Deck 25: Carbohydrates
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/60
Play
Full screen (f)
Deck 25: Carbohydrates
1
What are the R/S stereochemical designations of C2 and C3 of L-threose? 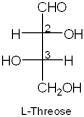
A)2R,3R
B)2R,3S
C)2S,3R
D)2S,3S

A)2R,3R
B)2R,3S
C)2S,3R
D)2S,3S
2R,3S
2
Which of the following is a disaccharide?
A)glucose
B)fructose
C)sucrose
D)mannitol
A)glucose
B)fructose
C)sucrose
D)mannitol
sucrose
3
What is (are) the major organic product(s) obtained from the following reaction? 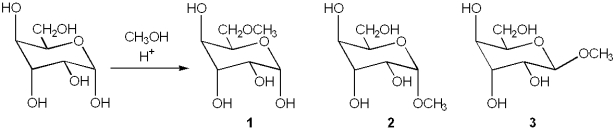
A)only 1
B)only 2
C)only 2 and 3
D)1, 2 and 3

A)only 1
B)only 2
C)only 2 and 3
D)1, 2 and 3
only 2 and 3
4
Which carbon in the following carbohydrate is the anomeric carbon? 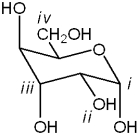
A)i
B)ii
C)iii
D)iv

A)i
B)ii
C)iii
D)iv

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
5
Which of the following structures represent the same carbohydrate? 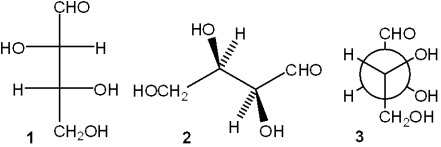
A)only 1 and 2
B)only 1 and 3
C)only 2 and 3
D)1, 2 and 3

A)only 1 and 2
B)only 1 and 3
C)only 2 and 3
D)1, 2 and 3

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
6
What is the major organic product obtained from the following reaction? 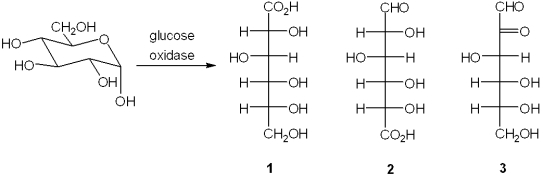
A)only 1
B)only 2
C)only 3
D)only 1 and 2

A)only 1
B)only 2
C)only 3
D)only 1 and 2

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
7
Which carbon in the following carbohydrate is the anomeric carbon? 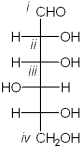
A)i
B)ii
C)iii
D)iv

A)i
B)ii
C)iii
D)iv

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
8
What is the relationship between D-erythrose and D-threose? 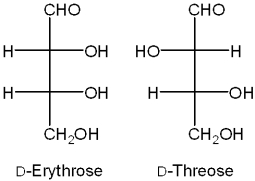
A)constitutional isomers
B)enantiomers
C)diastereomers
D)tautomers

A)constitutional isomers
B)enantiomers
C)diastereomers
D)tautomers

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
9
What is the relationship between D-arabinose and L-arabinose? 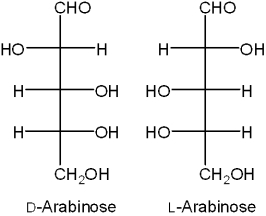
A)constitutional isomers
B)enantiomers
C)diastereomers
D)tautomers

A)constitutional isomers
B)enantiomers
C)diastereomers
D)tautomers

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
10
Which of the following represents a pyranose? 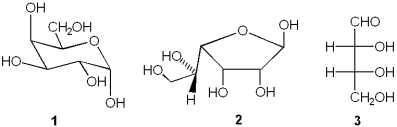
A)only 1
B)only 2
C)only 3
D)only 1 and 2

