Deck 11: Liquids and Intermolecular Forces
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/119
Play
Full screen (f)
Deck 11: Liquids and Intermolecular Forces
1
Of the following substances, __________ has the highest boiling point.
A)H2O
B)CO2
C)CH4
D)Kr
E)NH3
A)H2O
B)CO2
C)CH4
D)Kr
E)NH3
H2O
2
Which one of the following exhibits dipole-dipole attraction between molecules?
A)PH3
B)CCl4
C)Br2
D)CO2
E)C10H22
A)PH3
B)CCl4
C)Br2
D)CO2
E)C10H22
PH3
3
Crystalline solids __________.
A)have their particles arranged randomly
B)have highly ordered structures
C)are usually very soft
D)exist only at high temperatures
E)exist only at very low temperatures
A)have their particles arranged randomly
B)have highly ordered structures
C)are usually very soft
D)exist only at high temperatures
E)exist only at very low temperatures
have highly ordered structures
4
Of the following substances, only __________ has London dispersion forces as its only intermolecular force.
A)H2O
B)CCl4
C)HF
D)CH3COOH
E)PH3
A)H2O
B)CCl4
C)HF
D)CH3COOH
E)PH3

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
5
Together, liquids and solids constitute __________ phases of matter.
A)the compressible
B)the fluid
C)the condensed
D)all of the
E)the disordered
A)the compressible
B)the fluid
C)the condensed
D)all of the
E)the disordered

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
6
Of the following substances, __________ has the highest boiling point.
A)CH3CH2OH
B)C2H6
C)N2
D)F2
E)HOCH2CH2OH
A)CH3CH2OH
B)C2H6
C)N2
D)F2
E)HOCH2CH2OH

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
7
The strongest interparticle attractions exist between particles of a __________ and the weakest interparticle attractions exist between particles of a __________.
A)solid, liquid
B)solid, gas
C)liquid, gas
D)liquid, solid
E)gas, solid
A)solid, liquid
B)solid, gas
C)liquid, gas
D)liquid, solid
E)gas, solid

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
8
Which one of the following should have the lowest boiling point?
A)CH3OH
B)H2S
C)NH3
D)HCl
E)CH4
A)CH3OH
B)H2S
C)NH3
D)HCl
E)CH4

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
9
In which of the following molecules is hydrogen bonding likely to be the most significant component of the total intermolecular forces?
A)CH4
B)C5H11OH
C)C6H13NH2
D)CH3OH
E)CO2
A)CH4
B)C5H11OH
C)C6H13NH2
D)CH3OH
E)CO2

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
10
A gas is __________ and assumes __________ of its container whereas a liquid is __________ and assumes __________ of its container.
A)compressible, the volume and shape, not compressible, the shape of a portion
B)compressible, the shape, not compressible, the volume and shape
C)compressible, the volume and shape, compressible, the volume
D)condensed, the volume and shape, condensed, the volume and shape
E)condensed, the shape, compressible, the volume and shape
A)compressible, the volume and shape, not compressible, the shape of a portion
B)compressible, the shape, not compressible, the volume and shape
C)compressible, the volume and shape, compressible, the volume
D)condensed, the volume and shape, condensed, the volume and shape
E)condensed, the shape, compressible, the volume and shape

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
11
Of the following, __________ has the highest boiling point.
A)N2
B)Br2
C)H2
D)Cl2
E)O2
A)N2
B)Br2
C)H2
D)Cl2
E)O2

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
12
Which one of the following should have the lowest boiling point?
A)PH3
B)H2S
C)HCl
D)SiH4
E)H2O
A)PH3
B)H2S
C)HCl
D)SiH4
E)H2O

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
13
Of the following substances, only __________ has London dispersion forces as the only intermolecular force.
A)CH3OH
B)NH3
C)H2S
D)Kr
E)HCl
A)CH3OH
B)NH3
C)H2S
D)Kr
E)HCl

