Deck 3: Mass Relationships in Chemical Reactions
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/159
Play
Full screen (f)
Deck 3: Mass Relationships in Chemical Reactions
1
Sodium phosphate reacts with sulfuric acid to form sodium sulfate and phosphoric acid.What is the stoichiometric coefficient for sulfuric acid when the chemical equation is balanced using the lowest whole-number stoichiometric coefficients?
A)1
B)2
C)3
D)none of these
A)1
B)2
C)3
D)none of these
3
2
What is the molar mass of aspartic acid,C4O4H7N?
A)43 g/mol
B)70 g/mol
C)133 g/mol
D)197 g/mol
A)43 g/mol
B)70 g/mol
C)133 g/mol
D)197 g/mol
133 g/mol
3
How many sulfate ions are there in 5.00 g of FeSO4?
A)5.46 × 10-26 iron (II)ions
B)1.98 × 1022 iron (II)ions
C)1.83 × 1025 iron (II)ions
D)4.58 × 1026 iron (II)ions
A)5.46 × 10-26 iron (II)ions
B)1.98 × 1022 iron (II)ions
C)1.83 × 1025 iron (II)ions
D)4.58 × 1026 iron (II)ions
1.98 × 1022 iron (II)ions
4
Which one of the following statements about balanced equations is false? In a balanced reaction
A)atoms must be balanced on both sides of the reaction arrow.
B)mass must be conserved.
C)molecules must be balanced on both sides of the reaction arrow.
D)net charge must be balanced on both sides of the reaction arrow.
A)atoms must be balanced on both sides of the reaction arrow.
B)mass must be conserved.
C)molecules must be balanced on both sides of the reaction arrow.
D)net charge must be balanced on both sides of the reaction arrow.

Unlock Deck
Unlock for access to all 159 flashcards in this deck.
Unlock Deck
k this deck
5
Given the chemical equation: N2 + 3 H2 → 2 NH3.On a macroscopic level,what do the coefficients mean?
A)1 atom of nitrogen reacts with 3 atoms of hydrogen to give 2 atoms of ammonia.
B)1 mole of nitrogen reacts with 3 moles of hydrogen to give 2 moles of ammonia.
C)1 molecule of nitrogen reacts with 3 molecules of hydrogen to give 2 molecules of ammonia.
D)All of these are true.
A)1 atom of nitrogen reacts with 3 atoms of hydrogen to give 2 atoms of ammonia.
B)1 mole of nitrogen reacts with 3 moles of hydrogen to give 2 moles of ammonia.
C)1 molecule of nitrogen reacts with 3 molecules of hydrogen to give 2 molecules of ammonia.
D)All of these are true.

Unlock Deck
Unlock for access to all 159 flashcards in this deck.
Unlock Deck
k this deck
6
What is the sum of the coefficients when the following equation is balanced using the lowest whole-numbered coefficients? ________ B2O3(s)+ ________ HF(l)→ ________ BF3(g)+ ________ H2O(l)
A)8
B)11
C)15
D)none of these
A)8
B)11
C)15
D)none of these

Unlock Deck
Unlock for access to all 159 flashcards in this deck.
Unlock Deck
k this deck
7
Chemical equations are balanced in order to obey the law of
A)definite proportions.
B)mass action.
C)mass conservation.
D)multiple proportions.
A)definite proportions.
B)mass action.
C)mass conservation.
D)multiple proportions.

Unlock Deck
Unlock for access to all 159 flashcards in this deck.
Unlock Deck
k this deck
8
How many moles are in 1.50 g of ethylamine,CH3CH2NH2?
A)0.0222 mol
B)0.0332 mol
C)90.16 mol
D)45.08 mol
A)0.0222 mol
B)0.0332 mol
C)90.16 mol
D)45.08 mol

Unlock Deck
Unlock for access to all 159 flashcards in this deck.
Unlock Deck
k this deck
9
Which contains Avogadro's number of formula units?
A)36.5 g of Cl
B)36.5 g of Cl2
C)36.5 g of HCl
D)All of these
A)36.5 g of Cl
B)36.5 g of Cl2
C)36.5 g of HCl
D)All of these

Unlock Deck
Unlock for access to all 159 flashcards in this deck.
Unlock Deck
k this deck
10
What is the mass of a single ozone molecule,O3?
A)2.656 × 10-23 g
B)7.969 × 10-22 g
C)16.0 g
D)48.0 g
A)2.656 × 10-23 g
B)7.969 × 10-22 g
C)16.0 g
D)48.0 g

Unlock Deck
Unlock for access to all 159 flashcards in this deck.
Unlock Deck
k this deck
11
Which one of the following statements about balanced equations is true? A reaction is balanced by
A)changing the charge on an ion.
B)changing the formula of the molecule.
C)multiplying by suitable coefficients.
D)rearranging atoms in a molecule.
A)changing the charge on an ion.
B)changing the formula of the molecule.
C)multiplying by suitable coefficients.
D)rearranging atoms in a molecule.

