Deck 21: Nuclear Chemistry
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/158
Play
Full screen (f)
Deck 21: Nuclear Chemistry
1
What is the missing product from this reaction? 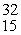 P →
P → 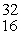 S + _____
S + _____
A)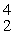 He
He
B)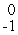 e
e
C)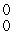 γ
γ
D)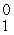 e
e
E)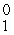 p
p
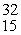 P →
P → 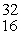 S + _____
S + _____A)
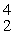 He
HeB)
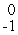 e
eC)
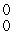 γ
γD)
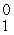 e
eE)
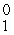 p
p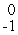 e
e 2
What happens to the mass number and the atomic number of an element when it undergoes beta decay?
A)Neither the mass number nor the atomic number change.
B)The mass number decreases by 4 and the atomic number decreases by 2.
C)The mass number does not change and the atomic number increases by 1.
D)The mass number does not change and the atomic number decreases by 2.
E)The mass number increases by 2 and the atomic number increases by 1.
A)Neither the mass number nor the atomic number change.
B)The mass number decreases by 4 and the atomic number decreases by 2.
C)The mass number does not change and the atomic number increases by 1.
D)The mass number does not change and the atomic number decreases by 2.
E)The mass number increases by 2 and the atomic number increases by 1.
The mass number does not change and the atomic number increases by 1.
3
Carbon-11 decays by __________.
A)alpha emission
B)beta emission
C)positron emission
D)electron capture
E)neutron capture
A)alpha emission
B)beta emission
C)positron emission
D)electron capture
E)neutron capture
positron emission
4
At approximately what number of protons, or neutrons, does the 1:1 ratio of protons to neutrons start to produce unstable nuclei?
A)10
B)20
C)30
D)50
E)80
A)10
B)20
C)30
D)50
E)80

Unlock Deck
Unlock for access to all 158 flashcards in this deck.
Unlock Deck
k this deck
5
Which one of the following is a correct representation of a positron?
A)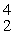 e
e
B)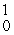 β
β
C)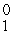 e
e
D)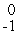 e
e
E)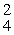 β
β
A)
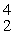 e
eB)
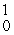 β
βC)
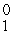 e
eD)
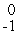 e
eE)
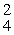 β
β
Unlock Deck
Unlock for access to all 158 flashcards in this deck.
Unlock Deck
k this deck
6
Of the following processes, which one changes the atomic number?
A)alpha emission
B)beta emission
C)electron capture
D)positron emission
E)All of these processes change the atomic numbers.
A)alpha emission
B)beta emission
C)electron capture
D)positron emission
E)All of these processes change the atomic numbers.

Unlock Deck
Unlock for access to all 158 flashcards in this deck.
Unlock Deck
k this deck
7
Atoms containing radioactive nuclei are called __________.
A)radionuclides
B)radioisotopes
C)nucleons
D)nuclides
E)radioisophores
A)radionuclides
B)radioisotopes
C)nucleons
D)nuclides
E)radioisophores

Unlock Deck
Unlock for access to all 158 flashcards in this deck.
Unlock Deck
k this deck
8
The formation of krypton from rubidium decay is a result of __________.
A)alpha emission
B)beta emission
C)positron emission
D)electron capture
E)neutron capture
A)alpha emission
B)beta emission
C)positron emission
D)electron capture
E)neutron capture

Unlock Deck
Unlock for access to all 158 flashcards in this deck.
Unlock Deck
k this deck
9
Which one of the following is a correct representation of a beta particle?
A)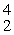 e
e
B)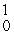 β
β
C)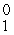 e
e
D)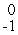 e
e
E)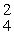 β
β
A)
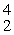 e
eB)
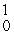 β
βC)
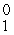 e
eD)
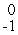 e
eE)
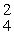 β
β
Unlock Deck
Unlock for access to all 158 flashcards in this deck.
Unlock Deck
k this deck
10
All atoms of a given element have the same __________.
A)mass number
B)number of nucleons
C)atomic mass
D)number of neutrons
E)atomic number
A)mass number
B)number of nucleons
C)atomic mass
D)number of neutrons
E)atomic number

Unlock Deck
Unlock for access to all 158 flashcards in this deck.
Unlock Deck
k this deck
11
In what type of radioactive decay does the atomic number of the product increase by one?
A)alpha
B)beta
C)gamma
D)positron emission
E)electron capture
A)alpha
B)beta
C)gamma
D)positron emission
E)electron capture

Unlock Deck
Unlock for access to all 158 flashcards in this deck.
Unlock Deck
k this deck
12
Atoms with the same atomic number and different mass numbers
A)do not exist.
B)are isomers.
C)are isotopes.
D)are allotropes
E)are resonance structures.
A)do not exist.
B)are isomers.
C)are isotopes.
D)are allotropes
E)are resonance structures.

Unlock Deck
Unlock for access to all 158 flashcards in this deck.
Unlock Deck
k this deck
13
Alpha decay produces a new nucleus whose __________ than those respectively of the original nucleus.
A)atomic number is 2 less and mass number is 2 less
B)atomic number is 1 less and mass number is 2 less
C)atomic number is 2 less and mass number is 4 less
D)atomic number is 2 more and mass number is 4 more
E)atomic number is 2 more and mass number is 2 less
A)atomic number is 2 less and mass number is 2 less
B)atomic number is 1 less and mass number is 2 less
C)atomic number is 2 less and mass number is 4 less
D)atomic number is 2 more and mass number is 4 more
E)atomic number is 2 more and mass number is 2 less

Unlock Deck
Unlock for access to all 158 flashcards in this deck.
Unlock Deck
k this deck
14
What happens to the mass number and the atomic number of an element when it emits gamma radiation?
A)The mass number remains unchanged while the atomic number decreases by one.
B)The mass number decreases by four and the atomic number decreases by two.
C)The mass number increases by four and the atomic number increases by two.
D)The mass number remains unchanged while the atomic number increases by one.
E)The mass number and atomic numbers remain unchanged.
A)The mass number remains unchanged while the atomic number decreases by one.
B)The mass number decreases by four and the atomic number decreases by two.
C)The mass number increases by four and the atomic number increases by two.
D)The mass number remains unchanged while the atomic number increases by one.
E)The mass number and atomic numbers remain unchanged.

Unlock Deck
Unlock for access to all 158 flashcards in this deck.
Unlock Deck
k this deck
15
Which one of the following is a correct representation of an alpha particle?
A)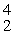 He
He
B)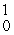 H
H
C)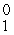 H
H
D)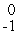 e
e
E)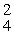 H
H
A)
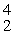 He
HeB)
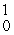 H
HC)
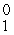 H
HD)
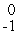 e
eE)
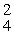 H
H
Unlock Deck
Unlock for access to all 158 flashcards in this deck.
Unlock Deck
k this deck
16
Which one of the following processes results in an increase in the atomic number?
A)gamma emission
B)positron emission
C)beta emission
D)alpha emission
E)corrosion
A)gamma emission
B)positron emission
C)beta emission
D)alpha emission
E)corrosion

Unlock Deck
Unlock for access to all 158 flashcards in this deck.
Unlock Deck
k this deck
17
What radioactive element is used to diagnose medical conditions of the heart and arteries?
A)cobalt-60
B)thallium-201
C)radium-226
D)radon-222
E)thorium-234
A)cobalt-60
B)thallium-201
C)radium-226
D)radon-222
E)thorium-234

Unlock Deck
Unlock for access to all 158 flashcards in this deck.
Unlock Deck
k this deck
18
What is the atomic number of a neutron?
A)3
B)1
C)2
D)0
E)4
A)3
B)1
C)2
D)0
E)4

Unlock Deck
Unlock for access to all 158 flashcards in this deck.
Unlock Deck
k this deck
19
Which type of radioactive decay results in no change in mass number and atomic number for the starting nucleus?
A)alpha
B)beta
C)positron emission
D)electron capture
E)gamma
A)alpha
B)beta
C)positron emission
D)electron capture
E)gamma

Unlock Deck
Unlock for access to all 158 flashcards in this deck.
Unlock Deck
k this deck
20
How many radioactive decay series exist in nature?
A)0
B)1
C)2
D)3
E)10
A)0
B)1
C)2
D)3
E)10

Unlock Deck
Unlock for access to all 158 flashcards in this deck.
Unlock Deck
k this deck
21
The mode of decay of 32P is __________.
A)alpha emission
B)beta emission
C)positron emission
D)electron capture
E)neutron capture
A)alpha emission
B)beta emission
C)positron emission
D)electron capture
E)neutron capture

Unlock Deck
Unlock for access to all 158 flashcards in this deck.
Unlock Deck
k this deck
22
Which one of the following requires a particle accelerator to occur?
A)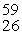 Fe →
Fe → 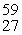 Co +
Co + 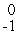 e
e
B)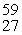 Co +
Co +  n →
n → 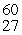 Co
Co
C)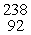 U +
U + 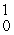 n →
n → 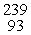 Np +
Np +  e
e
D)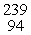 Pu +
Pu + 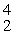 He →
He → 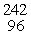 Cm +
Cm +  n
n
E)none of the above
A)
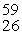 Fe →
Fe → 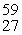 Co +
Co + 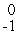 e
eB)
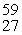 Co +
Co + 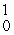 n →
n → 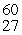 Co
CoC)
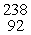 U +
U + 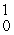 n →
n → 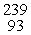 Np +
Np + 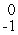 e
eD)
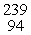 Pu +
Pu + 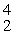 He →
He → 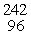 Cm +
Cm + 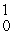 n
nE)none of the above

Unlock Deck
Unlock for access to all 158 flashcards in this deck.
Unlock Deck
k this deck
23
Transuranium elements have atomic numbers greater than __________.
A)90
B)91
C)92
D)93
E)94
A)90
B)91
C)92
D)93
E)94

Unlock Deck
Unlock for access to all 158 flashcards in this deck.
Unlock Deck
k this deck
24
The half-life of 218Po is 3.1 minutes. How much of a 155 gram sample remains after 0.40 hours?
A)0.00067 g
B)0.0072 g
C)0.72 g
D)0.0047 g
E)none of the above
A)0.00067 g
B)0.0072 g
C)0.72 g
D)0.0047 g
E)none of the above

Unlock Deck
Unlock for access to all 158 flashcards in this deck.
Unlock Deck
k this deck
25
The beta decay of cesium-137 has a half-life of 30.0 years. How many years must pass to reduce a 25 mg sample of cesium 137 to 8.7 mg?
A)46
B)32
C)3.2
D)50
E)52
A)46
B)32
C)3.2
D)50
E)52

Unlock Deck
Unlock for access to all 158 flashcards in this deck.
Unlock Deck
k this deck
26
The belt of nuclear stability ends with the element __________.
A)lead
B)polonium
C)radon
D)astatine
E)bismuth
A)lead
B)polonium
C)radon
D)astatine
E)bismuth

Unlock Deck
Unlock for access to all 158 flashcards in this deck.
Unlock Deck
k this deck
27
The product of the nuclear reaction in which 28Si is subjected to neutron capture followed by alpha emission is __________.
A)"31S"
B)"33S"
C)"23Mg"
D)"25Mg"
E)"25Al"
A)"31S"
B)"33S"
C)"23Mg"
D)"25Mg"
E)"25Al"

Unlock Deck
Unlock for access to all 158 flashcards in this deck.
Unlock Deck
k this deck
28
Which of these nuclides is most likely to be radioactive?
A)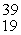 K
K
B)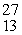 Al
Al
C)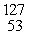 I
I
D)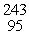 Am
Am
E)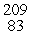 Bi
Bi
A)
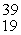 K
KB)
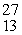 Al
AlC)
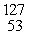 I
ID)
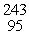 Am
AmE)
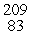 Bi
Bi
Unlock Deck
Unlock for access to all 158 flashcards in this deck.
Unlock Deck
k this deck
29
In the nuclear transmutation represented by 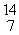 N(
N( 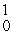 n,
n, 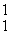 p)?, what is the product?
p)?, what is the product?
A)carbon-12
B)carbon-16
C)carbon-14
D)nitrogen-16
E)nitrogen-15
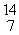 N(
N(  n,
n,  p)?, what is the product?
p)?, what is the product?A)carbon-12
B)carbon-16
C)carbon-14
D)nitrogen-16
E)nitrogen-15

Unlock Deck
Unlock for access to all 158 flashcards in this deck.
Unlock Deck
k this deck
30
Which one of the following can be done to shorten the half-life of the radioactive decay of uranium-238?
A)freeze it
B)heat it
C)convert it to UF6
D)oxidize it to the +2 oxidation state
E)none of the above
A)freeze it
B)heat it
C)convert it to UF6
D)oxidize it to the +2 oxidation state
E)none of the above

Unlock Deck
Unlock for access to all 158 flashcards in this deck.
Unlock Deck
k this deck
31
In the nuclear transmutation represented by 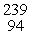 Pu(
Pu(  He,
He,  n)?, what is the product?
n)?, what is the product?
A)uranium-242
B)curium-245
C)curium-242
D)uranium-245
E)uranium-243
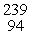 Pu(
Pu(  He,
He,  n)?, what is the product?
n)?, what is the product?A)uranium-242
B)curium-245
C)curium-242
D)uranium-245
E)uranium-243

Unlock Deck
Unlock for access to all 158 flashcards in this deck.
Unlock Deck
k this deck
32
Cesium-131 has a half-life of 9.7 days. What percent of a cesium-131 sample remains after 60 days?
A)100
B)0
C)1.4
D)98.6
E)more information is needed to solve the problem
A)100
B)0
C)1.4
D)98.6
E)more information is needed to solve the problem

Unlock Deck
Unlock for access to all 158 flashcards in this deck.
Unlock Deck
k this deck
33
In the nuclear transmutation represented by 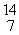 N(
N( 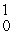 n,
n, 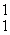 p)?, what is the emitted particle?
p)?, what is the emitted particle?
A)neutron
B)proton
C)positron
D)alpha particle
E)electron
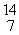 N(
N( 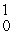 n,
n, 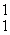 p)?, what is the emitted particle?
p)?, what is the emitted particle?A)neutron
B)proton
C)positron
D)alpha particle
E)electron

Unlock Deck
Unlock for access to all 158 flashcards in this deck.
Unlock Deck
k this deck
34
What is emitted in the nuclear transmutation, 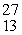 Al(n, ?)
Al(n, ?) 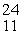 Na?
Na?
A)an alpha particle
B)a beta particle
C)a neutron
D)a proton
E)a gamma photon
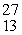 Al(n, ?)
Al(n, ?) 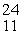 Na?
Na?A)an alpha particle
B)a beta particle
C)a neutron
D)a proton
E)a gamma photon

Unlock Deck
Unlock for access to all 158 flashcards in this deck.
Unlock Deck
k this deck
35
Which of the following correctly represents the transmutation in which a curium-242 nucleus is bombarded with an alpha particle to produce a californium-245 nucleus?
A)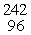 Cm(
Cm( 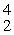 He,
He, 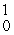 n)
n) 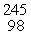 Cf
Cf
B)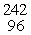 Cm(
Cm( 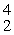 He,
He, 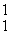 p)
p) 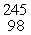 Cf
Cf
C)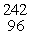 Cm(
Cm( 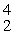 He,
He, 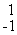 e)
e) 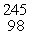 Cf
Cf
D)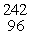 Cm(
Cm(  n,
n,  He)
He) 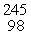 Cf
Cf
E)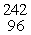 Cm(
Cm( 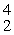 He, 2
He, 2 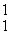 p)
p) 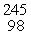 Cf
Cf
A)
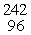 Cm(
Cm( 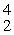 He,
He, 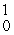 n)
n) 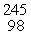 Cf
CfB)
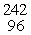 Cm(
Cm( 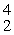 He,
He, 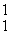 p)
p) 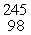 Cf
CfC)
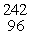 Cm(
Cm( 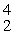 He,
He, 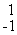 e)
e) 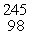 Cf
CfD)
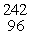 Cm(
Cm( 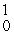 n,
n, 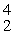 He)
He) 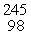 Cf
CfE)
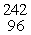 Cm(
Cm( 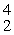 He, 2
He, 2 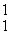 p)
p) 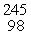 Cf
Cf
Unlock Deck
Unlock for access to all 158 flashcards in this deck.
Unlock Deck
k this deck
36
Bombardment of uranium-238 with a deuteron (hydrogen-2)generates neptunium-237 and __________ neutrons.
A)1
B)2
C)3
D)4
E)5
A)1
B)2
C)3
D)4
E)5

Unlock Deck
Unlock for access to all 158 flashcards in this deck.
Unlock Deck
k this deck
37
Cobalt-60 is produced by a three reaction process involving neutron capture, beta-emission, and neutron capture. The initial reactant in the production of cobalt-60 is __________.
A)"59Co"
B)"56Fe"
C)"58Fe"
D)"61Co"
E)"60Fe"
A)"59Co"
B)"56Fe"
C)"58Fe"
D)"61Co"
E)"60Fe"

Unlock Deck
Unlock for access to all 158 flashcards in this deck.
Unlock Deck
k this deck
38
Which of the following correctly represents the transmutation in which neptunium-239 is produced via bombardment of uranium-238 with a neutron?
A)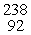 U(
U( 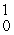 n,
n, 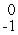 e)
e) 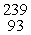 Np
Np
B)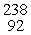 U(
U( 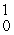 n,
n, 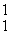 p)
p) 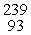 Np
Np
C)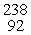 U(
U(  n, γ)
n, γ) 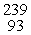 Np
Np
D)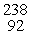 U(
U(  n,
n,  α)
α) 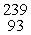 Np
Np
E)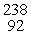 U(
U( 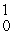 n,
n, 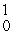 n)
n) 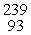 Np
Np
A)
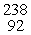 U(
U(  n,
n,  e)
e) 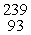 Np
NpB)
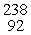 U(
U( 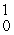 n,
n, 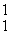 p)
p) 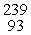 Np
NpC)
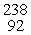 U(
U(  n, γ)
n, γ) 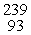 Np
NpD)
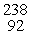 U(
U( 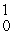 n,
n, 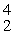 α)
α)  Np
NpE)
 U(
U( 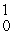 n,
n, 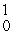 n)
n) 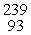 Np
Np
Unlock Deck
Unlock for access to all 158 flashcards in this deck.
Unlock Deck
k this deck
39
What is required for a nuclear transmutation to occur?
A)very high temperature
B)a corrosive environment
C)a particle to collide with a nucleus
D)spontaneous nuclear decay
E)gamma emission
A)very high temperature
B)a corrosive environment
C)a particle to collide with a nucleus
D)spontaneous nuclear decay
E)gamma emission

Unlock Deck
Unlock for access to all 158 flashcards in this deck.
Unlock Deck
k this deck
40
In the nuclear transmutation, 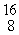 O (p, α)
O (p, α) 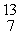 N, what is the bombarding particle?
N, what is the bombarding particle?
A)an alpha particle
B)a beta particle
C)a gamma photon
D)a proton
E)a phosphorus nucleus
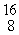 O (p, α)
O (p, α) 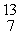 N, what is the bombarding particle?
N, what is the bombarding particle?A)an alpha particle
B)a beta particle
C)a gamma photon
D)a proton
E)a phosphorus nucleus

Unlock Deck
Unlock for access to all 158 flashcards in this deck.
Unlock Deck
k this deck
41
Which one of the following is true?
A)Some spontaneous nuclear reactions are exothermic.
B)Some spontaneous nuclear reactions are endothermic.
C)All spontaneous nuclear reactions are exothermic.
D)There is no relationship between exothermicity and spontaneity in nuclear reactions.
E)All spontaneous nuclear reactions are endothermic.
A)Some spontaneous nuclear reactions are exothermic.
B)Some spontaneous nuclear reactions are endothermic.
C)All spontaneous nuclear reactions are exothermic.
D)There is no relationship between exothermicity and spontaneity in nuclear reactions.
E)All spontaneous nuclear reactions are endothermic.

Unlock Deck
Unlock for access to all 158 flashcards in this deck.
Unlock Deck
k this deck
42
Which one of the following is used as a radiotracer to study blood?
A)iron-59
B)technetium-99
C)sodium-23
D)iodine-131
E)phosphorus-32
A)iron-59
B)technetium-99
C)sodium-23
D)iodine-131
E)phosphorus-32

Unlock Deck
Unlock for access to all 158 flashcards in this deck.
Unlock Deck
k this deck
43
What is the rate constant (in min-1)for the decay of this radionuclide?
A)45
B)32
C)0.024
D)0.032
E)0.022
A)45
B)32
C)0.024
D)0.032
E)0.022

Unlock Deck
Unlock for access to all 158 flashcards in this deck.
Unlock Deck
k this deck
44
The mass of a proton is 1.00728 amu and that of a neutron is 1.00867 amu. What is the binding energy (in J)of a 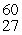 Co nucleus? (The mass of a cobalt-60 nucleus is 59.9338 amu.)
Co nucleus? (The mass of a cobalt-60 nucleus is 59.9338 amu.)
A)2.74 × 10-19
B)9.12 × 10-28
C)4.94 × 10-13
D)8.20 × 10-11
E)2.74 × 10-16
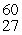 Co nucleus? (The mass of a cobalt-60 nucleus is 59.9338 amu.)
Co nucleus? (The mass of a cobalt-60 nucleus is 59.9338 amu.)A)2.74 × 10-19
B)9.12 × 10-28
C)4.94 × 10-13
D)8.20 × 10-11
E)2.74 × 10-16

Unlock Deck
Unlock for access to all 158 flashcards in this deck.
Unlock Deck
k this deck
45
Which one of the following devices converts radioactive emissions to light for detection?
A)Geiger counter
B)photographic film
C)scintillation counter
D)none of the above
E)radiotracer
A)Geiger counter
B)photographic film
C)scintillation counter
D)none of the above
E)radiotracer

Unlock Deck
Unlock for access to all 158 flashcards in this deck.
Unlock Deck
k this deck
46
Cesium-137 undergoes beta decay and has a half-life of 30.0 years. How many beta particles are emitted by a 14.0-g sample of cesium-137 in three minutes?
A)6.1 × 1013
B)6.2 × 1022
C)8.4 × 1015
D)1.3 × 10-8
E)8.1 × 1015
A)6.1 × 1013
B)6.2 × 1022
C)8.4 × 1015
D)1.3 × 10-8
E)8.1 × 1015

Unlock Deck
Unlock for access to all 158 flashcards in this deck.
Unlock Deck
k this deck
47
The half-life of 131I is 0.220 years. How much of a 500.0 mg sample remains after 24 hours?
A)496 mg
B)560 mg
C)219 mg
D)405 mg
E)337 mg
A)496 mg
B)560 mg
C)219 mg
D)405 mg
E)337 mg

Unlock Deck
Unlock for access to all 158 flashcards in this deck.
Unlock Deck
k this deck
48
The half-life of a radionuclide
A)is constant.
B)gets shorter with passing time.
C)gets longer with passing time.
D)gets shorter with increased temperature.
E)gets longer with increased temperature.
A)is constant.
B)gets shorter with passing time.
C)gets longer with passing time.
D)gets shorter with increased temperature.
E)gets longer with increased temperature.

Unlock Deck
Unlock for access to all 158 flashcards in this deck.
Unlock Deck
k this deck
49
The mass of a proton is 1.673 × 10-24 g. The mass of a neutron is 1.675 × 10-24 g. The mass of the nucleus of an 56Fe atom is 9.289 × 10-23 g. What is the nuclear binding energy (in J)for a 56Fe nucleus? (c = 3.00 × 108 m/s)
A)2.57 × 10-16
B)7.72 × 10-8
C)8.36 × 10-9
D)7.65 × 10-11
E)6.07 × 106
A)2.57 × 10-16
B)7.72 × 10-8
C)8.36 × 10-9
D)7.65 × 10-11
E)6.07 × 106

Unlock Deck
Unlock for access to all 158 flashcards in this deck.
Unlock Deck
k this deck
50
What is the half-life (in min)of this radionuclide?
A)0.024
B)0.022
C)32
D)0.032
E)45
A)0.024
B)0.022
C)32
D)0.032
E)45

Unlock Deck
Unlock for access to all 158 flashcards in this deck.
Unlock Deck
k this deck
51
The half-life of carbon-11 is 20.3 minutes. How much of a 100.0 mg sample remains after 1.50 hours?
A)8.48 mg
B)4.63 mg
C)12.9 mg
D)22.6 mg
E)7.70 mg
A)8.48 mg
B)4.63 mg
C)12.9 mg
D)22.6 mg
E)7.70 mg

Unlock Deck
Unlock for access to all 158 flashcards in this deck.
Unlock Deck
k this deck
52
The half-life of 223Ra is 11.4 days. How much of a 200.0 mg sample remains after 600 hours?
A)0.219 mg
B)21.9 mg
C).0302 mg
D)43.8 mg
E)6.04 mg
A)0.219 mg
B)21.9 mg
C).0302 mg
D)43.8 mg
E)6.04 mg

Unlock Deck
Unlock for access to all 158 flashcards in this deck.
Unlock Deck
k this deck
53
The carbon-14 dating method can be used to determine the age of a
A)flint arrowhead.
B)papyrus scroll.
C)stone axe head.
D)clay pot.
E)rock.
A)flint arrowhead.
B)papyrus scroll.
C)stone axe head.
D)clay pot.
E)rock.

Unlock Deck
Unlock for access to all 158 flashcards in this deck.
Unlock Deck
k this deck
54
The curie is a measure of the
A)number of disintegrations per second of a radioactive substance.
B)total energy absorbed by an object exposed to a radioactive source.
C)lethal threshold for radiation exposure.
D)number of alpha particles emitted by exactly one gram of a radioactive substance.
E)None of the above is correct.
A)number of disintegrations per second of a radioactive substance.
B)total energy absorbed by an object exposed to a radioactive source.
C)lethal threshold for radiation exposure.
D)number of alpha particles emitted by exactly one gram of a radioactive substance.
E)None of the above is correct.

Unlock Deck
Unlock for access to all 158 flashcards in this deck.
Unlock Deck
k this deck
55
The half-life for beta decay of strontium-90 is 28.8 years. A milk sample is found to contain 10.3 ppm strontium-90. How many years would pass before the strontium-90 concentration would drop to 1.0 ppm?
A)92.3
B)0.112
C)186
D)96.9
E)131
A)92.3
B)0.112
C)186
D)96.9
E)131

Unlock Deck
Unlock for access to all 158 flashcards in this deck.
Unlock Deck
k this deck
56
When two atoms of 2H are fused to form one atom of 4He, the total energy evolved is 3.83 × 10-12 J. What is the total change in mass (in kg)for this reaction? (C = 3.00 × 108 m/s)
A)1.28 × 10-17
B)4.26 × 10-26
C)3.45 × 108
D)1.15
E)4.26 × 10-29
A)1.28 × 10-17
B)4.26 × 10-26
C)3.45 × 108
D)1.15
E)4.26 × 10-29

Unlock Deck
Unlock for access to all 158 flashcards in this deck.
Unlock Deck
k this deck
57
210Pb has a half-life of 22.3 years and decays to produce 206Hg . If you start with 7.50 g of 210Pb, how many grams of 206Hg will you have after 17.5 years?
A)4.35
B)3.15
C)3.09
D)0.0600
E)1.71
A)4.35
B)3.15
C)3.09
D)0.0600
E)1.71

Unlock Deck
Unlock for access to all 158 flashcards in this deck.
Unlock Deck
k this deck
58
The half-life of 222Rn is 3.80 days. If a sample contains 36.0 g of Rn-222, how many years will it take for the sample to be reduced to 1.00 mg of Rn-222?
A)19.7
B)0.1597
C)8.53
D)0.0234
E)none of the above
A)19.7
B)0.1597
C)8.53
D)0.0234
E)none of the above

Unlock Deck
Unlock for access to all 158 flashcards in this deck.
Unlock Deck
k this deck
59
The basis for the carbon-14 dating method is that
A)the amount of carbon-14 in all objects is the same.
B)carbon-14 is very unstable and is readily lost from the atmosphere.
C)the ratio of carbon-14 to carbon-12 in the atmosphere is a constant.
D)living tissue will not absorb carbon-14 but will absorb carbon-12.
E)All of the above are correct.
A)the amount of carbon-14 in all objects is the same.
B)carbon-14 is very unstable and is readily lost from the atmosphere.
C)the ratio of carbon-14 to carbon-12 in the atmosphere is a constant.
D)living tissue will not absorb carbon-14 but will absorb carbon-12.
E)All of the above are correct.

Unlock Deck
Unlock for access to all 158 flashcards in this deck.
Unlock Deck
k this deck
60
What is a phosphor?
A)an oxide of phosphorus
B)a substance that thermally reduces to phosphorus
C)a bioluminescent substance
D)a substance that emits light when excited by radiation
E)an alkali metal phosphide
A)an oxide of phosphorus
B)a substance that thermally reduces to phosphorus
C)a bioluminescent substance
D)a substance that emits light when excited by radiation
E)an alkali metal phosphide

Unlock Deck
Unlock for access to all 158 flashcards in this deck.
Unlock Deck
k this deck
61
The missing product from this reaction is __________. 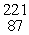 Fr →
Fr → 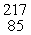 N + _____
N + _____
A)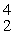 He
He
B)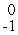 e
e
C)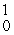 n
n
D)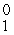 e
e
E)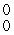 γ
γ
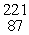 Fr →
Fr → 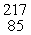 N + _____
N + _____A)
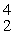 He
HeB)
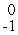 e
eC)
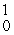 n
nD)
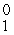 e
eE)
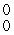 γ
γ
Unlock Deck
Unlock for access to all 158 flashcards in this deck.
Unlock Deck
k this deck
62
In balancing the nuclear reaction 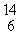 C → E +
C → E + 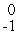 e, the identity of element E is __________.
e, the identity of element E is __________.
A)B
B)N
C)C
D)O
E)none of the above
 C → E +
C → E + 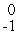 e, the identity of element E is __________.
e, the identity of element E is __________.A)B
B)N
C)C
D)O
E)none of the above

Unlock Deck
Unlock for access to all 158 flashcards in this deck.
Unlock Deck
k this deck
63
What type of reaction is known as a thermonuclear reaction?
A)fission
B)fusion
C)transmutation
D)beta emission
E)neutron emission
A)fission
B)fusion
C)transmutation
D)beta emission
E)neutron emission

Unlock Deck
Unlock for access to all 158 flashcards in this deck.
Unlock Deck
k this deck
64
This reaction is an example of __________. 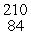 Po →
Po → 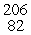 Pb + _____
Pb + _____
A)alpha decay
B)beta emission
C)gamma emission
D)positron emission
E)electron capture
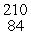 Po →
Po → 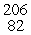 Pb + _____
Pb + _____A)alpha decay
B)beta emission
C)gamma emission
D)positron emission
E)electron capture

Unlock Deck
Unlock for access to all 158 flashcards in this deck.
Unlock Deck
k this deck
65
What percentage of electricity generated in the U.S. is from commercial nuclear plants?
A)1%
B)10%
C)19%
D)50%
E)90%
A)1%
B)10%
C)19%
D)50%
E)90%

Unlock Deck
Unlock for access to all 158 flashcards in this deck.
Unlock Deck
k this deck
66
The mass of a proton is 1.00728 amu and that of a neutron is 1.00867 amu. What is the binding energy per nucleon (in J)of a 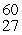 Co nucleus? (The mass of a cobalt-60 nucleus is 59.9338 amu.)
Co nucleus? (The mass of a cobalt-60 nucleus is 59.9338 amu.)
A)1.37 × 10-12
B)3.04 × 10-12
C)2.49 × 10-12
D)9.43 × 10-13
E)7.01 × 10-14
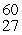 Co nucleus? (The mass of a cobalt-60 nucleus is 59.9338 amu.)
Co nucleus? (The mass of a cobalt-60 nucleus is 59.9338 amu.)A)1.37 × 10-12
B)3.04 × 10-12
C)2.49 × 10-12
D)9.43 × 10-13
E)7.01 × 10-14

Unlock Deck
Unlock for access to all 158 flashcards in this deck.
Unlock Deck
k this deck
67
The missing product from this reaction is __________. 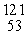 I →
I → 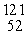 Te + _____
Te + _____
A)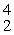 He
He
B)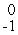 e
e
C)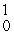 n
n
D)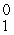 e
e
E)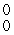 γ
γ
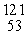 I →
I → 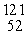 Te + _____
Te + _____A)
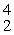 He
HeB)
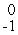 e
eC)
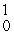 n
nD)
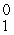 e
eE)
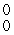 γ
γ
Unlock Deck
Unlock for access to all 158 flashcards in this deck.
Unlock Deck
k this deck
68
The alpha decay of what isotope of what element produces lead-206?

Unlock Deck
Unlock for access to all 158 flashcards in this deck.
Unlock Deck
k this deck
69
Which one of the following forms of radiation can penetrate the deepest into body tissue?
A)alpha
B)beta
C)gamma
D)positron
E)proton
A)alpha
B)beta
C)gamma
D)positron
E)proton

Unlock Deck
Unlock for access to all 158 flashcards in this deck.
Unlock Deck
k this deck
70
Which one of the following is not true concerning radon?
A)It decays by alpha emission.
B)It decays to polonium-218, an alpha emitter.
C)It is chemically active in human lungs.
D)It has been implicated in lung cancer.
E)It is generated as uranium decays.
A)It decays by alpha emission.
B)It decays to polonium-218, an alpha emitter.
C)It is chemically active in human lungs.
D)It has been implicated in lung cancer.
E)It is generated as uranium decays.

Unlock Deck
Unlock for access to all 158 flashcards in this deck.
Unlock Deck
k this deck
71
The main scientific difficulty in achieving a controlled fusion process is the
A)enormous repulsion between nuclei being fused.
B)enormous repulsion between the electrons of atoms being fused.
C)very large number of positrons emitted.
D)very large number of x-rays emitted.
E)very large number of gamma rays emitted.
A)enormous repulsion between nuclei being fused.
B)enormous repulsion between the electrons of atoms being fused.
C)very large number of positrons emitted.
D)very large number of x-rays emitted.
E)very large number of gamma rays emitted.

Unlock Deck
Unlock for access to all 158 flashcards in this deck.
Unlock Deck
k this deck
72
The missing product from this reaction is __________. 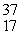 Cl + _____ →
Cl + _____ → 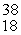 Ar +
Ar + 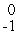 e
e
A)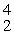 He
He
B)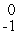 e
e
C)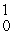 n
n
D)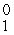 e
e
E)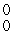 γ
γ
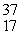 Cl + _____ →
Cl + _____ → 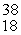 Ar +
Ar + 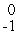 e
eA)
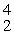 He
HeB)
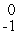 e
eC)
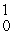 n
nD)
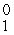 e
eE)
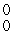 γ
γ
Unlock Deck
Unlock for access to all 158 flashcards in this deck.
Unlock Deck
k this deck
73
In terms of binding energy per nucleon, what element divides fission and fusion processes?
A)H
B)He
C)C
D)Fe
E)U
A)H
B)He
C)C
D)Fe
E)U

Unlock Deck
Unlock for access to all 158 flashcards in this deck.
Unlock Deck
k this deck
74
In balancing the nuclear reaction 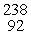 U →
U → 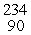 E +
E + 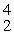 He, the identity of element E is __________.
He, the identity of element E is __________.
A)Pu
B)Np
C)U
D)Pa
E)Th
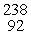 U →
U → 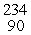 E +
E + 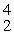 He, the identity of element E is __________.
He, the identity of element E is __________.A)Pu
B)Np
C)U
D)Pa
E)Th

Unlock Deck
Unlock for access to all 158 flashcards in this deck.
Unlock Deck
k this deck
75
The mass of a proton is 1.00728 amu and that of a neutron is 1.00867 amu. What is the mass defect (in amu)of a 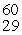 Ni nucleus? (The mass of a nickel-60 nucleus is 59.9308 amu.)
Ni nucleus? (The mass of a nickel-60 nucleus is 59.9308 amu.)
A)0.5449
B)1.2374
C)0.5491
D)28.7930
E)1.3066
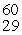 Ni nucleus? (The mass of a nickel-60 nucleus is 59.9308 amu.)
Ni nucleus? (The mass of a nickel-60 nucleus is 59.9308 amu.)A)0.5449
B)1.2374
C)0.5491
D)28.7930
E)1.3066

Unlock Deck
Unlock for access to all 158 flashcards in this deck.
Unlock Deck
k this deck
76
The missing product from this reaction is __________. 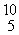 B + _____ →
B + _____ → 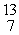 N +
N + 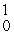 n
n
A)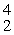 He
He
B)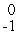 e
e
C)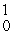 n
n
D)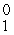 e
e
E)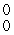 γ
γ
 B + _____ →
B + _____ → 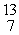 N +
N + 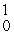 n
nA)
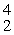 He
HeB)
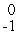 e
eC)
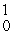 n
nD)
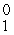 e
eE)
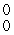 γ
γ
Unlock Deck
Unlock for access to all 158 flashcards in this deck.
Unlock Deck
k this deck
77
What exposure level to radiation is fatal to most humans?
A)100 rem
B)200 rem
C)600 rem
D)300 rem
E)1000 rem
A)100 rem
B)200 rem
C)600 rem
D)300 rem
E)1000 rem

Unlock Deck
Unlock for access to all 158 flashcards in this deck.
Unlock Deck
k this deck
78
By what process does thorium-230 decay to radium-226?
A)gamma emission
B)alpha emission
C)beta emission
D)electron capture
E)positron emission
A)gamma emission
B)alpha emission
C)beta emission
D)electron capture
E)positron emission

Unlock Deck
Unlock for access to all 158 flashcards in this deck.
Unlock Deck
k this deck
79
In balancing the nuclear reaction 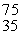 Br → E +
Br → E + 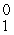 e, the identity of element E is __________.
e, the identity of element E is __________.
A)Kr
B)Br
C)U
D)Se
E)none of the above
 Br → E +
Br → E + 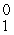 e, the identity of element E is __________.
e, the identity of element E is __________.A)Kr
B)Br
C)U
D)Se
E)none of the above

Unlock Deck
Unlock for access to all 158 flashcards in this deck.
Unlock Deck
k this deck
80
The missing product from this reaction is __________. 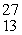 Al +
Al + 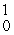 n →
n → 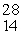 Si + _____
Si + _____
A)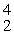 He
He
B)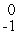 e
e
C)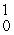 n
n
D)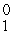 e
e
E)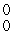 γ
γ
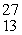 Al +
Al + 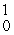 n →
n → 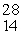 Si + _____
Si + _____A)
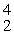 He
HeB)
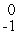 e
eC)
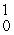 n
nD)
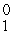 e
eE)
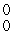 γ
γ
Unlock Deck
Unlock for access to all 158 flashcards in this deck.
Unlock Deck
k this deck