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
14
In liquids, the attractive intermolecular forces are __________.
A)very weak compared with kinetic energies of the molecules
B)strong enough to hold molecules relatively close together
C)strong enough to keep the molecules confined to vibrating about their fixed lattice points
D)not strong enough to keep molecules from moving past each other
E)strong enough to hold molecules relatively close together but not strong enough to keep molecules from moving past each other
A)very weak compared with kinetic energies of the molecules
B)strong enough to hold molecules relatively close together
C)strong enough to keep the molecules confined to vibrating about their fixed lattice points
D)not strong enough to keep molecules from moving past each other
E)strong enough to hold molecules relatively close together but not strong enough to keep molecules from moving past each other

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
15
Of the following substances, only __________ has London dispersion forces as its only intermolecular force.
A)CH3OH
B)NH3
C)H2S
D)CH4
E)HCl
A)CH3OH
B)NH3
C)H2S
D)CH4
E)HCl

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
16
Which of the following has dispersion forces as its only intermolecular force?
A)CH4
B)HCl
C)C6H13NH2
D)NaCl
E)CH3Cl
A)CH4
B)HCl
C)C6H13NH2
D)NaCl
E)CH3Cl

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
17
As a solid element melts, the atoms become __________ and they have __________ attraction for one another.
A)more separated, more
B)more separated, less
C)closer together, more
D)closer together, less
E)larger, greater
A)more separated, more
B)more separated, less
C)closer together, more
D)closer together, less
E)larger, greater

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
18
Which statement is true about liquids but not true about solids?
A)They flow and are highly ordered.
B)They are highly ordered and not compressible.
C)They flow and are compressible.
D)They assume both the volume and the shape of their containers.
E)They flow and are not compressible.
A)They flow and are highly ordered.
B)They are highly ordered and not compressible.
C)They flow and are compressible.
D)They assume both the volume and the shape of their containers.
E)They flow and are not compressible.

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
19
Which one of the following exhibits dipole-dipole attraction between molecules?
A)XeF4
B)AsH3
C)CO2
D)BCl3
E)Cl2
A)XeF4
B)AsH3
C)CO2
D)BCl3
E)Cl2

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
20
As a gaseous element condenses, the atoms become __________ and they have __________ attraction for one another.
A)more separated, more
B)more separated, less
C)closer together, more
D)closer together, less
E)larger, greater
A)more separated, more
B)more separated, less
C)closer together, more
D)closer together, less
E)larger, greater

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
21
Which of the following molecules has hydrogen bonding as its only intermolecular force?
A)NH3
B)H2O
C)C3H7OH
D)HOCH2CH2OH
E)None, all of the above exhibit dispersion forces.
A)NH3
B)H2O
C)C3H7OH
D)HOCH2CH2OH
E)None, all of the above exhibit dispersion forces.

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
22
The intermolecular force(s)responsible for the fact that CH4 has the lowest boiling point in the set CH4, SiH4, GeH4, SnH4 is/are __________.
A)hydrogen bonding
B)dipole-dipole interactions
C)London dispersion forces
D)mainly hydrogen bonding but also dipole-dipole interactions
E)mainly London-dispersion forces but also dipole-dipole interactions
A)hydrogen bonding
B)dipole-dipole interactions
C)London dispersion forces
D)mainly hydrogen bonding but also dipole-dipole interactions
E)mainly London-dispersion forces but also dipole-dipole interactions

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
23
__________ are particularly polarizable.
A)Small nonpolar molecules
B)Small polar molecules
C)Large nonpolar molecules
D)Large polar molecules
E)Large molecules, regardless of their polarity,
A)Small nonpolar molecules
B)Small polar molecules
C)Large nonpolar molecules
D)Large polar molecules
E)Large molecules, regardless of their polarity,

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
24
What is the predominant intermolecular force in CBr4?
A)London-dispersion forces
B)ion-dipole attraction
C)ionic bonding
D)dipole-dipole attraction
E)hydrogen-bonding
A)London-dispersion forces
B)ion-dipole attraction
C)ionic bonding
D)dipole-dipole attraction
E)hydrogen-bonding