Unlock Deck
Unlock for access to all 159 flashcards in this deck.
Unlock Deck
k this deck
12
Which conducts electricity?
A)a large collection of iron atoms
B)a single iron atom
C)both a large collection of iron atoms and a single iron atom
D)neither a large collection of iron atoms nor a single iron atom
A)a large collection of iron atoms
B)a single iron atom
C)both a large collection of iron atoms and a single iron atom
D)neither a large collection of iron atoms nor a single iron atom

Unlock Deck
Unlock for access to all 159 flashcards in this deck.
Unlock Deck
k this deck
13
How many sodium atoms are in 3.00 g of sodium dichromate,Na2Cr2O7?
A)0.023 oxygen atoms
B)2.82 × 1020 oxygen atoms
C)1.97 × 1021 oxygen atoms
D)1.38 × 1022 oxygen atoms
A)0.023 oxygen atoms
B)2.82 × 1020 oxygen atoms
C)1.97 × 1021 oxygen atoms
D)1.38 × 1022 oxygen atoms

Unlock Deck
Unlock for access to all 159 flashcards in this deck.
Unlock Deck
k this deck
14
What is the molar mass of calcium permanganate?
A)159 g/mol
B)199 g/mol
C)216 g/mol
D)278 g/mol
A)159 g/mol
B)199 g/mol
C)216 g/mol
D)278 g/mol

Unlock Deck
Unlock for access to all 159 flashcards in this deck.
Unlock Deck
k this deck
15
1.00 mole of O2 contains the same number of oxygen atoms as
A)0.667 mole of O3.
B)1.00 mole of CH3CO2H.
C)2.00 mole of CH3CH2OH.
D)All of the above
A)0.667 mole of O3.
B)1.00 mole of CH3CO2H.
C)2.00 mole of CH3CH2OH.
D)All of the above

Unlock Deck
Unlock for access to all 159 flashcards in this deck.
Unlock Deck
k this deck
16
What is the sum of the coefficients when the following equation is balanced using the lowest whole-numbered coefficients? ________ PH3(g)+ ________ O2(g)→ ________ P4O10(s)+ ________ H2O(g)
A)10
B)12
C)19
D)22
A)10
B)12
C)19
D)22

Unlock Deck
Unlock for access to all 159 flashcards in this deck.
Unlock Deck
k this deck
17
Given the chemical equation: N2 + 3 H2 → 2 NH3.On a molecular level,what do the coefficients mean?
A)1 atom of nitrogen reacts with 3 atoms of hydrogen to give 2 atoms of ammonia.
B)28 g of nitrogen reacts with 6 grams of hydrogen to give 34 grams of ammonia.
C)1 mole of nitrogen reacts with 3 moles of hydrogen to give 2 moles of ammonia.
D)1 molecule of nitrogen reacts with 3 molecules of hydrogen to give 2 molecules of ammonia.
A)1 atom of nitrogen reacts with 3 atoms of hydrogen to give 2 atoms of ammonia.
B)28 g of nitrogen reacts with 6 grams of hydrogen to give 34 grams of ammonia.
C)1 mole of nitrogen reacts with 3 moles of hydrogen to give 2 moles of ammonia.
D)1 molecule of nitrogen reacts with 3 molecules of hydrogen to give 2 molecules of ammonia.

Unlock Deck
Unlock for access to all 159 flashcards in this deck.
Unlock Deck
k this deck
18
What is the mass of 0.0500 mol of dichlorodifluoromethane,CF2Cl2?
A)4.14 × 10-4 g
B)6.05 g
C)12.1 g
D)24.2 g
A)4.14 × 10-4 g
B)6.05 g
C)12.1 g
D)24.2 g

Unlock Deck
Unlock for access to all 159 flashcards in this deck.
Unlock Deck
k this deck
19
What is the molar mass of iodine?
A)126.9 g/mol
B)253.8 g/mol
C)6.02 × 1023 g/mol
D)1.20 × 1024 g/mol
A)126.9 g/mol
B)253.8 g/mol
C)6.02 × 1023 g/mol
D)1.20 × 1024 g/mol

Unlock Deck
Unlock for access to all 159 flashcards in this deck.
Unlock Deck
k this deck
20
What is the molar mass of Co(NO3)2?
A)90 g/mol
B)121 g/mol
C)152 g/mol
D)183 g/mol
A)90 g/mol
B)121 g/mol
C)152 g/mol
D)183 g/mol

Unlock Deck
Unlock for access to all 159 flashcards in this deck.
Unlock Deck
k this deck
21
How many grams of calcium chloride are needed to produce 10.0 g of potassium chloride? CaCl2(aq)+ K2CO3(aq)→ 2 KCl(aq)+ CaCO3(aq)
A)0.134 g
B)7.44 g
C)9.28 g
D)18.56 g
A)0.134 g
B)7.44 g
C)9.28 g
D)18.56 g

Unlock Deck
Unlock for access to all 159 flashcards in this deck.
Unlock Deck
k this deck
22
If the percent yield for the following reaction is 60.0%,and 45.0 g of NO2 are consumed in the reaction,how many grams of nitric acid,HNO3(aq),are produced?
3 NO2(g)+ H2O(l)→ 2 HNO3(aq)+ NO(g)
A)24.6 g
B)41.1 g
C)54.8 g
D)69.3 g
3 NO2(g)+ H2O(l)→ 2 HNO3(aq)+ NO(g)
A)24.6 g
B)41.1 g
C)54.8 g
D)69.3 g

Unlock Deck
Unlock for access to all 159 flashcards in this deck.
Unlock Deck
k this deck
23
What is the identity of substance X if 0.380 mol of X weighs 17.5 g?
A)NO2
B)NO3
C)N2O
D)N2O4
A)NO2
B)NO3
C)N2O
D)N2O4

Unlock Deck
Unlock for access to all 159 flashcards in this deck.
Unlock Deck
k this deck
24
What mass of sulfur hexafluoride,SF6,has the same number of fluorine atoms as 50.0 g of oxygen difluoride,OF2?
A)202.7 g
B)8.33 g
C)43.0 g
D)135.1 g
A)202.7 g
B)8.33 g
C)43.0 g
D)135.1 g

Unlock Deck
Unlock for access to all 159 flashcards in this deck.
Unlock Deck
k this deck
25
Which of the following has the greatest mass?
A)6.02 × 1023 molecules of Cl2
B)35.45 g of Cl2
C)0.500 mol of Cl2
D)All of these have the same mass.
A)6.02 × 1023 molecules of Cl2
B)35.45 g of Cl2
C)0.500 mol of Cl2
D)All of these have the same mass.