A)only 1
B)only 2
C)only 3
D)only 1 and 2

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
11
Which of the following structures represent the same carbohydrate? 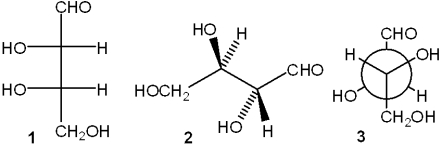
A)only 1 and 2
B)only 1 and 3
C)only 2 and 3
D)1, 2 and 3

A)only 1 and 2
B)only 1 and 3
C)only 2 and 3
D)1, 2 and 3

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
12
What is the major organic product obtained from the following reaction? 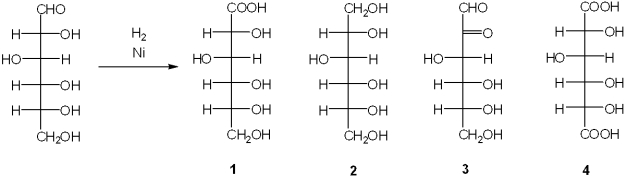
A)1
B)2
C)3
D)4

A)1
B)2
C)3
D)4

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
13
Which of the following represents a furanose? 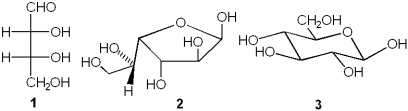
A)only 1
B)only 2
C)only 3
D)only 2 and 3

A)only 1
B)only 2
C)only 3
D)only 2 and 3

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
14
Which of the following has a D-configuration? 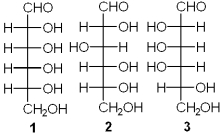
A)only 1 and 2
B)only 1 and 3
C)only 2 and 3
D)only 1, 2 and 3

A)only 1 and 2
B)only 1 and 3
C)only 2 and 3
D)only 1, 2 and 3

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
15
What is the relationship between D-ribose and D-xylose? 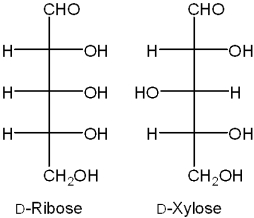
A)constitutional isomers
B)enantiomers
C)diastereomers
D)tautomers

A)constitutional isomers
B)enantiomers
C)diastereomers
D)tautomers

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
16
Which of the following has a D-configuration? 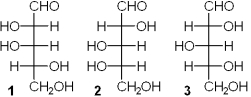
A)only 1 and 2
B)only 1 and 3
C)only 2 and 3
D)only 1, 2 and 3

A)only 1 and 2
B)only 1 and 3
C)only 2 and 3
D)only 1, 2 and 3

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
17
Which of the following represent a ketose? 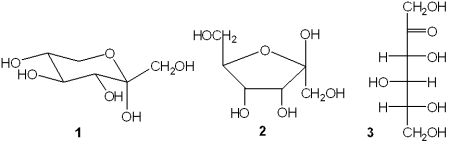
A)only 1 and 2
B)only 1 and 3
C)only 2 and 3
D)1, 2 and 3

A)only 1 and 2
B)only 1 and 3
C)only 2 and 3
D)1, 2 and 3

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
18
What are the R/S stereochemical designations of C2 and C3 of D-erythrose? 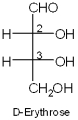
A)2R,3R
B)2R,3S
C)2S,3R
D)2S,3S

A)2R,3R
B)2R,3S
C)2S,3R
D)2S,3S

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
19
Which of the following represent an aldose? 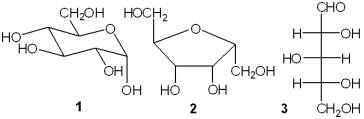
A)only 1 and 2
B)only 1 and 3
C)only 2 and 3
D)1, 2 and 3

A)only 1 and 2
B)only 1 and 3
C)only 2 and 3
D)1, 2 and 3

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
20
What is the relationship between D-threose and L-threose? 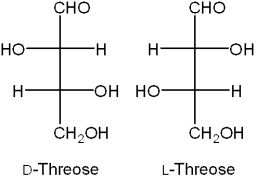
A)constitutional isomers
B)enantiomers
C)diastereomers
D)tautomers