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
25
What type(s)of intermolecular forces exist between Cl2 and CO3-2?
A)dispersion forces
B)dispersion forces and ion-dipole
C)dispersion forces, ion-dipole, and induced dipole - induced dipole
D)dispersion forces and ion-induced dipole
E)dispersion forces, ion-dipole, dipole-dipole, and ion-induced dipole
A)dispersion forces
B)dispersion forces and ion-dipole
C)dispersion forces, ion-dipole, and induced dipole - induced dipole
D)dispersion forces and ion-induced dipole
E)dispersion forces, ion-dipole, dipole-dipole, and ion-induced dipole

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
26
The predominant intermolecular force in AsH3 is __________.
A)London dispersion forces
B)ion-dipole forces
C)ionic bonding
D)dipole-dipole forces
E)hydrogen bonding
A)London dispersion forces
B)ion-dipole forces
C)ionic bonding
D)dipole-dipole forces
E)hydrogen bonding

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
27
What types of intermolecular forces exist between HI and H2S?
A)dipole-dipole and ion-dipole
B)dispersion forces, dipole-dipole, and ion-dipole
C)dispersion forces, hydrogen bonding, dipole-dipole, and ion-dipole
D)dispersion forces and dipole-dipole
A)dipole-dipole and ion-dipole
B)dispersion forces, dipole-dipole, and ion-dipole
C)dispersion forces, hydrogen bonding, dipole-dipole, and ion-dipole
D)dispersion forces and dipole-dipole

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
28
Which one of the following substances will not have hydrogen bonding as one of its intermolecular forces?
A)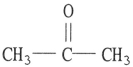
B)
C)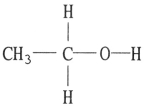
D)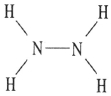
E)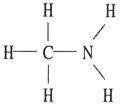
A)
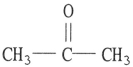
B)

C)

D)

E)


Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
29
When NaCl dissolves in water, aqueous Na+ and Cl- ions result. The force of attraction that exists between Na+ and H2O is called a(n)__________ interaction.
A)dipole-dipole
B)ion-ion
C)hydrogen bonding
D)ion-dipole
E)London dispersion force
A)dipole-dipole
B)ion-ion
C)hydrogen bonding
D)ion-dipole
E)London dispersion force

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
30
Hydrogen bonding is a special case of __________.
A)London-dispersion forces
B)ion-dipole attraction
C)dipole-dipole attractions
D)ion-ion interactions
E)none of the above
A)London-dispersion forces
B)ion-dipole attraction
C)dipole-dipole attractions
D)ion-ion interactions
E)none of the above

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
31
Which of the following molecules has hydrogen bonding as its only intermolecular force?
A)HF
B)H2O
C)C6H13NH2
D)C5H11OH
E)None, all of the above exhibit dispersion forces.
A)HF
B)H2O
C)C6H13NH2
D)C5H11OH
E)None, all of the above exhibit dispersion forces.

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
32
What type(s)of intermolecular forces exist between Br2 and CCl4?
A)dispersion forces
B)dispersion forces and ion-dipole
C)dispersion forces and dipole-dipole
D)dispersion forces, ion-dipole, and dipole-dipole
E)None. Since both are gases at room temperature, they do not interact with each other.
A)dispersion forces
B)dispersion forces and ion-dipole
C)dispersion forces and dipole-dipole
D)dispersion forces, ion-dipole, and dipole-dipole
E)None. Since both are gases at room temperature, they do not interact with each other.