Unlock Deck
Unlock for access to all 159 flashcards in this deck.
Unlock Deck
k this deck
26
What is the molar mass of aspirin if 5.19 × 1016 molecules of aspirin weigh 15.53 μg?
A)180 g/mol
B)80.6 g/mol
C)133.8 g/mol
D)200 g/mol
A)180 g/mol
B)80.6 g/mol
C)133.8 g/mol
D)200 g/mol

Unlock Deck
Unlock for access to all 159 flashcards in this deck.
Unlock Deck
k this deck
27
When methane,CH4,undergoes combustion with oxygen,the usual products are carbon dioxide and water.Carbon monoxide is formed when the limiting reactant is
A)carbon dioxide.
B)methane.
C)oxygen.
D)water.
A)carbon dioxide.
B)methane.
C)oxygen.
D)water.

Unlock Deck
Unlock for access to all 159 flashcards in this deck.
Unlock Deck
k this deck
28
How many cations are in 0.500 g of MgBr2?
A)1.37 × 1021 anions
B)1.64 × 1021 anions
C)2.22 × 1026 anions
D)4.43 × 1026 anions
A)1.37 × 1021 anions
B)1.64 × 1021 anions
C)2.22 × 1026 anions
D)4.43 × 1026 anions

Unlock Deck
Unlock for access to all 159 flashcards in this deck.
Unlock Deck
k this deck
29
Balance the chemical equation given below,and determine the number of moles of iodine that reacts with 5.0 g of aluminum. ________ Al(s)+ ________ I2(s)→ ________ Al2I6(s)
A)0.185 mol
B)0.278 mol
C)0.139 mol
D)0.834 mol
A)0.185 mol
B)0.278 mol
C)0.139 mol
D)0.834 mol

Unlock Deck
Unlock for access to all 159 flashcards in this deck.
Unlock Deck
k this deck
30
3.0 moles of nitrogen is reacted with 11.0 moles of hydrogen to produce ammonia according to the chemical equation shown below.Which one of the following statements is false? N2(g)+ 3 H2(g)→ 2 NH3(g)
A)2.0 moles of hydrogen are left over.
B)Hydrogen is the excess reactant.
C)Nitrogen is the limiting reactant.
D)12.0 moles of ammonia are produced.
A)2.0 moles of hydrogen are left over.
B)Hydrogen is the excess reactant.
C)Nitrogen is the limiting reactant.
D)12.0 moles of ammonia are produced.

Unlock Deck
Unlock for access to all 159 flashcards in this deck.
Unlock Deck
k this deck
31
The density of ethanol,C2H5OH,is 0.789 g/mL.How many milliliters of ethanol are needed to produce 5.00 g of CO2 according to the following chemical equation?
C2H5OH(l)+ 3 O2(g)→ 2 CO2(g)+ 3 H2O(l)
A)2.06 mL
B)3.32 mL
C)6.60 mL
D)13.3 mL
C2H5OH(l)+ 3 O2(g)→ 2 CO2(g)+ 3 H2O(l)
A)2.06 mL
B)3.32 mL
C)6.60 mL
D)13.3 mL

Unlock Deck
Unlock for access to all 159 flashcards in this deck.
Unlock Deck
k this deck
32
Which of the following has the smallest mass?
A)6.02 × 1023 molecules of I2
B)70.0 g of Cl2
C)2.00 mol of F2
D)0.040 kg of Br2
A)6.02 × 1023 molecules of I2
B)70.0 g of Cl2
C)2.00 mol of F2
D)0.040 kg of Br2

Unlock Deck
Unlock for access to all 159 flashcards in this deck.
Unlock Deck
k this deck
33
In the reaction between glucose and oxygen,10.0 g of glucose reacts and 7.50 L of carbon dioxide is formed.What is the percent yield if the density of CO2 is 1.26 g/L?
C6H12O6(s)+ 6 O2(g)→ 6 CO2(g)+ 6 H2O(l)
A)26.1%
B)40.6%
C)43.1%
D)64.5%
C6H12O6(s)+ 6 O2(g)→ 6 CO2(g)+ 6 H2O(l)
A)26.1%
B)40.6%
C)43.1%
D)64.5%

Unlock Deck
Unlock for access to all 159 flashcards in this deck.
Unlock Deck
k this deck
34
Balance the chemical equation given below,and determine the number of grams of Fe needed to produce 15.0 g of Fe2O3. ________ MgO(s)+ ________ Fe(s)→ ________ Fe2O3(s)+ ________ Mg(s)
A)11.36 g
B)3.78 g
C)5.245 g
D)10.49 g
A)11.36 g
B)3.78 g
C)5.245 g
D)10.49 g

Unlock Deck
Unlock for access to all 159 flashcards in this deck.
Unlock Deck
k this deck
35
How many anions are in 10.0 g of sodium phosphate?
A)3.67 × 1022 cations
B)1.10 × 1023 cations
C)9.87 × 1024 cations
D)2.96 × 1025 cations
A)3.67 × 1022 cations
B)1.10 × 1023 cations
C)9.87 × 1024 cations
D)2.96 × 1025 cations

Unlock Deck
Unlock for access to all 159 flashcards in this deck.
Unlock Deck
k this deck
36
Which of the following has the greatest mass?
A)6.0 × 1023 atoms of O
B)3.0 × 1023 molecules of O2
C)2.0 × 1023 molecules of O3
D)All have the same mass.
A)6.0 × 1023 atoms of O
B)3.0 × 1023 molecules of O2
C)2.0 × 1023 molecules of O3
D)All have the same mass.