A)constitutional isomers
B)enantiomers
C)diastereomers
D)tautomers

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
21
Consider the following structures. 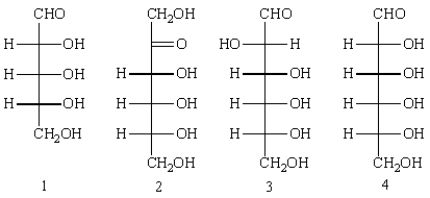 Answer the following questions by placing the appropriate number or indicated letter in the blank.
Answer the following questions by placing the appropriate number or indicated letter in the blank.
Structure ____ represents a aldopentose.
 Answer the following questions by placing the appropriate number or indicated letter in the blank.
Answer the following questions by placing the appropriate number or indicated letter in the blank.Structure ____ represents a aldopentose.

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
22
Which of the following is vitamin C?
A)D-glucose
B)D-mannitol
C)L-ascorbic acid
D)L-sorbose
A)D-glucose
B)D-mannitol
C)L-ascorbic acid
D)L-sorbose

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
23
Starch, cellulose, and surcrose are all classified as polysaccharides.

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
24
Which of the following is not a polysaccharide?
A)amylose
B)amylopectin
C)glycogen
D)ribulose
A)amylose
B)amylopectin
C)glycogen
D)ribulose

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
25
Oxidation of optically active D-erythrose with nitric acid produces optically inactive meso-tartaric acid (below). 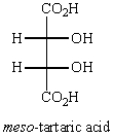 The structure of D-erythrose is shown below.
The structure of D-erythrose is shown below. 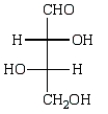
 The structure of D-erythrose is shown below.
The structure of D-erythrose is shown below. 

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
26
Consider the following Fisher projections.  They all convert to the same tetrahedral representation as shown below..
They all convert to the same tetrahedral representation as shown below.. 
 They all convert to the same tetrahedral representation as shown below..
They all convert to the same tetrahedral representation as shown below.. 

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
27
Consider the following structures.  Answer the following questions by placing the appropriate number or indicated letter in the blank.
Answer the following questions by placing the appropriate number or indicated letter in the blank.
The formula for maltose is: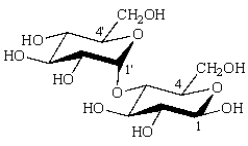 The number of the acetal carbon is _____.
The number of the acetal carbon is _____.
 Answer the following questions by placing the appropriate number or indicated letter in the blank.
Answer the following questions by placing the appropriate number or indicated letter in the blank.The formula for maltose is:
 The number of the acetal carbon is _____.
The number of the acetal carbon is _____.
Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
28
The following structure shows the correct assignment of the R or S configuration to each chirality center in sorbose. 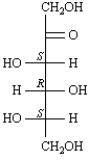


Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
29
The following compound will react with Tollens' solution. 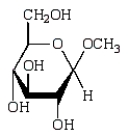


Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
30
Reduction of an optically active aldopentose with NaBH4 gives an optically inactive alditol. The aldopentose could be: 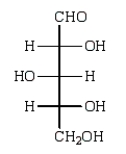


Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
31
Consider the following structures.  Answer the following questions by placing the appropriate number or indicated letter in the blank.
Answer the following questions by placing the appropriate number or indicated letter in the blank.
The structures that represent reducing sugars are:
a. only the ketoses.
b. only the aldoses.
c. all of these structures.
 Answer the following questions by placing the appropriate number or indicated letter in the blank.
Answer the following questions by placing the appropriate number or indicated letter in the blank.The structures that represent reducing sugars are:
a. only the ketoses.
b. only the aldoses.
c. all of these structures.

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
32
The following are two different methods of representing the same structure. 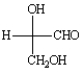
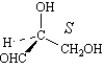



Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
33
A 1,4- -linkage between two glucose units produces a disaccharide called maltose.