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
33
What is the predominant intermolecular force in CH3CH2OH?
A)London-dispersion forces
B)ion-dipole attraction
C)ionic bonding
D)induced dipole-dipole attraction
E)hydrogen-bonding
A)London-dispersion forces
B)ion-dipole attraction
C)ionic bonding
D)induced dipole-dipole attraction
E)hydrogen-bonding

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
34
C12H26 molecules are held together by __________.
A)ion-ion interactions
B)hydrogen bonding
C)ion-dipole interactions
D)dipole-dipole interactions
E)dispersion forces
A)ion-ion interactions
B)hydrogen bonding
C)ion-dipole interactions
D)dipole-dipole interactions
E)dispersion forces

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
35
Which one of the following derivatives of ethane has the highest boiling point?
A)C2Br6
B)C2F6
C)C2I6
D)C2Cl6
E)C2H6
A)C2Br6
B)C2F6
C)C2I6
D)C2Cl6
E)C2H6

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
36
The ease with which the charge distribution in a molecule can be distorted by an external electrical field is called the __________.
A)electronegativity
B)hydrogen bonding
C)polarizability
D)volatility
E)viscosity
A)electronegativity
B)hydrogen bonding
C)polarizability
D)volatility
E)viscosity

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
37
Which one of the following substances will have hydrogen bonding as one of its intermolecular forces?
A)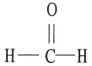
B)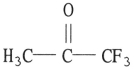
C)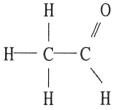
D)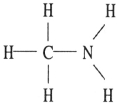
E)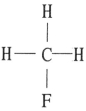
A)
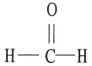
B)
C)

D)

E)
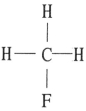

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
38
What intermolecular force is responsible for the fact that ice is less dense than liquid water?
A)London dispersion forces
B)dipole-dipole forces
C)ion-dipole forces
D)hydrogen bonding
E)ionic bonding
A)London dispersion forces
B)dipole-dipole forces
C)ion-dipole forces
D)hydrogen bonding
E)ionic bonding

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
39
Elemental iodine (I2)is a solid at room temperature. What is the major attractive force that exists among different I2 molecules in the solid?
A)London dispersion forces
B)dipole-dipole rejections
C)ionic-dipole interactions
D)covalent-ionic interactions
E)dipole-dipole attractions
A)London dispersion forces
B)dipole-dipole rejections
C)ionic-dipole interactions
D)covalent-ionic interactions
E)dipole-dipole attractions

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
40
The predominant intermolecular force in (CH3)2NH is __________.
A)London dispersion forces
B)ion-dipole forces
C)ionic bonding
D)dipole-dipole forces
E)hydrogen bonding
A)London dispersion forces
B)ion-dipole forces
C)ionic bonding
D)dipole-dipole forces
E)hydrogen bonding

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
41
Based on the following information, which compound has the strongest intermolecular forces? Substance ΔHvap(kJ/mol)
Argon (Ar)6.3
Benzene (C6H6)31.0
Ethanol (C2H5OH)39.3
Water (H2O)40.8
Methane (CH4)9.2
A)Argon
B)Benzene
C)Ethanol
D)Water
E)Methane
Argon (Ar)6.3
Benzene (C6H6)31.0
Ethanol (C2H5OH)39.3
Water (H2O)40.8
Methane (CH4)9.2
A)Argon
B)Benzene
C)Ethanol
D)Water
E)Methane

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
42
Viscosity is __________.
A)the "skin" on a liquid surface caused by intermolecular attraction
B)the resistance to flow
C)the same as density
D)inversely proportional to molar mass
E)unaffected by temperature
A)the "skin" on a liquid surface caused by intermolecular attraction
B)the resistance to flow
C)the same as density
D)inversely proportional to molar mass
E)unaffected by temperature

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
43
The critical temperature and pressure of CS2 are 279°C and 78 atm, respectively. At temperatures above 279°C and pressures above 78 atm, CS2 can only occur as a __________.
A)solid
B)liquid
C)liquid and gas
D)gas
E)supercritical fluid
A)solid
B)liquid
C)liquid and gas
D)gas
E)supercritical fluid

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
44
Of the following, __________ is the most volatile.
A)CBr4
B)CCl4
C)CF4
D)CH4
E)C6H14
A)CBr4
B)CCl4
C)CF4
D)CH4
E)C6H14