Unlock Deck
Unlock for access to all 159 flashcards in this deck.
Unlock Deck
k this deck
37
5.0 g of nitrogen is reacted with 5.0 g of hydrogen to produce ammonia according to the chemical equation shown below.Which one of the following statements is false?
N2(g)+ 3 H2(g)→ 2 NH3(g)
A)2.8 grams of hydrogen are left over.
B)Hydrogen is the excess reactant.
C)Nitrogen is the limiting reactant.
D)The theoretical yield of ammonia is 6.1 g.
N2(g)+ 3 H2(g)→ 2 NH3(g)
A)2.8 grams of hydrogen are left over.
B)Hydrogen is the excess reactant.
C)Nitrogen is the limiting reactant.
D)The theoretical yield of ammonia is 6.1 g.

Unlock Deck
Unlock for access to all 159 flashcards in this deck.
Unlock Deck
k this deck
38
What mass of carbon monoxide,CO,contains the same number of molecules as 3.00 g of trichlorofluoromethane,CCl3F?
A)3.00 g
B)0.612 g
C)1.63 g
D)9.33 g
A)3.00 g
B)0.612 g
C)1.63 g
D)9.33 g

Unlock Deck
Unlock for access to all 159 flashcards in this deck.
Unlock Deck
k this deck
39
Tablets of ascorbic acid,or Vitamin C,C6H8O6,are taken as a dietary supplement.If a typical tablet contains 500 mg,how many molecules of Vitamin C are in a tablet?
A)500 molecules
B)1.71 × 1024 molecules
C)3.0 × 1024 molecules
D)1.71 × 1021 molecules
A)500 molecules
B)1.71 × 1024 molecules
C)3.0 × 1024 molecules
D)1.71 × 1021 molecules

Unlock Deck
Unlock for access to all 159 flashcards in this deck.
Unlock Deck
k this deck
40
4.0 g of iron is reacted with 4.0 g of water according to the chemical equation shown below.Which one of the following statements is false? 3 Fe(s)+ 4 H2O(l)→ Fe3O4(s)+ 4 H2(g)
A)6.91 g of Fe3O4 are produced.
B)2.28 g of H2O are left over.
C)Mass is conserved in this reaction.
D)Fe is the limiting reactant.
A)6.91 g of Fe3O4 are produced.
B)2.28 g of H2O are left over.
C)Mass is conserved in this reaction.
D)Fe is the limiting reactant.

Unlock Deck
Unlock for access to all 159 flashcards in this deck.
Unlock Deck
k this deck
41
The sugar fructose has an empirical formula of CH2O.The mass spectrum shows a molecular ion peak at a mass of 179.9.What is the molecular formula of fructose?
A)CH2O
B)C2H4O4
C)C6H11O6
D)C6H12O6
A)CH2O
B)C2H4O4
C)C6H11O6
D)C6H12O6

Unlock Deck
Unlock for access to all 159 flashcards in this deck.
Unlock Deck
k this deck
42
Consider two reactants,A and B.The molar mass of A is greater han the molar mass of B.You add equal masses of A and B together and let them react.Which of the following statements must be true?
A)Reactant A must be limiting.
B)Reactant B must be limiting.
C)Reactant A is the excess reactant.
D)None of the above choices must be true.
A)Reactant A must be limiting.
B)Reactant B must be limiting.
C)Reactant A is the excess reactant.
D)None of the above choices must be true.

Unlock Deck
Unlock for access to all 159 flashcards in this deck.
Unlock Deck
k this deck
43
Which statement about elemental analysis by combustion is not correct?
A)Carbon is determined from the amount of CO2 formed.
B)Hydrogen is determined from the amount of H2O formed.
C)Oxygen is determined from the amount of H2O formed.
D)Only carbon and hydrogen can be determined directly from CO2 and H2O.
A)Carbon is determined from the amount of CO2 formed.
B)Hydrogen is determined from the amount of H2O formed.
C)Oxygen is determined from the amount of H2O formed.
D)Only carbon and hydrogen can be determined directly from CO2 and H2O.

Unlock Deck
Unlock for access to all 159 flashcards in this deck.
Unlock Deck
k this deck
44
How many grams of the excess reagent are left over when 6.00 g of CS2 gas react with 10.0 g of Cl2 gas in the following reaction? CS2(g)+ 3 Cl2(g)→ CCl4(l)+ S2Cl2(l)
A)2.42 g
B)2.77 g
C)3.58 g
D)4.00 g
A)2.42 g
B)2.77 g
C)3.58 g
D)4.00 g

Unlock Deck
Unlock for access to all 159 flashcards in this deck.
Unlock Deck
k this deck
45
What is the empirical formula for ethyl fluoride if the compound contains 49.97% carbon,10.51% hydrogen,and 39.52% fluorine by mass?
A)C2H5F
B)C4H10F2
C)C4H10F4
D)C25F2
A)C2H5F
B)C4H10F2
C)C4H10F4
D)C25F2

Unlock Deck
Unlock for access to all 159 flashcards in this deck.
Unlock Deck
k this deck
46
Which of the following statements about mass spectrometry is false?
A)Mass spectrometry can be used to determine the molecular weight of a compound.
B)The curvature of the path in a magnetic field is determined by the mass of the ion.
C)The paths of heavier ions are deflected more strongly than the paths of lighter ions.
D)The sample is changed into positively charged ions.
A)Mass spectrometry can be used to determine the molecular weight of a compound.
B)The curvature of the path in a magnetic field is determined by the mass of the ion.
C)The paths of heavier ions are deflected more strongly than the paths of lighter ions.
D)The sample is changed into positively charged ions.

Unlock Deck
Unlock for access to all 159 flashcards in this deck.
Unlock Deck
k this deck
47
Which one of the following contains 38.7% carbon by mass?
A)C2H2
B)CH4
C)CH3NH2
D)CO2
A)C2H2
B)CH4
C)CH3NH2
D)CO2

Unlock Deck
Unlock for access to all 159 flashcards in this deck.
Unlock Deck
k this deck
48
In the combustion analysis of an unknown compound containing only carbon,hydrogen,and oxygen,the grams of oxygen are found from the grams of
A)CO2 only.
B)H2O only.
C)CO2 and H2O only.
D)CO2,H2O and unknown compound.
A)CO2 only.
B)H2O only.
C)CO2 and H2O only.
D)CO2,H2O and unknown compound.

Unlock Deck
Unlock for access to all 159 flashcards in this deck.
Unlock Deck
k this deck
49
A compound responsible for the odor of garlic has a molecular weight of 146 g/mol.A 0.650 g sample of the compound contains 0.321 g of carbon,0.044 g of hydrogen,and 0.285 g of sulfur.What is the molecular formula of the compound?
A)CH5S
B)C3H5S
C)C3H15S3
D)C6H10S2
A)CH5S
B)C3H5S
C)C3H15S3
D)C6H10S2

Unlock Deck
Unlock for access to all 159 flashcards in this deck.
Unlock Deck
k this deck
50
When silver nitrate reacts with barium chloride,silver chloride and barium nitrate are formed.How many grams of silver chloride are formed when 8.0 g of silver nitrate reacts with 15.0 g of barium chloride?
A)6.752 g
B)9.40 g
C)11.9 g
D)18.8 g
A)6.752 g
B)9.40 g
C)11.9 g
D)18.8 g

Unlock Deck
Unlock for access to all 159 flashcards in this deck.
Unlock Deck
k this deck
51
Which of the following statements is false concerning the formula of a compound?
A)The empirical formula is the simplest whole numbered ratio of atoms in a compound.
B)The molecular formula is the true ratio of atoms in a compound.
C)The molecular formula and empirical formula can be identical.
D)The number of atoms in a molecular formula is always greater than the number of atoms in an empirical formula.
A)The empirical formula is the simplest whole numbered ratio of atoms in a compound.
B)The molecular formula is the true ratio of atoms in a compound.
C)The molecular formula and empirical formula can be identical.
D)The number of atoms in a molecular formula is always greater than the number of atoms in an empirical formula.

Unlock Deck
Unlock for access to all 159 flashcards in this deck.
Unlock Deck
k this deck
52
Combustion analysis of an unknown compound containing only carbon and hydrogen produced 2.845 g of CO2 and 1.744 g of H2O.What is the empirical formula of the compound?
A)CH2
B)CH3
C)C4H10
D)C5H2
A)CH2
B)CH3
C)C4H10
D)C5H2

Unlock Deck
Unlock for access to all 159 flashcards in this deck.
Unlock Deck
k this deck
53
Each molecule of cortisone contains 21 atoms of carbon (plus other atoms).The mass percentage of carbon in cortisone is 69.98%.What is the molar mass of cortisone?
A)176.5 g/mol
B)252.2 g/mol
C)287.6 g/mol
D)360.4 g/mol
A)176.5 g/mol
B)252.2 g/mol
C)287.6 g/mol
D)360.4 g/mol

Unlock Deck
Unlock for access to all 159 flashcards in this deck.
Unlock Deck
k this deck
54
![<strong> Acetone has the formula C<sub>3</sub>H<sub>6</sub>O.Which ball and stick model shown above represents acetone? [gray spheres = C,black spheres = O,unshaded spheres = H]</strong> A)model a) B)model b) C)model c) D)model d)](https://d2lvgg3v3hfg70.cloudfront.net/TB4940/11ea7e2d_d09e_aff7_a2f7_11edcd81f00c_TB4940_00_TB4940_00_TB4940_00_TB4940_00.jpg)
Acetone has the formula C3H6O.Which ball and stick model shown above represents acetone? [gray spheres = C,black spheres = O,unshaded spheres = H]
A)model a)
B)model b)
C)model c)
D)model d)

Unlock Deck
Unlock for access to all 159 flashcards in this deck.
Unlock Deck
k this deck
55
When iron(III)oxide reacts with hydrochloric acid,iron(III)chloride and water are formed.How many grams of iron(III)chloride are formed from 10.0 g of iron(III)oxide and 10.0 g of hydrochloric acid?
A)11.1 g
B)14.8 g
C)20.3 g
D)35.1 g
A)11.1 g
B)14.8 g
C)20.3 g
D)35.1 g

Unlock Deck
Unlock for access to all 159 flashcards in this deck.
Unlock Deck
k this deck
56
Molecular mass can be determined by
A)combustion analysis.
B)mass spectrometry.
C)titration.
D)weighing with an analytical balance.
A)combustion analysis.
B)mass spectrometry.
C)titration.
D)weighing with an analytical balance.

Unlock Deck
Unlock for access to all 159 flashcards in this deck.
Unlock Deck
k this deck
57
Combustion analysis of 2.796 g of an unknown compound containing carbon,hydrogen,and oxygen produced 5.597 g of CO2 and 2.268 g of H2O.What is the empirical formula of the compound?
A)C2H5O
B)C2H5O2
C)C2H10O3
D)C2H4O
A)C2H5O
B)C2H5O2
C)C2H10O3
D)C2H4O

Unlock Deck
Unlock for access to all 159 flashcards in this deck.
Unlock Deck
k this deck
58
Isoeugenol is the compound which gives the characteristic odor to nutmeg and contains carbon,hydrogen,and oxygen.If a 0.500 g sample of isoeugenol is combusted it gives 1.341 g of CO2 and 0.329 g of H2O.Isoeugenol has a molecular weight of 164 g/mol.What is the molecular formula of isoeugenol?
A)C2HO
B)C5H6O
C)C8H4O4
D)C10H12O2
A)C2HO
B)C5H6O
C)C8H4O4
D)C10H12O2

Unlock Deck
Unlock for access to all 159 flashcards in this deck.
Unlock Deck
k this deck
59
Balance the chemical equation given below,and calculate the volume of nitrogen monoxide gas produced when 8.00 g of ammonia is reacted with 14.0 g of oxygen at 25°C? The density of nitrogen monoxide at 25°C is 1.23 g/L. ________ NH3(g)+ ________ O2(g)→ ________ NO(g)+ ________ H2O(l)
A)8.54 L
B)11.1 L
C)11.5 L
D)18.8 L
A)8.54 L
B)11.1 L
C)11.5 L
D)18.8 L

Unlock Deck
Unlock for access to all 159 flashcards in this deck.
Unlock Deck
k this deck
60
Combustion analysis of a 0.675 g sample of an unknown compound that contains only carbon,hydrogen,and oxygen gives 0.627 g of CO2 and 1.534 g of H2O.The molecular mass of the unknown is
A)C3H6O.
B)C6H12O2.
C)C9H18O3.
D)unable to be determined from this data.
A)C3H6O.
B)C6H12O2.
C)C9H18O3.
D)unable to be determined from this data.

Unlock Deck
Unlock for access to all 159 flashcards in this deck.
Unlock Deck
k this deck
61
The following diagram represents the reaction of A (unshaded spheres)with B (shaded spheres).What is the balanced chemical equation for this reaction,and what is the limiting reactant? 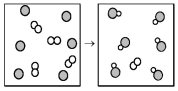
A)A2 + 2B → 2AB;A2 is the limiting reactant.
B)A2 + 2B → 2AB;B is the limiting reactant.
C)4A2 + 6B → 6AB;A2 is the limiting reactant.
D)4A2 + 6B → 6AB;B is the limiting reactant.

A)A2 + 2B → 2AB;A2 is the limiting reactant.
B)A2 + 2B → 2AB;B is the limiting reactant.
C)4A2 + 6B → 6AB;A2 is the limiting reactant.
D)4A2 + 6B → 6AB;B is the limiting reactant.

Unlock Deck
Unlock for access to all 159 flashcards in this deck.
Unlock Deck
k this deck
62
What is the balanced chemical equation for the reaction of element A (unshaded spheres)with element B (shaded spheres)as represented below? 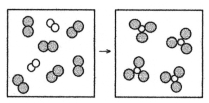
A)A + B → AB
B)A + 3B → 2AB
C)A2 + B2 → AB3
D)A2 + 3B2 → 2AB3

A)A + B → AB
B)A + 3B → 2AB
C)A2 + B2 → AB3
D)A2 + 3B2 → 2AB3

Unlock Deck
Unlock for access to all 159 flashcards in this deck.
Unlock Deck
k this deck
63
![<strong> 2-Propanol has the molecular formula C<sub>3</sub>H<sub>8</sub>O.Which ball and stick model shown above represents 2-propanol? [gray spheres = C,black spheres = O,unshaded spheres = H]</strong> A)model a) B)model b) C)model c) D)model d)](https://d2lvgg3v3hfg70.cloudfront.net/TB4940/11ea7e2d_d09e_aff7_a2f7_11edcd81f00c_TB4940_00_TB4940_00_TB4940_00_TB4940_00.jpg)
2-Propanol has the molecular formula C3H8O.Which ball and stick model shown above represents 2-propanol? [gray spheres = C,black spheres = O,unshaded spheres = H]
A)model a)
B)model b)
C)model c)
D)model d)

Unlock Deck
Unlock for access to all 159 flashcards in this deck.
Unlock Deck
k this deck
64
What is the balanced chemical equation for the reaction of element A (unshaded spheres)with element B (shaded spheres)as represented below? 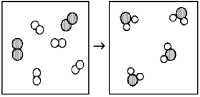
A)A + B → AB
B)4A + 2B → 4AB
C)A2 + B2 → A2B
D)2A2 + B2 → 2A2B

A)A + B → AB
B)4A + 2B → 4AB
C)A2 + B2 → A2B
D)2A2 + B2 → 2A2B

Unlock Deck
Unlock for access to all 159 flashcards in this deck.
Unlock Deck
k this deck
65
Reaction of A (unshaded spheres)with B2 (shaded spheres)is shown schematically in the following diagram.Which equation best describes the stoichiometry of the reaction? 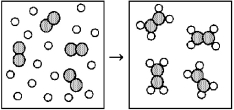
A)4 A + B2 → 8 A2B
B)4 A + B2 → A4B2
C)16 A + 4 B2 → 8 A2B
D)16 A + 4 B2 → 4 A4B2

A)4 A + B2 → 8 A2B
B)4 A + B2 → A4B2
C)16 A + 4 B2 → 8 A2B
D)16 A + 4 B2 → 4 A4B2

Unlock Deck
Unlock for access to all 159 flashcards in this deck.
Unlock Deck
k this deck
66
The following diagram represents the reaction of A2 (unshaded spheres)with B2 (shaded spheres).How many moles of product can be produced from the reaction of 1.0 mol of A2 and 1.0 mol of B2? 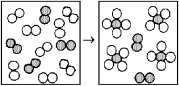
A)0.5 mol of product
B)1.0 mol of product
C)2.0 mol of product
D)4.0 mol of product

A)0.5 mol of product
B)1.0 mol of product
C)2.0 mol of product
D)4.0 mol of product

Unlock Deck
Unlock for access to all 159 flashcards in this deck.
Unlock Deck
k this deck
67
What is the stoichiometric coefficient for oxygen when the following equation is balanced using the lowest whole-number coefficients? ________ C3H6O2(l)+ ________ O2(g)→ ________ CO2(g)+ ________ H2O(l)
A)1
B)3
C)5
D)7
A)1
B)3
C)5
D)7

Unlock Deck
Unlock for access to all 159 flashcards in this deck.
Unlock Deck
k this deck
68
The following diagrams represent the reaction of A2 (shaded spheres)with B2 (unshaded spheres).How many moles of product can be made from 1.0 mol of A2 and 1.0 mol of B2? 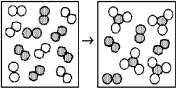
A)0.67 mol product
B)1.0 mol product
C)2.0 mol product
D)3.0 mol product

A)0.67 mol product
B)1.0 mol product
C)2.0 mol product
D)3.0 mol product

Unlock Deck
Unlock for access to all 159 flashcards in this deck.
Unlock Deck
k this deck
69
The following diagrams represent the reaction of A2 (shaded spheres)with B2 (unshaded spheres).Identify the limiting reactant and write a balanced equation for the reaction. 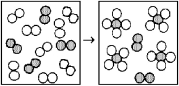
A)A2 is the limiting reactant;A + 4 B → AB4.
B)A2 is the limiting reactant;A2 + 4 B2 → 2 AB4.
C)B2 is the limiting reactant;A + 4 B → AB4.
D)B2 is the limiting reactant;A2 + 4 B2 → 2 AB4.

A)A2 is the limiting reactant;A + 4 B → AB4.
B)A2 is the limiting reactant;A2 + 4 B2 → 2 AB4.
C)B2 is the limiting reactant;A + 4 B → AB4.
D)B2 is the limiting reactant;A2 + 4 B2 → 2 AB4.

Unlock Deck
Unlock for access to all 159 flashcards in this deck.
Unlock Deck
k this deck
70
The following diagram represents the reaction of A2 (unshaded spheres)with B (shaded spheres).What is the balanced chemical equation for this reaction,and what is the limiting reactant? 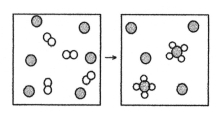
A)2A2 + B → A4B;A2 is the limiting reactant.
B)2A2 + B → A4B;B is the limiting reactant.
C)4A2 + 6B → 2A4B;A2 is the limiting reactant.
D)4A2 + 6B → 2A4B;B is the limiting reactant.

A)2A2 + B → A4B;A2 is the limiting reactant.
B)2A2 + B → A4B;B is the limiting reactant.
C)4A2 + 6B → 2A4B;A2 is the limiting reactant.
D)4A2 + 6B → 2A4B;B is the limiting reactant.

Unlock Deck
Unlock for access to all 159 flashcards in this deck.
Unlock Deck
k this deck
71
![<strong> Diethyl ether has the molecular formula C<sub>4</sub>H<sub>10</sub>O.Which ball and stick model shown above represents diethyl ether? [gray spheres = C,black spheres = O,unshaded spheres = H]</strong> A)model a) B)model b) C)model c) D)model d)](https://d2lvgg3v3hfg70.cloudfront.net/TB4940/11ea7e2d_d09e_aff7_a2f7_11edcd81f00c_TB4940_00_TB4940_00_TB4940_00_TB4940_00.jpg)
Diethyl ether has the molecular formula C4H10O.Which ball and stick model shown above represents diethyl ether? [gray spheres = C,black spheres = O,unshaded spheres = H]
A)model a)
B)model b)
C)model c)
D)model d)

Unlock Deck
Unlock for access to all 159 flashcards in this deck.
Unlock Deck
k this deck
72
What is the balanced chemical equation for the reaction of element A (unshaded spheres)with element B (shaded spheres)as represented below? 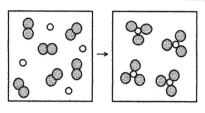
A)A + B → AB
B)2A + 3B → 2AB
C)A + B2 → AB3
D)2A + 3B2 → 2AB3

A)A + B → AB
B)2A + 3B → 2AB
C)A + B2 → AB3
D)2A + 3B2 → 2AB3

Unlock Deck
Unlock for access to all 159 flashcards in this deck.
Unlock Deck
k this deck
73
![<strong> Ethanol has the molecular formula C<sub>2</sub>H<sub>6</sub>O.Which ball and stick model shown above represents ethanol? [gray spheres = C,black spheres = O,unshaded spheres = H]</strong> A)model a) B)model b) C)model c) D)model d)](https://d2lvgg3v3hfg70.cloudfront.net/TB4940/11ea7e2d_d09e_aff7_a2f7_11edcd81f00c_TB4940_00_TB4940_00_TB4940_00_TB4940_00.jpg)
Ethanol has the molecular formula C2H6O.Which ball and stick model shown above represents ethanol? [gray spheres = C,black spheres = O,unshaded spheres = H]
A)model a)
B)model b)
C)model c)
D)model d)

Unlock Deck
Unlock for access to all 159 flashcards in this deck.
Unlock Deck
k this deck
74
The following diagram represents the reaction of A2 (unshaded spheres)with B (shaded spheres).How many moles of product can be produced from the reaction of 1.0 mol of A2 and 1.0 mol of B? 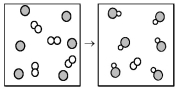
A)0.5 mol of product
B)1.0 mol of product
C)3.0 mol of product
D)6.0 mol of product

A)0.5 mol of product
B)1.0 mol of product
C)3.0 mol of product
D)6.0 mol of product

Unlock Deck
Unlock for access to all 159 flashcards in this deck.
Unlock Deck
k this deck
75
Aluminum metal reacts with aqueous copper(II)sulfate to form aqueous aluminum sulfate and copper metal.What is the stoichiometric coefficient for aluminum when the chemical equation is balanced using the lowest whole-number stoichiometric coefficients?
A)1
B)2
C)3
D)4
A)1
B)2
C)3
D)4

Unlock Deck
Unlock for access to all 159 flashcards in this deck.
Unlock Deck
k this deck
76
Reaction of A (unshaded spheres)with B2 (shaded spheres)is shown schematically in the following diagram.Which equation best describes the stoichiometry of the reaction? 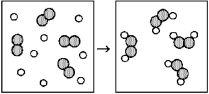
A)A2 + 2 B → A2B2
B)8 A + 4 B2 → 4 A2B2
C)2 A + B2 → A2B2
D)4 A + 4 B2 → 4 A2B2

A)A2 + 2 B → A2B2
B)8 A + 4 B2 → 4 A2B2
C)2 A + B2 → A2B2
D)4 A + 4 B2 → 4 A2B2

Unlock Deck
Unlock for access to all 159 flashcards in this deck.
Unlock Deck
k this deck
77
If unshaded spheres represent nitrogen atoms and shaded spheres represent oxygen atoms,which box represents reactants and which represents products for the reaction 2 NO2(g)→ 2 NO(g)+ O2(g)? 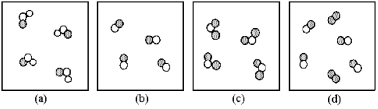
A)box (a)reactants and box (b)products
B)box (a)reactants and box (d)products
C)box (c)reactants and box (b)products
D)box (c)reactants and box (d)products

A)box (a)reactants and box (b)products
B)box (a)reactants and box (d)products
C)box (c)reactants and box (b)products
D)box (c)reactants and box (d)products

Unlock Deck
Unlock for access to all 159 flashcards in this deck.
Unlock Deck
k this deck
78
The following diagrams represent the reaction of A2 (shaded spheres)with B2 (unshaded spheres).Identify the limiting reactant and write a balanced equation for the reaction. 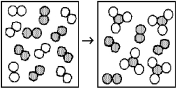
A)A2 is the limiting reactant;A + 3 B → AB3.
B)A2 is the limiting reactant;A2 + 3 B2 → 2 AB3.
C)B2 is the limiting reactant;A + 3 B → AB3.
D)B2 is the limiting reactant;A2 + 3 B2 → 2 AB3.

A)A2 is the limiting reactant;A + 3 B → AB3.
B)A2 is the limiting reactant;A2 + 3 B2 → 2 AB3.
C)B2 is the limiting reactant;A + 3 B → AB3.
D)B2 is the limiting reactant;A2 + 3 B2 → 2 AB3.

Unlock Deck
Unlock for access to all 159 flashcards in this deck.
Unlock Deck
k this deck
79
If unshaded spheres represent nitrogen atoms and shaded spheres represent oxygen atoms,which box represents reactants and which represents products for the reaction 2 N2O(g)→ 2 N2(g)+ O2(g)? 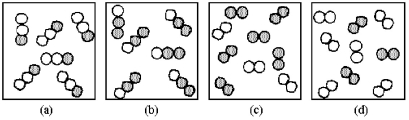
A)box (a)reactants and box (c)products
B)box (a)reactants and box (d)products
C)box (b)reactants and box (c)products
D)box (b)reactants and box (d)products

A)box (a)reactants and box (c)products
B)box (a)reactants and box (d)products
C)box (b)reactants and box (c)products
D)box (b)reactants and box (d)products

Unlock Deck
Unlock for access to all 159 flashcards in this deck.
Unlock Deck
k this deck
80
What is the balanced chemical equation for the reaction of element A (unshaded spheres)with element B (shaded spheres)as represented below? 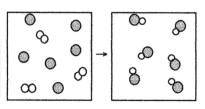
A)A + B → AB
B)A + 3B → 3AB
C)A2 + B → AB
D)A2 + 2B → 2AB

A)A + B → AB
B)A + 3B → 3AB
C)A2 + B → AB
D)A2 + 2B → 2AB

Unlock Deck
Unlock for access to all 159 flashcards in this deck.
Unlock Deck
k this deck