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
34
Consider the structure shown below. 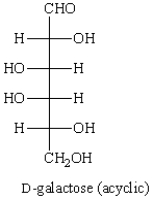 The Haworth projection formula for the pyranose form of -D-galactose is given below.
The Haworth projection formula for the pyranose form of -D-galactose is given below. 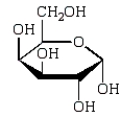
 The Haworth projection formula for the pyranose form of -D-galactose is given below.
The Haworth projection formula for the pyranose form of -D-galactose is given below. 

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
35
Consider the following structures.  Answer the following questions by placing the appropriate number or indicated letter in the blank.
Answer the following questions by placing the appropriate number or indicated letter in the blank.
Structure ____ represents a ketohexose.
 Answer the following questions by placing the appropriate number or indicated letter in the blank.
Answer the following questions by placing the appropriate number or indicated letter in the blank.Structure ____ represents a ketohexose.

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
36
Consider the following structures.  Answer the following questions by placing the appropriate number or indicated letter in the blank.
Answer the following questions by placing the appropriate number or indicated letter in the blank.
The number of hydroxyl groups in the pyranose form of glucose is _____.
 Answer the following questions by placing the appropriate number or indicated letter in the blank.
Answer the following questions by placing the appropriate number or indicated letter in the blank.The number of hydroxyl groups in the pyranose form of glucose is _____.

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
37
Consider the following structures.  Answer the following questions by placing the appropriate number or indicated letter in the blank.
Answer the following questions by placing the appropriate number or indicated letter in the blank.
The number of the anomeric carbon in the following structure is _____.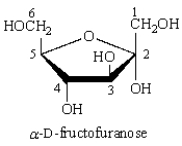
 Answer the following questions by placing the appropriate number or indicated letter in the blank.
Answer the following questions by placing the appropriate number or indicated letter in the blank.The number of the anomeric carbon in the following structure is _____.


Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
38
A glycoside is formed upon reaction of glucopyranose with NaBH4.

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
39
The difference between the pyranose and furanose forms of a given aldohexose is the ring size.

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
40
Which of the following is an alditol?
A)glucose
B)fructose
C)glucaric acid
D)mannitol
A)glucose
B)fructose
C)glucaric acid
D)mannitol

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
41
What is the major organic product obtained from the following reduction of glucose? 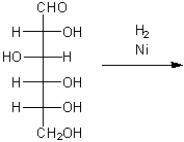


Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
42
From the sugars below, choose the one that best fits each description below. Place the letter of the sugar in the blank to the left of the description. There is only one correct answer for each question.
_____ is a sugar with three chirality centers.
A)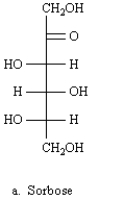
B)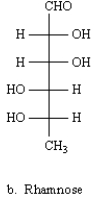
C)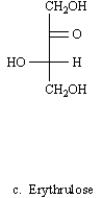
D)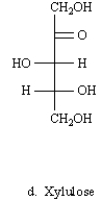
_____ is a sugar with three chirality centers.
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
43
From the sugars below, choose the one that best fits each description below. Place the letter of the sugar in the blank to the left of the description. There is only one correct answer for each question.
_____ is a deoxy sugar.
A)
B)
C)
D)
_____ is a deoxy sugar.
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
44
The textile rayon is produced by treatment of cellulose with carbon disulfide in aqueous sodium hydroxide. What type of functional group is introduced on the cellulose under these conditions? 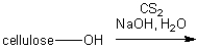

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
45
Complete the structure on the right so that it represents the pyranose form of the carbohydrate shown on the left as a Fischer projection. Place the substituents carefully so that their position in axial or equatorial positions accurately reflects the stereochemistry of the carbohydrate. 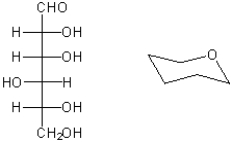


Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
46
What are the major organic products obtained from treatment of a methyl glycoside with periodic acid in water? 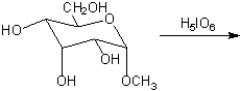


Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
47
From the sugars below, choose the one that best fits each description below. Place the letter of the sugar in the blank to the left of the description. There is only one correct answer for each question.
_____ is a sugar which yields a single alditol upon reduction.
A)
B)
C)
D)
_____ is a sugar which yields a single alditol upon reduction.
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
48
The textile cellulose acetate is produced by treatment of cellulose with acetic anhydride. What type of functional group is introduced on the cellulose under these conditions? 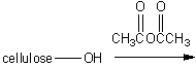


Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
49
Consider the following structures.  Answer the following questions by placing the appropriate number or indicated letter in the blank.
Answer the following questions by placing the appropriate number or indicated letter in the blank.
The following monosaccharide has ________ equatorial hydroxyl groups.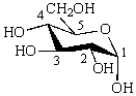
 Answer the following questions by placing the appropriate number or indicated letter in the blank.
Answer the following questions by placing the appropriate number or indicated letter in the blank.The following monosaccharide has ________ equatorial hydroxyl groups.


Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
50
Complete the structure on the right so that it represents the pyranose form of the carbohydrate shown on the left as a Fischer projection. Place the substituents carefully so that their position in axial or equatorial positions accurately reflects the stereochemistry of the carbohydrate. 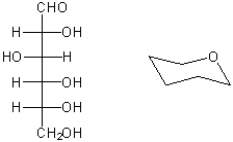


Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
51
Refer to the equilibrium below to answer the following question(s). 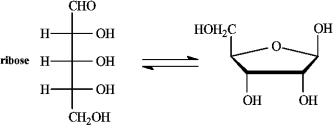
-The correct name for the cyclic structure is__________________________.

-The correct name for the cyclic structure is__________________________.

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
52
What is the major organic product obtained from the reaction of glucose with glucose oxidase? 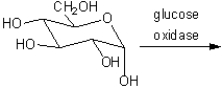


Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
53
From the sugars below, choose the one that best fits each description below. Place the letter of the sugar in the blank to the left of the description. There is only one correct answer for each question.
_____ is a D-sugar.
A)
B)
C)
D)
_____ is a D-sugar.
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
54
Refer to the equilibrium below to answer the following question(s). 
The enantiomer of ribose is drawn is _______. (D or L)

The enantiomer of ribose is drawn is _______. (D or L)

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
55
From the sugars below, choose the one that best fits each description below. Place the letter of the sugar in the blank to the left of the description. There is only one correct answer for each question.
The fundamental difference between the monosaccharide, disaccharide, and polysaccharide classifications of carbohydrates is the number of _________________present.
The fundamental difference between the monosaccharide, disaccharide, and polysaccharide classifications of carbohydrates is the number of _________________present.

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
56
Refer to the equilibrium below to answer the following question(s). 
-The anomer of ribose is drawn is _______. (alpha or beta)

-The anomer of ribose is drawn is _______. (alpha or beta)

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
57
What are the major organic products obtained from treatment of a 1,2,3-triol with periodic acid in water? 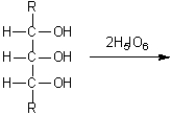


Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
58
From the sugars below, choose the one that best fits each description below. Place the letter of the sugar in the blank to the left of the description. There is only one correct answer for each question.
The name of the following Fischer projection including stereochemistry is _____________________.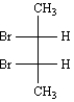
The name of the following Fischer projection including stereochemistry is _____________________.


Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
59
What are the two major organic products obtained from treatment of an
-hydroxyketone with periodic acid in water?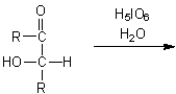
-hydroxyketone with periodic acid in water?


Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
60
What are the two major organic products obtained from the following reaction? What is the relationship between them? (What type of isomers are they?) 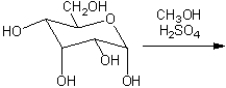


Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck