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
45
What types of intermolecular forces exist between NH3 and CBr4?
A)dispersion forces and dipole-dipole forces
B)dispersion forces and dipole-induced dipole forces
C)dispersion forces and hydrogen bonds
D)dispersion forces, hydrogen bonds, and induced dipole-induced dipole forces
E)dispersion forces, hydrogen bonds, and dipole-induced dipole forces
A)dispersion forces and dipole-dipole forces
B)dispersion forces and dipole-induced dipole forces
C)dispersion forces and hydrogen bonds
D)dispersion forces, hydrogen bonds, and induced dipole-induced dipole forces
E)dispersion forces, hydrogen bonds, and dipole-induced dipole forces

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
46
Which statements about viscosity are true? (i)Viscosity increases as temperature decreases.
(ii)Viscosity increases as molecular weight increases.
(iii)Viscosity increases as intermolecular forces increase.
A)(i)only
B)(ii)and (iii)
C)(i)and (iii)
D)none
E)all
(ii)Viscosity increases as molecular weight increases.
(iii)Viscosity increases as intermolecular forces increase.
A)(i)only
B)(ii)and (iii)
C)(i)and (iii)
D)none
E)all

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
47
How high a liquid will rise up a narrow tube as a result of capillary action depends on __________.
A)the magnitudes of cohesive forces in the liquid and adhesive forces between the liquid and the tube, and gravity
B)gravity alone
C)only the magnitude of adhesive forces between the liquid and the tube
D)the viscosity of the liquid
E)only the magnitude of cohesive forces in the liquid
A)the magnitudes of cohesive forces in the liquid and adhesive forces between the liquid and the tube, and gravity
B)gravity alone
C)only the magnitude of adhesive forces between the liquid and the tube
D)the viscosity of the liquid
E)only the magnitude of cohesive forces in the liquid

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
48
A substance that expands to fill its container yet has a density approaching that of a liquid, and that can behave as a solvent is called a(n)__________.
A)plasma
B)gas
C)liquid
D)amorphous solid
E)supercritical fluid and gas
A)plasma
B)gas
C)liquid
D)amorphous solid
E)supercritical fluid and gas

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
49
The phase changes B → C and D → E are not associated with temperature increases because the heat energy is used up to __________.
A)increase distances between molecules
B)break intramolecular bonds
C)rearrange atoms within molecules
D)increase the velocity of molecules
E)increase the density of the sample
A)increase distances between molecules
B)break intramolecular bonds
C)rearrange atoms within molecules
D)increase the velocity of molecules
E)increase the density of the sample

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
50
Which compound has the strongest intermolecular forces?
A)CBr4
B)C12H26
C)CI4
D)N2
E)O2
A)CBr4
B)C12H26
C)CI4
D)N2
E)O2

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
51
Which of the following statements is false?
A)The absolute value of the heat of sublimation is equal to the absolute value of the heat of deposition.
B)The heat of sublimation is equal to the sum of the heat of vaporization and the heat of melting.
C)The heat of sublimation is equal to the sum of the heat of vaporization and the heat of freezing.
D)The absolute value of the heat of sublimation is equal to the absolute value of the sum of the heat of condensation and the heat of freezing.
E)The absolute value of the heat of deposition is equal to sum of the absolute value of the heat of vaporization and the absolute value of the heat of freezing.
A)The absolute value of the heat of sublimation is equal to the absolute value of the heat of deposition.
B)The heat of sublimation is equal to the sum of the heat of vaporization and the heat of melting.
C)The heat of sublimation is equal to the sum of the heat of vaporization and the heat of freezing.
D)The absolute value of the heat of sublimation is equal to the absolute value of the sum of the heat of condensation and the heat of freezing.
E)The absolute value of the heat of deposition is equal to sum of the absolute value of the heat of vaporization and the absolute value of the heat of freezing.

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
52
Of the following, __________ is an exothermic process.
A)melting
B)subliming
C)freezing
D)boiling
E)All of the above are exothermic.
A)melting
B)subliming
C)freezing
D)boiling
E)All of the above are exothermic.

