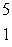 H
H
 Li +
Li +  n
n  ____ +
____ + 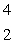 He
HeA)
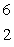 He
HeB)
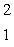 H
HC)
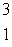 H
HD)
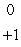
E)
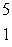 H
H
Unlock Deck
Unlock for access to all 133 flashcards in this deck.
Unlock Deck
k this deck
14
As a result of alpha emission, the product nucleus is
A)one atomic number lower than the original element.
B)two atomic numbers higher than the original element.
C)one atomic number higher than the original element.
D)two atomic numbers lower than the original element.
E)four atomic numbers lower than the original element.
A)one atomic number lower than the original element.
B)two atomic numbers higher than the original element.
C)one atomic number higher than the original element.
D)two atomic numbers lower than the original element.
E)four atomic numbers lower than the original element.

Unlock Deck
Unlock for access to all 133 flashcards in this deck.
Unlock Deck
k this deck
15
When atoms of beryllium-9 are bombarded with alpha particles, neutrons are produced.What new isotope is also formed? 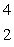 He +
He +  Be
Be 
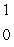 n + _____
n + _____
A)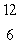 C
C
B)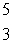 Li
Li
C)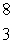 Li
Li
D)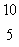 B
B
E)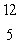 B
B
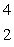 He +
He + 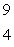 Be
Be 
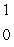 n + _____
n + _____A)
 C
CB)
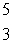 Li
LiC)
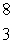 Li
LiD)
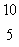 B
BE)
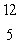 B
B
Unlock Deck
Unlock for access to all 133 flashcards in this deck.
Unlock Deck
k this deck
16
Which of the following represents a rule for balancing a nuclear equation? I.The total number of protons plus neutrons in the products and reactants must be the same
II.The total number of each type of element in the products and reactants must be the same
III.The total number of nuclear charges in the products and reactants must be the same
IV.The total number of each type of elementary particle in the products and reactants must be the same
A)I, II, III, and IV
B)I, II, and IV
C)I, III, and IV
D)III and IV
E)I and III
II.The total number of each type of element in the products and reactants must be the same
III.The total number of nuclear charges in the products and reactants must be the same
IV.The total number of each type of elementary particle in the products and reactants must be the same
A)I, II, III, and IV
B)I, II, and IV
C)I, III, and IV
D)III and IV
E)I and III

Unlock Deck
Unlock for access to all 133 flashcards in this deck.
Unlock Deck
k this deck
17
Consider the following decay series: 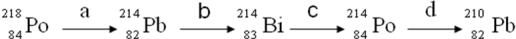 Which type of nuclear process occurs in steps c and d, respectively?
Which type of nuclear process occurs in steps c and d, respectively?
A)( emission, emission)
B)( emission, emission)
C)( emission, positron emission)
D)( emission, emission)
E)(positron emission, emission)
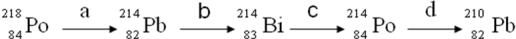 Which type of nuclear process occurs in steps c and d, respectively?
Which type of nuclear process occurs in steps c and d, respectively?A)( emission, emission)
B)( emission, emission)
C)( emission, positron emission)
D)( emission, emission)
E)(positron emission, emission)

Unlock Deck
Unlock for access to all 133 flashcards in this deck.
Unlock Deck
k this deck
18
Radium-226 decays by alpha emission.What is its decay product?
A)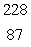 Fr
Fr
B)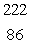 Rn
Rn
C)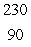 Th
Th
D)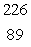 Ac
Ac
E)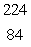 Po
Po
A)
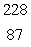 Fr
FrB)
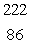 Rn
RnC)
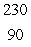 Th
ThD)
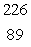 Ac
AcE)
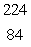 Po
Po
Unlock Deck
Unlock for access to all 133 flashcards in this deck.
Unlock Deck
k this deck
19
Sulfur-35 decays by beta emission.The decay product is
A)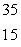 P
P
B)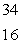 S
S
C)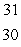 Si
Si
D)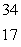 Cl
Cl
E)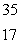 Cl
Cl
A)
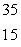 P
PB)
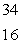 S
SC)
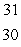 Si
SiD)
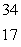 Cl
ClE)
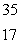 Cl
Cl
Unlock Deck
Unlock for access to all 133 flashcards in this deck.
Unlock Deck
k this deck
20
Beta particles are identical to
A)protons.
B)helium atoms.
C)hydrogen atoms.
D)helium nuclei.
E)electrons.
A)protons.
B)helium atoms.
C)hydrogen atoms.
D)helium nuclei.
E)electrons.

Unlock Deck
Unlock for access to all 133 flashcards in this deck.
Unlock Deck
k this deck
21
Consider the following decay series: 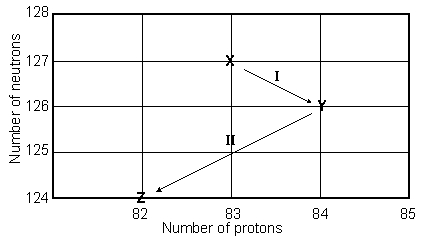 What is the symbol for the product labeled as Z?
What is the symbol for the product labeled as Z?
A)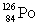
B)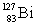
C)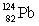
D)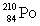
E)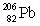
 What is the symbol for the product labeled as Z?
What is the symbol for the product labeled as Z?A)
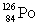
B)
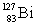
C)
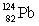
D)
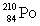
E)
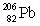

Unlock Deck
Unlock for access to all 133 flashcards in this deck.
Unlock Deck
k this deck
22
Consider the following decay series: 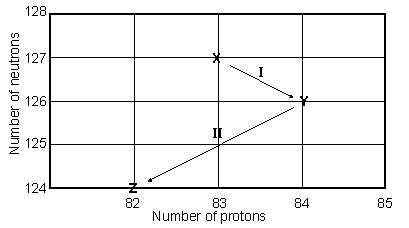 What type of nuclear process occurs at the transformation labeled I?
What type of nuclear process occurs at the transformation labeled I?
A)( emission)
B)( emission)
C)positron emission
D)electron capture
E)gamma radiation
 What type of nuclear process occurs at the transformation labeled I?
What type of nuclear process occurs at the transformation labeled I?A)( emission)
B)( emission)
C)positron emission
D)electron capture
E)gamma radiation

Unlock Deck
Unlock for access to all 133 flashcards in this deck.
Unlock Deck
k this deck
23
Determine how much energy is released when thorium-230 decays according to ![<strong>Determine how much energy is released when thorium-230 decays according to <sup> </sup> .[Atomic masses: thorium-230 = 230.033127 amu; helium-4 = 4.002603 amu; radium-226 = 226.025403 amu]</strong> A)3.98 × 10<sup>9</sup> kJ/mol B)4.60 × 10<sup>8</sup> kJ/mol C)7.20 × 10<sup>11</sup> kJ/mol D)4.90 × 10<sup>9</sup> kJ/mol E)7.15 × 10<sup>11</sup> kJ/mol](https://d2lvgg3v3hfg70.cloudfront.net/TB3246/11ea7cbf_8f3e_3dd0_a2ab_c563d2a78863_TB3246_11.jpg) .[Atomic masses: thorium-230 = 230.033127 amu; helium-4 = 4.002603 amu; radium-226 = 226.025403 amu]
.[Atomic masses: thorium-230 = 230.033127 amu; helium-4 = 4.002603 amu; radium-226 = 226.025403 amu]
A)3.98 × 109 kJ/mol
B)4.60 × 108 kJ/mol
C)7.20 × 1011 kJ/mol
D)4.90 × 109 kJ/mol
E)7.15 × 1011 kJ/mol
![<strong>Determine how much energy is released when thorium-230 decays according to <sup> </sup> .[Atomic masses: thorium-230 = 230.033127 amu; helium-4 = 4.002603 amu; radium-226 = 226.025403 amu]</strong> A)3.98 × 10<sup>9</sup> kJ/mol B)4.60 × 10<sup>8</sup> kJ/mol C)7.20 × 10<sup>11</sup> kJ/mol D)4.90 × 10<sup>9</sup> kJ/mol E)7.15 × 10<sup>11</sup> kJ/mol](https://d2lvgg3v3hfg70.cloudfront.net/TB3246/11ea7cbf_8f3e_3dd0_a2ab_c563d2a78863_TB3246_11.jpg) .[Atomic masses: thorium-230 = 230.033127 amu; helium-4 = 4.002603 amu; radium-226 = 226.025403 amu]
.[Atomic masses: thorium-230 = 230.033127 amu; helium-4 = 4.002603 amu; radium-226 = 226.025403 amu]A)3.98 × 109 kJ/mol
B)4.60 × 108 kJ/mol
C)7.20 × 1011 kJ/mol
D)4.90 × 109 kJ/mol
E)7.15 × 1011 kJ/mol

Unlock Deck
Unlock for access to all 133 flashcards in this deck.
Unlock Deck
k this deck
24
What is the nuclear binding energy per nucleon, in joules, for ![<strong>What is the nuclear binding energy per nucleon, in joules, for (atomic mass 24.985839 amu). [Data: (atomic mass)= 1.007825 amu; (mass)= 1.008665 amu; 1 kg = 6.022 × 10<sup>26</sup> amu; c = 3.00 × 10<sup>8</sup> m/s]</strong> A)0.22076 J/nucleon B)3.30 × 10<sup>-11</sup> J/nucleon C)1.32 × 10<sup>-12</sup> J/nucleon D)0.999 J/nucleon E)None of the above.](https://d2lvgg3v3hfg70.cloudfront.net/TB3246/11ea7cbf_8f3d_5369_a2ab_d72687d979d2_TB3246_11.jpg) (atomic mass 24.985839 amu). [Data:
(atomic mass 24.985839 amu). [Data: ![<strong>What is the nuclear binding energy per nucleon, in joules, for (atomic mass 24.985839 amu). [Data: (atomic mass)= 1.007825 amu; (mass)= 1.008665 amu; 1 kg = 6.022 × 10<sup>26</sup> amu; c = 3.00 × 10<sup>8</sup> m/s]</strong> A)0.22076 J/nucleon B)3.30 × 10<sup>-11</sup> J/nucleon C)1.32 × 10<sup>-12</sup> J/nucleon D)0.999 J/nucleon E)None of the above.](https://d2lvgg3v3hfg70.cloudfront.net/TB3246/11ea7cbf_8f3d_7a7a_a2ab_9f07c9c9a677_TB3246_11.jpg) (atomic mass)= 1.007825 amu;
(atomic mass)= 1.007825 amu; ![<strong>What is the nuclear binding energy per nucleon, in joules, for (atomic mass 24.985839 amu). [Data: (atomic mass)= 1.007825 amu; (mass)= 1.008665 amu; 1 kg = 6.022 × 10<sup>26</sup> amu; c = 3.00 × 10<sup>8</sup> m/s]</strong> A)0.22076 J/nucleon B)3.30 × 10<sup>-11</sup> J/nucleon C)1.32 × 10<sup>-12</sup> J/nucleon D)0.999 J/nucleon E)None of the above.](https://d2lvgg3v3hfg70.cloudfront.net/TB3246/11ea7cbf_8f3d_7a7b_a2ab_f7edb8a75f7d_TB3246_11.jpg) (mass)= 1.008665 amu; 1 kg = 6.022 × 1026 amu; c = 3.00 × 108 m/s]
(mass)= 1.008665 amu; 1 kg = 6.022 × 1026 amu; c = 3.00 × 108 m/s]
A)0.22076 J/nucleon
B)3.30 × 10-11 J/nucleon
C)1.32 × 10-12 J/nucleon
D)0.999 J/nucleon
E)None of the above.
![<strong>What is the nuclear binding energy per nucleon, in joules, for (atomic mass 24.985839 amu). [Data: (atomic mass)= 1.007825 amu; (mass)= 1.008665 amu; 1 kg = 6.022 × 10<sup>26</sup> amu; c = 3.00 × 10<sup>8</sup> m/s]</strong> A)0.22076 J/nucleon B)3.30 × 10<sup>-11</sup> J/nucleon C)1.32 × 10<sup>-12</sup> J/nucleon D)0.999 J/nucleon E)None of the above.](https://d2lvgg3v3hfg70.cloudfront.net/TB3246/11ea7cbf_8f3d_5369_a2ab_d72687d979d2_TB3246_11.jpg) (atomic mass 24.985839 amu). [Data:
(atomic mass 24.985839 amu). [Data: ![<strong>What is the nuclear binding energy per nucleon, in joules, for (atomic mass 24.985839 amu). [Data: (atomic mass)= 1.007825 amu; (mass)= 1.008665 amu; 1 kg = 6.022 × 10<sup>26</sup> amu; c = 3.00 × 10<sup>8</sup> m/s]</strong> A)0.22076 J/nucleon B)3.30 × 10<sup>-11</sup> J/nucleon C)1.32 × 10<sup>-12</sup> J/nucleon D)0.999 J/nucleon E)None of the above.](https://d2lvgg3v3hfg70.cloudfront.net/TB3246/11ea7cbf_8f3d_7a7a_a2ab_9f07c9c9a677_TB3246_11.jpg) (atomic mass)= 1.007825 amu;
(atomic mass)= 1.007825 amu; ![<strong>What is the nuclear binding energy per nucleon, in joules, for (atomic mass 24.985839 amu). [Data: (atomic mass)= 1.007825 amu; (mass)= 1.008665 amu; 1 kg = 6.022 × 10<sup>26</sup> amu; c = 3.00 × 10<sup>8</sup> m/s]</strong> A)0.22076 J/nucleon B)3.30 × 10<sup>-11</sup> J/nucleon C)1.32 × 10<sup>-12</sup> J/nucleon D)0.999 J/nucleon E)None of the above.](https://d2lvgg3v3hfg70.cloudfront.net/TB3246/11ea7cbf_8f3d_7a7b_a2ab_f7edb8a75f7d_TB3246_11.jpg) (mass)= 1.008665 amu; 1 kg = 6.022 × 1026 amu; c = 3.00 × 108 m/s]
(mass)= 1.008665 amu; 1 kg = 6.022 × 1026 amu; c = 3.00 × 108 m/s]A)0.22076 J/nucleon
B)3.30 × 10-11 J/nucleon
C)1.32 × 10-12 J/nucleon
D)0.999 J/nucleon
E)None of the above.

Unlock Deck
Unlock for access to all 133 flashcards in this deck.
Unlock Deck
k this deck
25
Which of the following nuclear processes does not change the mass number in the product element formed? I.Alpha emission
II.Beta emission
III.Positron emission
IV.Electron capture
A)I and II
B)III and IV
C)I, II, and III
D)II, III, and IV
E)IV only
II.Beta emission
III.Positron emission
IV.Electron capture
A)I and II
B)III and IV
C)I, II, and III
D)II, III, and IV
E)IV only

Unlock Deck
Unlock for access to all 133 flashcards in this deck.
Unlock Deck
k this deck
26
Calculate the energy released in joules when one mole of polonium-214 decays according to the equation ![<strong>Calculate the energy released in joules when one mole of polonium-214 decays according to the equation Po Pb + He. [Atomic masses: Pb-210 = 209.98284 amu, Po-214 = 213.99519 amu, He-4 = 4.00260 amu.]</strong> A)8.78 × 10<sup>14</sup> J/mol B)7.2 × 10<sup>14</sup> J/mol C)8.78 × 10<sup>11</sup> J/mol D)-9.75 × 10<sup>-3</sup> J/mol E)1.46 × 10<sup>-9</sup> J/mol](https://d2lvgg3v3hfg70.cloudfront.net/TB3246/11ea7cbf_8f3d_efac_a2ab_659e81ceb826_TB3246_11.jpg) Po
Po ![<strong>Calculate the energy released in joules when one mole of polonium-214 decays according to the equation Po Pb + He. [Atomic masses: Pb-210 = 209.98284 amu, Po-214 = 213.99519 amu, He-4 = 4.00260 amu.]</strong> A)8.78 × 10<sup>14</sup> J/mol B)7.2 × 10<sup>14</sup> J/mol C)8.78 × 10<sup>11</sup> J/mol D)-9.75 × 10<sup>-3</sup> J/mol E)1.46 × 10<sup>-9</sup> J/mol](https://d2lvgg3v3hfg70.cloudfront.net/TB3246/11ea7cbf_8f3d_efad_a2ab_51c2383d410c_TB3246_11.jpg)
![<strong>Calculate the energy released in joules when one mole of polonium-214 decays according to the equation Po Pb + He. [Atomic masses: Pb-210 = 209.98284 amu, Po-214 = 213.99519 amu, He-4 = 4.00260 amu.]</strong> A)8.78 × 10<sup>14</sup> J/mol B)7.2 × 10<sup>14</sup> J/mol C)8.78 × 10<sup>11</sup> J/mol D)-9.75 × 10<sup>-3</sup> J/mol E)1.46 × 10<sup>-9</sup> J/mol](https://d2lvgg3v3hfg70.cloudfront.net/TB3246/11ea7cbf_8f3e_16be_a2ab_99924e5bfde6_TB3246_11.jpg) Pb +
Pb + ![<strong>Calculate the energy released in joules when one mole of polonium-214 decays according to the equation Po Pb + He. [Atomic masses: Pb-210 = 209.98284 amu, Po-214 = 213.99519 amu, He-4 = 4.00260 amu.]</strong> A)8.78 × 10<sup>14</sup> J/mol B)7.2 × 10<sup>14</sup> J/mol C)8.78 × 10<sup>11</sup> J/mol D)-9.75 × 10<sup>-3</sup> J/mol E)1.46 × 10<sup>-9</sup> J/mol](https://d2lvgg3v3hfg70.cloudfront.net/TB3246/11ea7cbf_8f3e_16bf_a2ab_edc130e4adfd_TB3246_11.jpg) He. [Atomic masses: Pb-210 = 209.98284 amu, Po-214 = 213.99519 amu, He-4 = 4.00260 amu.]
He. [Atomic masses: Pb-210 = 209.98284 amu, Po-214 = 213.99519 amu, He-4 = 4.00260 amu.]
A)8.78 × 1014 J/mol
B)7.2 × 1014 J/mol
C)8.78 × 1011 J/mol
D)-9.75 × 10-3 J/mol
E)1.46 × 10-9 J/mol
![<strong>Calculate the energy released in joules when one mole of polonium-214 decays according to the equation Po Pb + He. [Atomic masses: Pb-210 = 209.98284 amu, Po-214 = 213.99519 amu, He-4 = 4.00260 amu.]</strong> A)8.78 × 10<sup>14</sup> J/mol B)7.2 × 10<sup>14</sup> J/mol C)8.78 × 10<sup>11</sup> J/mol D)-9.75 × 10<sup>-3</sup> J/mol E)1.46 × 10<sup>-9</sup> J/mol](https://d2lvgg3v3hfg70.cloudfront.net/TB3246/11ea7cbf_8f3d_efac_a2ab_659e81ceb826_TB3246_11.jpg) Po
Po ![<strong>Calculate the energy released in joules when one mole of polonium-214 decays according to the equation Po Pb + He. [Atomic masses: Pb-210 = 209.98284 amu, Po-214 = 213.99519 amu, He-4 = 4.00260 amu.]</strong> A)8.78 × 10<sup>14</sup> J/mol B)7.2 × 10<sup>14</sup> J/mol C)8.78 × 10<sup>11</sup> J/mol D)-9.75 × 10<sup>-3</sup> J/mol E)1.46 × 10<sup>-9</sup> J/mol](https://d2lvgg3v3hfg70.cloudfront.net/TB3246/11ea7cbf_8f3d_efad_a2ab_51c2383d410c_TB3246_11.jpg)
![<strong>Calculate the energy released in joules when one mole of polonium-214 decays according to the equation Po Pb + He. [Atomic masses: Pb-210 = 209.98284 amu, Po-214 = 213.99519 amu, He-4 = 4.00260 amu.]</strong> A)8.78 × 10<sup>14</sup> J/mol B)7.2 × 10<sup>14</sup> J/mol C)8.78 × 10<sup>11</sup> J/mol D)-9.75 × 10<sup>-3</sup> J/mol E)1.46 × 10<sup>-9</sup> J/mol](https://d2lvgg3v3hfg70.cloudfront.net/TB3246/11ea7cbf_8f3e_16be_a2ab_99924e5bfde6_TB3246_11.jpg) Pb +
Pb + ![<strong>Calculate the energy released in joules when one mole of polonium-214 decays according to the equation Po Pb + He. [Atomic masses: Pb-210 = 209.98284 amu, Po-214 = 213.99519 amu, He-4 = 4.00260 amu.]</strong> A)8.78 × 10<sup>14</sup> J/mol B)7.2 × 10<sup>14</sup> J/mol C)8.78 × 10<sup>11</sup> J/mol D)-9.75 × 10<sup>-3</sup> J/mol E)1.46 × 10<sup>-9</sup> J/mol](https://d2lvgg3v3hfg70.cloudfront.net/TB3246/11ea7cbf_8f3e_16bf_a2ab_edc130e4adfd_TB3246_11.jpg) He. [Atomic masses: Pb-210 = 209.98284 amu, Po-214 = 213.99519 amu, He-4 = 4.00260 amu.]
He. [Atomic masses: Pb-210 = 209.98284 amu, Po-214 = 213.99519 amu, He-4 = 4.00260 amu.]A)8.78 × 1014 J/mol
B)7.2 × 1014 J/mol
C)8.78 × 1011 J/mol
D)-9.75 × 10-3 J/mol
E)1.46 × 10-9 J/mol

Unlock Deck
Unlock for access to all 133 flashcards in this deck.
Unlock Deck
k this deck
27
Which of the following nuclear processes results in an increase of atomic number in the product element formed? I.Alpha emission
II.Beta emission
III.Positron emission
IV.Electron capture
A)I and II
B)III and IV
C)I, II, and III
D)II only
E)IV only
II.Beta emission
III.Positron emission
IV.Electron capture
A)I and II
B)III and IV
C)I, II, and III
D)II only
E)IV only

Unlock Deck
Unlock for access to all 133 flashcards in this deck.
Unlock Deck
k this deck
28
Find the nuclear binding energy of uranium-234 (atomic mass = 234.040947 amu)in units of joules per nucleon.[Data: neutron mass = 1.674928 × 10-24 g; proton mass = 1.672623 × 10-24 g; electron mass = 9.109387 × 10-28 g; NA = 6.0221367 × 1023 /mol; c = 2.99792458 × 108 m/s]
A)2.97 × 10-10 J/nucleon
B)3.04 × 10-10 J/nucleon
C)1.30 × 10-12 J/nucleon
D)1.22 × 10-12 J/nucleon
E)1.27 × 10-12 J/nucleon
A)2.97 × 10-10 J/nucleon
B)3.04 × 10-10 J/nucleon
C)1.30 × 10-12 J/nucleon
D)1.22 × 10-12 J/nucleon
E)1.27 × 10-12 J/nucleon

Unlock Deck
Unlock for access to all 133 flashcards in this deck.
Unlock Deck
k this deck
29
Which of the following nuclear processes results in an increase in the number of neutrons in the product element? I.Alpha emission
II.Beta emission
III.Positron emission
IV.Electron capture
A)I and II
B)III and IV
C)I, II, and III
D)II, III, and IV
E)I, II, III, and IV
II.Beta emission
III.Positron emission
IV.Electron capture
A)I and II
B)III and IV
C)I, II, and III
D)II, III, and IV
E)I, II, III, and IV

Unlock Deck
Unlock for access to all 133 flashcards in this deck.
Unlock Deck
k this deck
30
The energy equivalent of a mass defect of - 0.1710 amu is which of the following?
A)- 5.130 x 107 J
B)- 1.539 x 1016 J
C)- 2.556 x 10-8 J
D)- 2.556 x 10-11 J
E)- 8.519 x 10-20 J
A)- 5.130 x 107 J
B)- 1.539 x 1016 J
C)- 2.556 x 10-8 J
D)- 2.556 x 10-11 J
E)- 8.519 x 10-20 J

Unlock Deck
Unlock for access to all 133 flashcards in this deck.
Unlock Deck
k this deck
31
The energy equivalent of a mass defect of - 0.1620 amu is which of the following?
A)- 1.458 x 1016 J
B)- 2.421 x 10-8 J
C)- 2.421 x 10-11 J
D)- 2.421 x 1013 J
E)- 1.458 x 10-11 J
A)- 1.458 x 1016 J
B)- 2.421 x 10-8 J
C)- 2.421 x 10-11 J
D)- 2.421 x 1013 J
E)- 1.458 x 10-11 J

Unlock Deck
Unlock for access to all 133 flashcards in this deck.
Unlock Deck
k this deck
32
A balanced nuclear equation representing the beta emission of iodine-131 is which of the following?
A)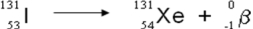
B)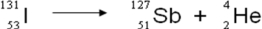
C)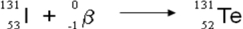
D)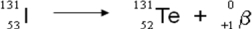
E)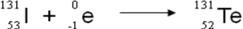
A)
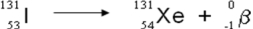
B)
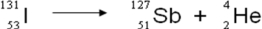
C)
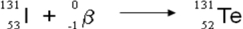
D)
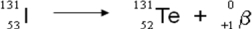
E)
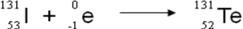

Unlock Deck
Unlock for access to all 133 flashcards in this deck.
Unlock Deck
k this deck
33
The isotope with the greatest nuclear binding energy per nucleon is
A)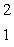 H
H
B)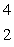 He
He
C)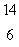 C
C
D)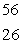 Fe
Fe
E)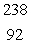 U
U
A)
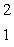 H
HB)
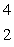 He
HeC)
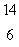 C
CD)
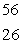 Fe
FeE)
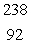 U
U
Unlock Deck
Unlock for access to all 133 flashcards in this deck.
Unlock Deck
k this deck
34
A balanced nuclear equation representing the alpha emission of curium-242 is which of the following?
A)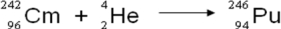
B)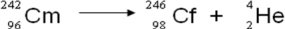
C)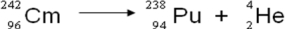
D)
E)
A)
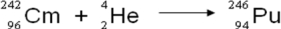
B)
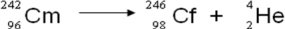
C)
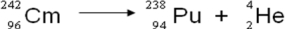
D)

E)


Unlock Deck
Unlock for access to all 133 flashcards in this deck.
Unlock Deck
k this deck
35
The only stable isotope of iodine is iodine-127.Predict the mode of decay of 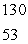 I.
I.
A)alpha emission
B)beta emission
C)positron emission
D)electron capture
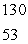 I.
I.A)alpha emission
B)beta emission
C)positron emission
D)electron capture

Unlock Deck
Unlock for access to all 133 flashcards in this deck.
Unlock Deck
k this deck
36
Find the nuclear binding energy of potassium-40 (atomic mass = 39.9632591 amu)in units of joules per nucleon.[Data: neutron mass = 1.674928 × 10-24 g; proton mass = 1.672623 × 10-24g; electron mass = 9.109387 × 10-28 g; NA = 6.0221367 × 1023 /mol; c = 2.99792458 × 108 m/s]
A)1.37 × 10-12 J/nucleon
B)5.48 × 10-11 J/nucleon
C)5.64 × 10-11 J/nucleon
D)1.41 × 10-12 J/nucleon
E)2.97 × 10-12 J/nucleon
A)1.37 × 10-12 J/nucleon
B)5.48 × 10-11 J/nucleon
C)5.64 × 10-11 J/nucleon
D)1.41 × 10-12 J/nucleon
E)2.97 × 10-12 J/nucleon

Unlock Deck
Unlock for access to all 133 flashcards in this deck.
Unlock Deck
k this deck
37
A typical radius of an atomic nucleus is about
A)100 µm
B)5000 mm
C)100 nm
D)5 × 10-3 pm
E)500 pm
A)100 µm
B)5000 mm
C)100 nm
D)5 × 10-3 pm
E)500 pm

Unlock Deck
Unlock for access to all 133 flashcards in this deck.
Unlock Deck
k this deck
38
The only stable isotope of aluminum is aluminum-27.What type of radioactive decay should be expected from 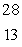 Al?
Al?
A)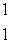 H
H
B)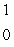 n
n
C)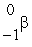
D)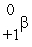
E)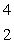 He
He
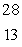 Al?
Al?A)
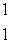 H
HB)
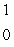 n
nC)
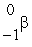
D)
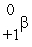
E)
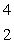 He
He
Unlock Deck
Unlock for access to all 133 flashcards in this deck.
Unlock Deck
k this deck
39
Consider the following decay series: 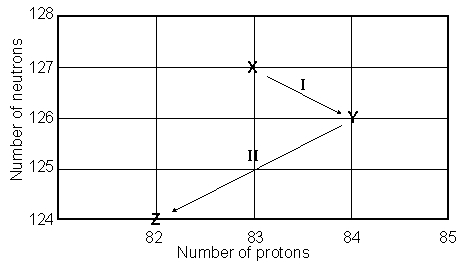 What is the symbol for the product labeled as Y?
What is the symbol for the product labeled as Y?
A)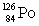
B)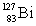
C)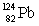
D)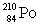
E)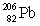
 What is the symbol for the product labeled as Y?
What is the symbol for the product labeled as Y?A)
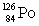
B)
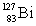
C)
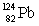
D)
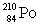
E)
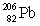

Unlock Deck
Unlock for access to all 133 flashcards in this deck.
Unlock Deck
k this deck
40
Consider the following decay series: 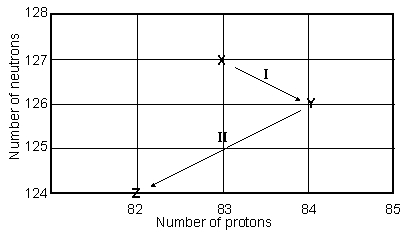 What type of nuclear process occurs at the transformation labeled II?
What type of nuclear process occurs at the transformation labeled II?
A)( emission)
B)( emission)
C)positron emission
D)electron capture
E)gamma radiation
 What type of nuclear process occurs at the transformation labeled II?
What type of nuclear process occurs at the transformation labeled II?A)( emission)
B)( emission)
C)positron emission
D)electron capture
E)gamma radiation

Unlock Deck
Unlock for access to all 133 flashcards in this deck.
Unlock Deck
k this deck
41
How old is a bottle of wine if the tritium (3H)content is 25% that of a new wine? The half-life of tritium is 12.5 years.
A)0.25 yr
B)3.1 yr
C)25 yr
D)38 yr
E)50.yr
A)0.25 yr
B)3.1 yr
C)25 yr
D)38 yr
E)50.yr

Unlock Deck
Unlock for access to all 133 flashcards in this deck.
Unlock Deck
k this deck
42
The half-life of 90Sr is 29 years.What fraction of the atoms in a sample of 90Sr would remain 175 years later?
A)0.17
B)0.12
C)0.062
D)0.015
E)0.50
A)0.17
B)0.12
C)0.062
D)0.015
E)0.50

Unlock Deck
Unlock for access to all 133 flashcards in this deck.
Unlock Deck
k this deck
43
Cobalt-60 is a beta emitter with a half-life of 5.3 years.Approximately what fraction of the cobalt-60 atoms in a particular sample will remain after 32 years?
A)1/6
B)1/8
C)1/16
D)1/32
E)1/64
A)1/6
B)1/8
C)1/16
D)1/32
E)1/64

Unlock Deck
Unlock for access to all 133 flashcards in this deck.
Unlock Deck
k this deck
44
Cobalt-60 is a beta emitter with a half-life of 5.3 years.Approximately what fraction of cobalt-60 atoms will remain in a particular sample after 26.5 years?
A)1/5
B)1/16
C)1/26
D)1/32
E)1/64
A)1/5
B)1/16
C)1/26
D)1/32
E)1/64

Unlock Deck
Unlock for access to all 133 flashcards in this deck.
Unlock Deck
k this deck
45
Polonium-208 is an alpha emitter with a half-life of 2.90 years.How many milligrams of polonium from an original sample of 2.00 mg will remain after 8.00 years?
A)0.147 mg
B)0.296 mg
C)0.725 mg
D)6.77 mg
E)1.90 mg
A)0.147 mg
B)0.296 mg
C)0.725 mg
D)6.77 mg
E)1.90 mg

Unlock Deck
Unlock for access to all 133 flashcards in this deck.
Unlock Deck
k this deck
46
If 12% of a certain radioisotope decays in 5.2 years, what is the half-life of this isotope?
A)0.59 yr
B)1.7 yr
C)22 yr
D)28 yr
E)32 yr
A)0.59 yr
B)1.7 yr
C)22 yr
D)28 yr
E)32 yr

Unlock Deck
Unlock for access to all 133 flashcards in this deck.
Unlock Deck
k this deck
47
The radioisotope potassium-40 decays to argon-40 by positron emission with a half-life of 1.3 × 109 yr.A sample of moon rock was found to contain 78 argon-40 atoms for every 22 potassium-40 atoms.The age of the rock is
A)8.1 × 10-10 yr
B)2.4 × 109 yr
C)2.8 × 109 yr
D)4.6 × 109 yr
E)6.8 × 109 yr
A)8.1 × 10-10 yr
B)2.4 × 109 yr
C)2.8 × 109 yr
D)4.6 × 109 yr
E)6.8 × 109 yr

Unlock Deck
Unlock for access to all 133 flashcards in this deck.
Unlock Deck
k this deck
48
The Rb-87/Sr-87 method of dating rocks is often used by geologists: 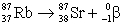 t1/2 = 6.0 × 1010 yr Estimate the age of a rock sample in which the present-day mole ratio of Rb-87 to Sr-87 is 36:1.
t1/2 = 6.0 × 1010 yr Estimate the age of a rock sample in which the present-day mole ratio of Rb-87 to Sr-87 is 36:1.
A)2.4 × 109 yr
B)1.7 × 109 yr
C)3.1 × 1011 yr
D)4.1 × 10-11 yr
E)3.6 × 1011 yr
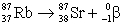 t1/2 = 6.0 × 1010 yr Estimate the age of a rock sample in which the present-day mole ratio of Rb-87 to Sr-87 is 36:1.
t1/2 = 6.0 × 1010 yr Estimate the age of a rock sample in which the present-day mole ratio of Rb-87 to Sr-87 is 36:1.A)2.4 × 109 yr
B)1.7 × 109 yr
C)3.1 × 1011 yr
D)4.1 × 10-11 yr
E)3.6 × 1011 yr

Unlock Deck
Unlock for access to all 133 flashcards in this deck.
Unlock Deck
k this deck
49
Determine how much energy is released when polonium-210 decays according to ![<strong>Determine how much energy is released when polonium-210 decays according to <sup> </sup> .[Atomic masses: polonium-210 = 209.982857 amu; helium-4 = 4.002603 amu; lead-206 = 205.974449 amu]</strong> A)4.14 × 10<sup>9</sup> kJ/mol B)7.20 × 10<sup>11</sup> kJ/mol C)5.22 × 10<sup>8</sup> kJ/mol D)4.66 × 10<sup>9</sup> kJ/mol E)6.43 × 10<sup>12</sup> kJ/mol](https://d2lvgg3v3hfg70.cloudfront.net/TB3246/11ea7cbf_8f3e_64e1_a2ab_f3d95254b569_TB3246_11.jpg) .[Atomic masses: polonium-210 = 209.982857 amu; helium-4 = 4.002603 amu; lead-206 = 205.974449 amu]
.[Atomic masses: polonium-210 = 209.982857 amu; helium-4 = 4.002603 amu; lead-206 = 205.974449 amu]
A)4.14 × 109 kJ/mol
B)7.20 × 1011 kJ/mol
C)5.22 × 108 kJ/mol
D)4.66 × 109 kJ/mol
E)6.43 × 1012 kJ/mol
![<strong>Determine how much energy is released when polonium-210 decays according to <sup> </sup> .[Atomic masses: polonium-210 = 209.982857 amu; helium-4 = 4.002603 amu; lead-206 = 205.974449 amu]</strong> A)4.14 × 10<sup>9</sup> kJ/mol B)7.20 × 10<sup>11</sup> kJ/mol C)5.22 × 10<sup>8</sup> kJ/mol D)4.66 × 10<sup>9</sup> kJ/mol E)6.43 × 10<sup>12</sup> kJ/mol](https://d2lvgg3v3hfg70.cloudfront.net/TB3246/11ea7cbf_8f3e_64e1_a2ab_f3d95254b569_TB3246_11.jpg) .[Atomic masses: polonium-210 = 209.982857 amu; helium-4 = 4.002603 amu; lead-206 = 205.974449 amu]
.[Atomic masses: polonium-210 = 209.982857 amu; helium-4 = 4.002603 amu; lead-206 = 205.974449 amu]A)4.14 × 109 kJ/mol
B)7.20 × 1011 kJ/mol
C)5.22 × 108 kJ/mol
D)4.66 × 109 kJ/mol
E)6.43 × 1012 kJ/mol

Unlock Deck
Unlock for access to all 133 flashcards in this deck.
Unlock Deck
k this deck
50
What would the atom ratio of 206Pb to 238U be in a uranium mineral from a rock that is 1.0 × 109 years old? t1/2(238U)= 4.5 × 109 yr.
A)0.14
B)0.16
C)0.22
D)0.86
E)1.16
A)0.14
B)0.16
C)0.22
D)0.86
E)1.16

Unlock Deck
Unlock for access to all 133 flashcards in this deck.
Unlock Deck
k this deck
51
What fraction of radioactive atoms remains in a sample after five half-lives?
A)zero
B)1/6
C)1/16
D)1/32
E)1/64
A)zero
B)1/6
C)1/16
D)1/32
E)1/64

Unlock Deck
Unlock for access to all 133 flashcards in this deck.
Unlock Deck
k this deck
52
Charcoal found under a stone at Stonehenge, England, has a carbon-14 activity that is 0.60 that of new wood.How old is the charcoal? (The half-life of carbon-14 is 5,730 years.)
A)Less than 5,730 yr
B)Between 5,730 and 11,460 yr
C)Between 11,460 and 17,190 yr
D)More than 17,190 yr
A)Less than 5,730 yr
B)Between 5,730 and 11,460 yr
C)Between 11,460 and 17,190 yr
D)More than 17,190 yr

Unlock Deck
Unlock for access to all 133 flashcards in this deck.
Unlock Deck
k this deck
53
Estimate the age of a bottled wine that has a tritium, 3H, content 60% that of freshly bottled wine.Tritium decays by beta decay and has a half-life of 12.3 yr. 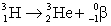
A)0.029 yr
B)7.4 yr
C)9.1 yr
D)16 yr
E)35 yr
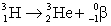
A)0.029 yr
B)7.4 yr
C)9.1 yr
D)16 yr
E)35 yr

Unlock Deck
Unlock for access to all 133 flashcards in this deck.
Unlock Deck
k this deck
54
Carbon-11 is a radioactive isotope of carbon.Its half-life is 20.3 minutes.What fraction of the initial number of carbon-11 atoms in a sample will remain after 81 minutes?
A)1/16
B)1/4
C)1/2
D)1/32
E)1/8
A)1/16
B)1/4
C)1/2
D)1/32
E)1/8

Unlock Deck
Unlock for access to all 133 flashcards in this deck.
Unlock Deck
k this deck
55
What fraction of radioactive atoms remains in a sample after six half-lives?
A)zero
B)1/6
C)1/16
D)1/32
E)1/64
A)zero
B)1/6
C)1/16
D)1/32
E)1/64

Unlock Deck
Unlock for access to all 133 flashcards in this deck.
Unlock Deck
k this deck
56
The only stable isotope of fluorine is 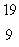 F.What type of radioactivity would you expect from the isotope
F.What type of radioactivity would you expect from the isotope 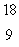 F, which has one fewer neutron?
F, which has one fewer neutron?
A)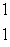 p
p
B)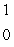 n
n
C)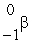
D)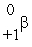
E)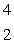 He
He
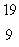 F.What type of radioactivity would you expect from the isotope
F.What type of radioactivity would you expect from the isotope 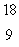 F, which has one fewer neutron?
F, which has one fewer neutron?A)
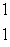 p
pB)
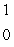 n
nC)
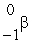
D)
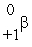
E)
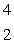 He
He
Unlock Deck
Unlock for access to all 133 flashcards in this deck.
Unlock Deck
k this deck
57
A rock contains 0.37 mg of Pb-206 and 0.95 mg of U-238.Approximately how many U-238 atoms were in the rock when it was formed billions of years ago? (The half life for 238U 206Pb is 4.5 × 109 yr.)
A)1.32 atoms
B)5.8 × 10-6 atoms
C)2.4 × 1018 atoms
D)3.5 × 1018 atoms
E)3.5 × 1021 atoms
A)1.32 atoms
B)5.8 × 10-6 atoms
C)2.4 × 1018 atoms
D)3.5 × 1018 atoms
E)3.5 × 1021 atoms

Unlock Deck
Unlock for access to all 133 flashcards in this deck.
Unlock Deck
k this deck
58
If 24% of a certain radioisotope decays in 6.5 years, what is the half-life of this isotope?
A)3.9 yr
B)16 yr
C)0.22 yr
D)2.2 yr
E)3.2 yr
A)3.9 yr
B)16 yr
C)0.22 yr
D)2.2 yr
E)3.2 yr

Unlock Deck
Unlock for access to all 133 flashcards in this deck.
Unlock Deck
k this deck
59
The heaviest known isotope of hydrogen is called tritium, 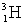 .It decays by beta emission, and has a half-life of 12.3 years.What fraction of a tritium sample will remain after 5.20 years?
.It decays by beta emission, and has a half-life of 12.3 years.What fraction of a tritium sample will remain after 5.20 years?
A)0.0210
B)0.746
C)3.41
D)0.254
E)0.423
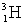 .It decays by beta emission, and has a half-life of 12.3 years.What fraction of a tritium sample will remain after 5.20 years?
.It decays by beta emission, and has a half-life of 12.3 years.What fraction of a tritium sample will remain after 5.20 years?A)0.0210
B)0.746
C)3.41
D)0.254
E)0.423

Unlock Deck
Unlock for access to all 133 flashcards in this deck.
Unlock Deck
k this deck
60
A rock contains 0.37 mg of Pb-206 and 0.95 mg of U-238.The half-life of the decay series U-238 Pb-206 is 4.5 × 109 yr.Assuming no Pb-206 was present in the rock initially, how old is the rock?
A)1.7 × 109 yr
B)5.2 × 109 yr
C)2.7 × 106 yr
D)4.5 × 109 yr
E)2.4 × 109 yr
A)1.7 × 109 yr
B)5.2 × 109 yr
C)2.7 × 106 yr
D)4.5 × 109 yr
E)2.4 × 109 yr

Unlock Deck
Unlock for access to all 133 flashcards in this deck.
Unlock Deck
k this deck
61
Present-day plant life has a carbon-14 decay rate of 16 disintegrations per minute (dpm)per gram of carbon.If a contemporary wooden chair were somehow preserved for the next 3,900 years, what 14C decay rate should be expected from the wood used to make the chair? (t1/2 = 5,730 yr)
A)26 dpm
B)12 dpm
C)11 dpm
D)10 dpm
E)8 dpm
A)26 dpm
B)12 dpm
C)11 dpm
D)10 dpm
E)8 dpm

Unlock Deck
Unlock for access to all 133 flashcards in this deck.
Unlock Deck
k this deck
62
The half-life of 14C is 5,730 yr.Assuming some charcoal from a campfire 29,000 years old was found, what fraction of the original C-14 would remain today?
A)3.0 × 10-2
B)0.197
C)3.51
D)33.3
E)None of these.
A)3.0 × 10-2
B)0.197
C)3.51
D)33.3
E)None of these.

Unlock Deck
Unlock for access to all 133 flashcards in this deck.
Unlock Deck
k this deck
63
The energy released by the sun is the result of
A)natural radioactivity.
B)nuclear fusion.
C)combustion of hydrogen.
D)photosynthesis.
E)nuclear fission.
A)natural radioactivity.
B)nuclear fusion.
C)combustion of hydrogen.
D)photosynthesis.
E)nuclear fission.

Unlock Deck
Unlock for access to all 133 flashcards in this deck.
Unlock Deck
k this deck
64
Which isotope, when bombarded with oxygen-18, yields the artificial isotope seaborgium-263 plus 4 neutrons?
A)nobelium-245
B)radium-259
C)californium-245
D)nobelium-249
E)californium-249
A)nobelium-245
B)radium-259
C)californium-245
D)nobelium-249
E)californium-249

Unlock Deck
Unlock for access to all 133 flashcards in this deck.
Unlock Deck
k this deck
65
A sample of a radioisotope shows an activity of 999 disintegrations per minute due to beta decay.If after 1.10 years the activity is 952 disintegrations per minute, what is the half-life of this radioisotope?
A)4.38 × 10-2 yr
B)11.4 yr
C)0.25 yr
D)15.8 yr
E)9.1 yr
A)4.38 × 10-2 yr
B)11.4 yr
C)0.25 yr
D)15.8 yr
E)9.1 yr

Unlock Deck
Unlock for access to all 133 flashcards in this deck.
Unlock Deck
k this deck
66
Petroleum is a fossil fuel containing many different carbon compounds.If the carbon atoms in petroleum have been in the ground for 100 million years, what fraction of the initial 14C atoms is still there? (t1/2 = 5,370 yr)
A)0
B)1 × 10-10
C)5.7 × 10-5
D)1.0 × 10-3
E)5.7 × 10-1
A)0
B)1 × 10-10
C)5.7 × 10-5
D)1.0 × 10-3
E)5.7 × 10-1

Unlock Deck
Unlock for access to all 133 flashcards in this deck.
Unlock Deck
k this deck
67
List the different types of nuclear radiation (alpha, beta, gamma)in order of increasing penetrating power.
A)alpha < beta < gamma
B)beta < alpha < gamma
C)gamma < alpha < beta
D)gamma < beta < alpha
E)alpha < gamma < beta
A)alpha < beta < gamma
B)beta < alpha < gamma
C)gamma < alpha < beta
D)gamma < beta < alpha
E)alpha < gamma < beta

Unlock Deck
Unlock for access to all 133 flashcards in this deck.
Unlock Deck
k this deck
68
Gamma-rays cause radiation damage when they interact with matter by producing
A)ions and free radicals.
B)isotopes.
C)daughter products.
D)oxidation.
E)reduction.
A)ions and free radicals.
B)isotopes.
C)daughter products.
D)oxidation.
E)reduction.

Unlock Deck
Unlock for access to all 133 flashcards in this deck.
Unlock Deck
k this deck
69
How many 14C atoms are in a charcoal sample that has a decay rate of 3,500 disintegrations per min? (For 14C, t1/2 = 5,730 yr.)
A)2.9 × 107 atoms
B)8.0 × 10-7 atoms
C)1.4 × 1014 atoms
D)1.5 × 1013 atoms
E)6.02 × 1020 atoms
A)2.9 × 107 atoms
B)8.0 × 10-7 atoms
C)1.4 × 1014 atoms
D)1.5 × 1013 atoms
E)6.02 × 1020 atoms

Unlock Deck
Unlock for access to all 133 flashcards in this deck.
Unlock Deck
k this deck
70
Rubidium-87 decays by beta decay with a half-life of 4.9 × 1010 yr.How many 87Rb atoms are in a moon rock sample that has a rubidium decay rate of 3,500 disintegrations per hour?
A)9.0 × 1016 atoms
B)4.3 × 10-4 atoms
C)2.2 × 1018 atoms
D)2.5 × 1014 atoms
E)1.7 × 1014 atoms
A)9.0 × 1016 atoms
B)4.3 × 10-4 atoms
C)2.2 × 1018 atoms
D)2.5 × 1014 atoms
E)1.7 × 1014 atoms

Unlock Deck
Unlock for access to all 133 flashcards in this deck.
Unlock Deck
k this deck
71
In passing through matter, alpha particles lose energy chiefly by causing
A)fermentation.
B)neutralization.
C)ionization.
D)condensation.
E)carbonation.
A)fermentation.
B)neutralization.
C)ionization.
D)condensation.
E)carbonation.

Unlock Deck
Unlock for access to all 133 flashcards in this deck.
Unlock Deck
k this deck
72
Which one of the following statements about fission and fusion is false?
A)Fission occurs among the heaviest isotopes, whereas fusion occurs more readily for light isotopes.
B)For a fission reaction the mass defect ( m)is negative, whereas for fusion m is positive.
C)In order for fusion reactions to occur, temperatures must be in the millions of degrees.
D)The fission of Pu-239 atoms produces a great number of isotopes of a large number of elements.
E)Neutron-induced fission processes can occur at room temperature, rather than at millions of degrees.
A)Fission occurs among the heaviest isotopes, whereas fusion occurs more readily for light isotopes.
B)For a fission reaction the mass defect ( m)is negative, whereas for fusion m is positive.
C)In order for fusion reactions to occur, temperatures must be in the millions of degrees.
D)The fission of Pu-239 atoms produces a great number of isotopes of a large number of elements.
E)Neutron-induced fission processes can occur at room temperature, rather than at millions of degrees.

Unlock Deck
Unlock for access to all 133 flashcards in this deck.
Unlock Deck
k this deck
73
The carbon-14 activity of some ancient Indian corn was found to be 7.0 disintegrations per minute (dpm)per gram of carbon.If present-day plant life has 16 dpm per gram of carbon, how old is the Indian corn? (t1/2 = 5,730 yr)
A)6,800 yr
B)2,500 yr
C)4,700 yr
D)10,000 yr
E)7,200 yr
A)6,800 yr
B)2,500 yr
C)4,700 yr
D)10,000 yr
E)7,200 yr

Unlock Deck
Unlock for access to all 133 flashcards in this deck.
Unlock Deck
k this deck
74
In the following reaction, identify X. 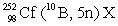
A)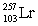
B)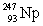
C)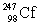
D)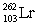
E)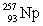
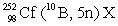
A)
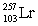
B)
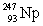
C)
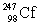
D)
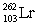
E)
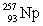

Unlock Deck
Unlock for access to all 133 flashcards in this deck.
Unlock Deck
k this deck
75
Which isotope, when bombarded with nitrogen-15, yields the artificial isotope dubnium-260 plus 4 neutrons?
A)californium-245
B)thorium-257
C)nobelium-245
D)californium-249
E)dubnium-249
A)californium-245
B)thorium-257
C)nobelium-245
D)californium-249
E)dubnium-249

Unlock Deck
Unlock for access to all 133 flashcards in this deck.
Unlock Deck
k this deck
76
The dose unit of ionizing radiation is called the rad.The rad is defined in terms of
A)the half-life of a radioisotope.
B)the energy deposited per gram of an object.
C)the biological damage produced.
D)the accumulation of fission products.
E)the number of ions per centimeter.
A)the half-life of a radioisotope.
B)the energy deposited per gram of an object.
C)the biological damage produced.
D)the accumulation of fission products.
E)the number of ions per centimeter.

Unlock Deck
Unlock for access to all 133 flashcards in this deck.
Unlock Deck
k this deck
77
What role does cadmium metal (Cd)play in a nuclear reactor?
A)slows down the fission neutrons (moderator)
B)transfers heat from the reactor to the heat exchanger (primary coolant)
C)controls chain reaction (control rods)
D)transfers heat from the condenser to the environment (cooling tower)
E)undergoes fission (fuel rods)
A)slows down the fission neutrons (moderator)
B)transfers heat from the reactor to the heat exchanger (primary coolant)
C)controls chain reaction (control rods)
D)transfers heat from the condenser to the environment (cooling tower)
E)undergoes fission (fuel rods)

Unlock Deck
Unlock for access to all 133 flashcards in this deck.
Unlock Deck
k this deck
78
Which type of nuclear process requires an extremely high temperature (millions of degrees)?
A)beta decay
B)fission reaction
C)fusion reaction
D)alpha decay
E)positron emission
A)beta decay
B)fission reaction
C)fusion reaction
D)alpha decay
E)positron emission

Unlock Deck
Unlock for access to all 133 flashcards in this deck.
Unlock Deck
k this deck
79
List the given types of nuclear radiation (cosmic rays, medical and dental X rays, and nuclear waste) in order of how much each contributes to the average yearly dose of nuclear radiation for AmericAns :
A)cosmic rays < medical and dental X rays < nuclear waste
B)medical and dental X rays < cosmic rays < nuclear waste
C)nuclear waste < cosmic rays < medical and dental X rays
D)cosmic rays < nuclear waste < medical and dental X rays
E)medical and dental X rays < nuclear waste < cosmic rays
A)cosmic rays < medical and dental X rays < nuclear waste
B)medical and dental X rays < cosmic rays < nuclear waste
C)nuclear waste < cosmic rays < medical and dental X rays
D)cosmic rays < nuclear waste < medical and dental X rays
E)medical and dental X rays < nuclear waste < cosmic rays

Unlock Deck
Unlock for access to all 133 flashcards in this deck.
Unlock Deck
k this deck
80
Which of the following is an example of a free radical?
A)H3O+
B)H2O2
C)HO2-
D)HO2·
E)H2O
A)H3O+
B)H2O2
C)HO2-
D)HO2·
E)H2O

Unlock Deck
Unlock for access to all 133 flashcards in this deck.
Unlock Deck
k this deck