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
53
__________ is the energy required to expand the surface area of a liquid by a unit amount of area.
A)Viscosity
B)Surface tension
C)Volatility
D)Meniscus
E)Capillary action
A)Viscosity
B)Surface tension
C)Volatility
D)Meniscus
E)Capillary action

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
54
Heat of sublimation can be approximated by adding together __________ and __________.
A)heat of fusion, heat of condensation
B)heat of fusion, heat of vaporization
C)heat of freezing (solidification), heat of condensation
D)heat of freezing (solidification), heat of vaporization
E)heat of deposition, heat of vaporization
A)heat of fusion, heat of condensation
B)heat of fusion, heat of vaporization
C)heat of freezing (solidification), heat of condensation
D)heat of freezing (solidification), heat of vaporization
E)heat of deposition, heat of vaporization

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
55
Large intermolecular forces in a substance are manifested by __________.
A)low vapor pressure
B)high boiling point
C)high heats of fusion and vaporization
D)high critical temperatures and pressures
E)all of the above
A)low vapor pressure
B)high boiling point
C)high heats of fusion and vaporization
D)high critical temperatures and pressures
E)all of the above

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
56
The property responsible for the "beading up" of water is __________.
A)density
B)viscosity
C)vapor pressure
D)surface tension
E)hydrogen bonding
A)density
B)viscosity
C)vapor pressure
D)surface tension
E)hydrogen bonding

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
57
What types of intermolecular forces exist between PH3 and N2?
A)dispersion forces and dipole-dipole forces
B)dispersion forces and dipole-induced dipole forces
C)dispersion forces and hydrogen bonds
D)dispersion forces, hydrogen bonds, and induced dipole-induced dipole forces
E)dispersion forces, hydrogen bonds, and dipole-induced dipole forces
A)dispersion forces and dipole-dipole forces
B)dispersion forces and dipole-induced dipole forces
C)dispersion forces and hydrogen bonds
D)dispersion forces, hydrogen bonds, and induced dipole-induced dipole forces
E)dispersion forces, hydrogen bonds, and dipole-induced dipole forces

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
58
The shape of a liquid's meniscus is determined by __________.
A)the viscosity of the liquid
B)the type of material the container is made of
C)the relative magnitudes of cohesive forces in the liquid and adhesive forces between the liquid and its container
D)the amount of hydrogen bonding in the liquid
E)the volume of the liquid
A)the viscosity of the liquid
B)the type of material the container is made of
C)the relative magnitudes of cohesive forces in the liquid and adhesive forces between the liquid and its container
D)the amount of hydrogen bonding in the liquid
E)the volume of the liquid

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
59
The substance with the largest heat of vaporization is __________.
A)I2
B)Br2
C)Cl2
D)F2
E)O2
A)I2
B)Br2
C)Cl2
D)F2
E)O2

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
60
Of the following, __________ should have the highest critical temperature.
A)CBr4
B)CCl4
C)CF4
D)CH4
E)H2
A)CBr4
B)CCl4
C)CF4
D)CH4
E)H2

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
61
On a phase diagram, the critical pressure is __________.
A)the pressure required to melt a solid
B)the pressure below which a substance is a solid at all temperatures
C)the pressure above which a substance is a liquid at all temperatures
D)the pressure at which a liquid changes to a gas
E)the pressure required to liquefy a gas at its critical temperature
A)the pressure required to melt a solid
B)the pressure below which a substance is a solid at all temperatures
C)the pressure above which a substance is a liquid at all temperatures
D)the pressure at which a liquid changes to a gas
E)the pressure required to liquefy a gas at its critical temperature

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
62
Of the following, __________ is the least volatile.
A)CBr4
B)CCl4
C)CF4
D)CH4
E)C6H14
A)CBr4
B)CCl4
C)CF4
D)CH4
E)C6H14

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
63
Cholesteric liquid crystals are colored because __________.
A)each molecule is a chromophore
B)of the slight twist between layers
C)of the large spacing between layers
D)of the large number of conjugated bonds
E)all of the molecules contain multiple benzene rings
A)each molecule is a chromophore
B)of the slight twist between layers
C)of the large spacing between layers
D)of the large number of conjugated bonds
E)all of the molecules contain multiple benzene rings

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
64
In the __________ liquid crystalline phase, the component molecules exhibit only one dimensional ordering.
A)nematic
B)smectic A
C)smectic B
D)smectic C
E)cholesteric
A)nematic
B)smectic A
C)smectic B
D)smectic C
E)cholesteric

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
65
When the phase diagram for a substance has a solid-liquid phase boundary line that has a negative slope (leans to the left), the substance __________.
A)can go from solid to liquid, within a small temperature range, via the application of pressure
B)sublimes rather than melts under ordinary conditions
C)cannot go from solid to liquid by application of pressure at any temperature
D)cannot be liquefied above its triple point
E)melts rather than sublimes under ordinary conditions
A)can go from solid to liquid, within a small temperature range, via the application of pressure
B)sublimes rather than melts under ordinary conditions
C)cannot go from solid to liquid by application of pressure at any temperature
D)cannot be liquefied above its triple point
E)melts rather than sublimes under ordinary conditions

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
66
Which of the following characteristics would prevent liquid crystal behavior?
A)long axial structure
B)ionic configuration
C)carbon-carbon single bonds
D)double bonding
E)polar groups
A)long axial structure
B)ionic configuration
C)carbon-carbon single bonds
D)double bonding
E)polar groups

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
67
On a phase diagram, the critical temperature is __________.
A)the temperature below which a gas cannot be liquefied
B)the temperature above which a gas cannot be liquefied
C)the temperature at which all three states are in equilibrium
D)the temperature required to melt a solid
E)the temperature required to cause sublimation of a solid
A)the temperature below which a gas cannot be liquefied
B)the temperature above which a gas cannot be liquefied
C)the temperature at which all three states are in equilibrium
D)the temperature required to melt a solid
E)the temperature required to cause sublimation of a solid

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
68
Molecules with only single bonds do not generally exhibit liquid-crystalline properties because __________.
A)molecules without multiple bonds lack the rigidity necessary for alignment
B)molecules without multiple bonds are too small to exhibit liquid-crystalline properties
C)molecules with only single bonds are gases
D)molecules with only single bonds are too big to exhibit liquid-crystalline properties
E)molecules without multiple bonds lack the flexibility necessary for alignment
A)molecules without multiple bonds lack the rigidity necessary for alignment
B)molecules without multiple bonds are too small to exhibit liquid-crystalline properties
C)molecules with only single bonds are gases
D)molecules with only single bonds are too big to exhibit liquid-crystalline properties
E)molecules without multiple bonds lack the flexibility necessary for alignment

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
69
On a phase diagram, the melting point is the same as __________.
A)the triple point
B)the critical point
C)the freezing point
D)the boiling point
E)the vapor-pressure curve
A)the triple point
B)the critical point
C)the freezing point
D)the boiling point
E)the vapor-pressure curve

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
70
The predominant intermolecular force in CaBr2 is __________.
A)London-dispersion forces
B)ion-dipole forces
C)ionic bonding
D)dipole-dipole forces
E)hydrogen bonding
A)London-dispersion forces
B)ion-dipole forces
C)ionic bonding
D)dipole-dipole forces
E)hydrogen bonding

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
71
Which of the following is most likely to exhibit liquid-crystalline behavior?
A)CH3CH2-C(CH3)2-CH2CH3
B)CH3CH2CH2CH2CH2CH2CH2CH3
C)CH3CH2CH2CH2CH2-Na+
D)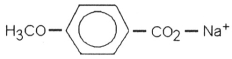
E)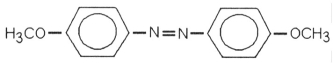
A)CH3CH2-C(CH3)2-CH2CH3
B)CH3CH2CH2CH2CH2CH2CH2CH3
C)CH3CH2CH2CH2CH2-Na+
D)

E)

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
72
Some things take longer to cook at high altitudes than at low altitudes because __________.
A)water boils at a lower temperature at high altitude than at low altitude
B)water boils at a higher temperature at high altitude than at low altitude
C)heat isn't conducted as well in low density air
D)natural gas flames don't burn as hot at high altitudes
E)there is a higher moisture content in the air at high altitude
A)water boils at a lower temperature at high altitude than at low altitude
B)water boils at a higher temperature at high altitude than at low altitude
C)heat isn't conducted as well in low density air
D)natural gas flames don't burn as hot at high altitudes
E)there is a higher moisture content in the air at high altitude

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
73
The vapor pressure of a liquid __________.
A)increases linearly with increasing temperature
B)increases nonlinearly with increasing temperature
C)decreases linearly with increasing temperature
D)decreases nonlinearly with increasing temperature
E)is totally unrelated to its molecular structure
A)increases linearly with increasing temperature
B)increases nonlinearly with increasing temperature
C)decreases linearly with increasing temperature
D)decreases nonlinearly with increasing temperature
E)is totally unrelated to its molecular structure

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
74
Volatility and vapor pressure are __________.
A)inversely proportional to one another
B)directly proportional to one another
C)not related
D)the same thing
E)both independent of temperature
A)inversely proportional to one another
B)directly proportional to one another
C)not related
D)the same thing
E)both independent of temperature

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
75
The vapor pressure of any substance at its normal boiling point is
A)1 Pa
B)1 torr
C)1 atm
D)equal to atmospheric pressure
E)equal to the vapor pressure of water
A)1 Pa
B)1 torr
C)1 atm
D)equal to atmospheric pressure
E)equal to the vapor pressure of water

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
76
The slope of a plot of the natural log of the vapor pressure of a substance versus 1/T is __________.
A)ΔHvap
B)-ΔHvap
C)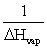
D)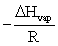
E)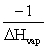
A)ΔHvap
B)-ΔHvap
C)
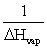
D)
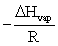
E)
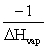

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
77
In general, the vapor pressure of a substance increases as __________ increases.
A)surface tension
B)molecular weight
C)hydrogen bonding
D)viscosity
E)temperature
A)surface tension
B)molecular weight
C)hydrogen bonding
D)viscosity
E)temperature

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
78
In the smectic A liquid-crystalline phase, __________.
A)the molecules are aligned along their long axes, with no ordering with respect to the ends of the molecules
B)the molecules are arranged in sheets, with their long axes parallel and their ends aligned as well
C)the molecules are aligned with their long axes tilted with respect to a line perpendicular to the plane in which the molecules are stacked
D)disk-shaped molecules are aligned through a stacking of the disks in layers
E)the molecules are oriented in a totally random fashion
A)the molecules are aligned along their long axes, with no ordering with respect to the ends of the molecules
B)the molecules are arranged in sheets, with their long axes parallel and their ends aligned as well
C)the molecules are aligned with their long axes tilted with respect to a line perpendicular to the plane in which the molecules are stacked
D)disk-shaped molecules are aligned through a stacking of the disks in layers
E)the molecules are oriented in a totally random fashion

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
79
A volatile liquid is one that __________.
A)is highly flammable
B)is highly viscous
C)is highly hydrogen-bonded
D)is highly cohesive
E)readily evaporates
A)is highly flammable
B)is highly viscous
C)is highly hydrogen-bonded
D)is highly cohesive
E)readily evaporates

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
80
In the ________ liquid crystalline phase, the component molecules are aligned along their long axis and are arranged in layers with the molecules in each plane twisted slightly in relation to the molecules in the planes above and below.
A)nematic
B)smectic A
C)smectic B
D)smectic C
E)cholesteric
A)nematic
B)smectic A
C)smectic B
D)smectic C
E)cholesteric

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck



