Deck 23: Connections to Organic and Biological Chemistry
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/290
Play
Full screen (f)
Deck 23: Connections to Organic and Biological Chemistry
1
When alkanes react with chlorine in the presence of ultraviolet light,chlorine atoms substitute for one or more alkane hydrogen atoms.What is the number of different chloroalkane compounds that can be formed by the reaction of C2H6 with chlorine?
A)3
B)6
C)9
D)12
A)3
B)6
C)9
D)12
9
2
Compounds that have the same formula but different molecular structures are called
A)isoelectronic.
B)isomers.
C)isotones.
D)isotopes.
A)isoelectronic.
B)isomers.
C)isotones.
D)isotopes.
isomers.
3
The cycloalkane represented by a square is called ________ and has the molecular formula ________.
A)cyclopropane,C3H6
B)cyclopropane,C3H8
C)cyclobutane,C4H4
D)cyclobutane,C4H8
A)cyclopropane,C3H6
B)cyclopropane,C3H8
C)cyclobutane,C4H4
D)cyclobutane,C4H8
cyclobutane,C4H8
4
Which compound is not an isomer of the other three?
A)cyclohexane
B)1,2-dimethylcyclobutane
C)1,2-dimethylcyclopropane
D)methylcyclopentane
A)cyclohexane
B)1,2-dimethylcyclobutane
C)1,2-dimethylcyclopropane
D)methylcyclopentane

Unlock Deck
Unlock for access to all 290 flashcards in this deck.
Unlock Deck
k this deck
5
What are the C-C-C bond angles in cyclopropane and cyclohexane?
A)60° in both molecules
B)60° in cyclopropane and 109.5° in cyclohexane
C)60° in cyclopropane and 120° in cyclohexane
D)109.5° in both molecules
A)60° in both molecules
B)60° in cyclopropane and 109.5° in cyclohexane
C)60° in cyclopropane and 120° in cyclohexane
D)109.5° in both molecules

Unlock Deck
Unlock for access to all 290 flashcards in this deck.
Unlock Deck
k this deck
6
Which is the condensed structure of a straight-chain hydrocarbon? 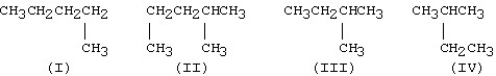
A)I
B)II
C)III
D)IV
A)I
B)II
C)III
D)IV

Unlock Deck
Unlock for access to all 290 flashcards in this deck.
Unlock Deck
k this deck
7
Which condensed structure below representing an alkane is not correct?
A)CH3CH2CH3
B)
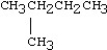
C)CH3CH2CH2CH3
D)
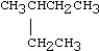
A)CH3CH2CH3
B)
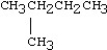
C)CH3CH2CH2CH3
D)
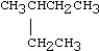

Unlock Deck
Unlock for access to all 290 flashcards in this deck.
Unlock Deck
k this deck
8
Another term for alkanes is
A)alkenes.
B)alkynes.
C)saturated hydrocarbons.
D)unsaturated hydrocarbons.
A)alkenes.
B)alkynes.
C)saturated hydrocarbons.
D)unsaturated hydrocarbons.

Unlock Deck
Unlock for access to all 290 flashcards in this deck.
Unlock Deck
k this deck
9
Which of the structures below are identical? 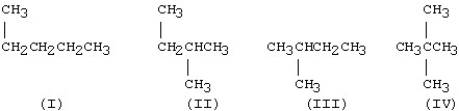
A)I and II
B)II and III
C)III and IV
D)I and IV

A)I and II
B)II and III
C)III and IV
D)I and IV

Unlock Deck
Unlock for access to all 290 flashcards in this deck.
Unlock Deck
k this deck
10
Which of the following statements about properties of organic compounds is not correct?
A)Most organic compounds are not soluble in water.
B)Most organic compounds do not conduct electricity.
C)Organic compounds have higher melting and boiling points than ionic compounds.
D)Organic compounds have weak intermolecular forces.
A)Most organic compounds are not soluble in water.
B)Most organic compounds do not conduct electricity.
C)Organic compounds have higher melting and boiling points than ionic compounds.
D)Organic compounds have weak intermolecular forces.

Unlock Deck
Unlock for access to all 290 flashcards in this deck.
Unlock Deck
k this deck
11
Which one of the structures below is different from the other three? 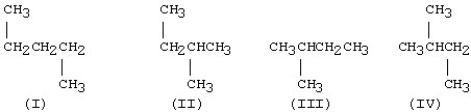
A)I
B)II
C)III
D)IV

A)I
B)II
C)III
D)IV

Unlock Deck
Unlock for access to all 290 flashcards in this deck.
Unlock Deck
k this deck
12
Which of the following statements is false regarding functional groups?
A)The chemical properties of the functional group dictate the chemistry of the larger molecule.
B)Each functional group has a characteristic chemical behavior.
C)A functional group consists of an atom or a group of atoms that is part of a larger molecule.
D)A functional group consists of only carbon and hydrogen atoms.
A)The chemical properties of the functional group dictate the chemistry of the larger molecule.
B)Each functional group has a characteristic chemical behavior.
C)A functional group consists of an atom or a group of atoms that is part of a larger molecule.
D)A functional group consists of only carbon and hydrogen atoms.

Unlock Deck
Unlock for access to all 290 flashcards in this deck.
Unlock Deck
k this deck
13
The most important characteristic of carbon atoms for forming organic molecules is
A)ability to bond together to form long chains.
B)ability to form multiple covalent bonds.
C)use of hybrid orbitals.
D)A and B
A)ability to bond together to form long chains.
B)ability to form multiple covalent bonds.
C)use of hybrid orbitals.
D)A and B

Unlock Deck
Unlock for access to all 290 flashcards in this deck.
Unlock Deck
k this deck
14
Some of the products from reaction of bromine and propane are shown below.(The hydrogen atoms are not shown in the structures. )How many are identical structures? 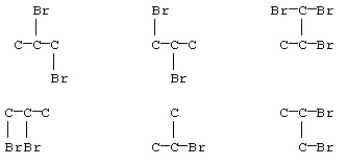
A)2
B)3
C)4
D)All are isomers of each other.

A)2
B)3
C)4
D)All are isomers of each other.

Unlock Deck
Unlock for access to all 290 flashcards in this deck.
Unlock Deck
k this deck
15
Which of the following statements about isomers is false?
A)All alkanes have branched chain isomers.
B)As the number of carbon atoms increase in a compound,so do the number of possible isomers.
C)Isomers have different physical properties.
D)Isomers have the same formula but different molecular structures.
A)All alkanes have branched chain isomers.
B)As the number of carbon atoms increase in a compound,so do the number of possible isomers.
C)Isomers have different physical properties.
D)Isomers have the same formula but different molecular structures.

Unlock Deck
Unlock for access to all 290 flashcards in this deck.
Unlock Deck
k this deck
16
How many isomers are there for C5H12?
A)3
B)4
C)5
D)6
A)3
B)4
C)5
D)6

Unlock Deck
Unlock for access to all 290 flashcards in this deck.
Unlock Deck
k this deck
17
Which of the following statements about cycloalkanes is true?
A)The bonds in cyclopropane and cyclobutane are weaker than other cycloalkanes.
B)Cycloalkanes are also called "acyclic" compounds.
C)Cyclopropane has 109° bond angles.
D)Most cycloalkanes are planar molecules.
A)The bonds in cyclopropane and cyclobutane are weaker than other cycloalkanes.
B)Cycloalkanes are also called "acyclic" compounds.
C)Cyclopropane has 109° bond angles.
D)Most cycloalkanes are planar molecules.

Unlock Deck
Unlock for access to all 290 flashcards in this deck.
Unlock Deck
k this deck
18
Identify the functional group: 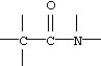
A)alcohol
B)amine
C)amide
D)ester

A)alcohol
B)amine
C)amide
D)ester

Unlock Deck
Unlock for access to all 290 flashcards in this deck.
Unlock Deck
k this deck
19
Which of the following condensed structures represent the same molecule? 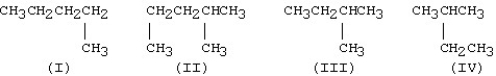
A)I and II
B)II and IV
C)III and IV
D)II and III
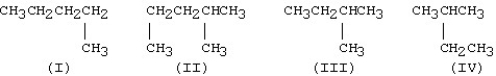
A)I and II
B)II and IV
C)III and IV
D)II and III

Unlock Deck
Unlock for access to all 290 flashcards in this deck.
Unlock Deck
k this deck
20
Identify the functional group: 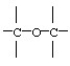
A)aldehyde
B)ketone
C)ester
D)ether

A)aldehyde
B)ketone
C)ester
D)ether

Unlock Deck
Unlock for access to all 290 flashcards in this deck.
Unlock Deck
k this deck
21
Which one of the following molecules is the most polar?
A)acetaldehyde
B)acetic acid
C)ethane
D)ethylene
A)acetaldehyde
B)acetic acid
C)ethane
D)ethylene

Unlock Deck
Unlock for access to all 290 flashcards in this deck.
Unlock Deck
k this deck
22
What is the name for this alkyl group? 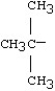
A)isobutyl
B)tert-butyl
C)tributyl
D)trimethyl
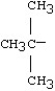
A)isobutyl
B)tert-butyl
C)tributyl
D)trimethyl

Unlock Deck
Unlock for access to all 290 flashcards in this deck.
Unlock Deck
k this deck
23
Which statement below is false concerning nomenclature rules for alkanes?
A)Carbon atoms are numbered starting with the atom farthest from the branching point.
B)Each branching group is assigned a number referring to its point of attachment in the parent chain.
C)The name of the alkane is written as a single word.
D)The parent name is taken from the longest continuous chain of carbon atoms.
A)Carbon atoms are numbered starting with the atom farthest from the branching point.
B)Each branching group is assigned a number referring to its point of attachment in the parent chain.
C)The name of the alkane is written as a single word.
D)The parent name is taken from the longest continuous chain of carbon atoms.

Unlock Deck
Unlock for access to all 290 flashcards in this deck.
Unlock Deck
k this deck
24
Which class of hydrocarbons has the general formula CnH2n+2?
A)alkanes
B)alkenes
C)alkynes
D)cylcloalkanes
A)alkanes
B)alkenes
C)alkynes
D)cylcloalkanes

Unlock Deck
Unlock for access to all 290 flashcards in this deck.
Unlock Deck
k this deck
25
An alkyl group is named for the part of the alkane group that remains after a ________ is removed.
A)C atom
B)CH3 group
C)CH2 group
D)H atom
A)C atom
B)CH3 group
C)CH2 group
D)H atom

Unlock Deck
Unlock for access to all 290 flashcards in this deck.
Unlock Deck
k this deck
26
How many grams of Ca(OH)2 are needed to neutralize 5.00 g of 3-methylbutanoic acid?
A)1.81 g
B)2.10 g
C)3.63 g
D)4.20 g
A)1.81 g
B)2.10 g
C)3.63 g
D)4.20 g

Unlock Deck
Unlock for access to all 290 flashcards in this deck.
Unlock Deck
k this deck
27
What is the common name for the simplest ketone,propanone?
A)acetal
B)acetone
C)carbanone
D)formalin
A)acetal
B)acetone
C)carbanone
D)formalin

Unlock Deck
Unlock for access to all 290 flashcards in this deck.
Unlock Deck
k this deck
28
The H-C-H bond angles in acetic acid,shown below,are closest to O  CH3C-OH
CH3C-OH
A)90°.
B)109.5°.
C)120°.
D)180°.
 CH3C-OH
CH3C-OHA)90°.
B)109.5°.
C)120°.
D)180°.

Unlock Deck
Unlock for access to all 290 flashcards in this deck.
Unlock Deck
k this deck
29
How many ether isomers have the formula C4H10O?
A)2
B)3
C)4
D)5
A)2
B)3
C)4
D)5

Unlock Deck
Unlock for access to all 290 flashcards in this deck.
Unlock Deck
k this deck
30
Which of the functional groups below are not characterized by π bonds?
A)alkenes
B)alkynes
C)aldehydes
D)alcohols
A)alkenes
B)alkynes
C)aldehydes
D)alcohols

Unlock Deck
Unlock for access to all 290 flashcards in this deck.
Unlock Deck
k this deck
31
If the point of attachment to an alkane is by the middle carbon in a propyl group,the group is called
A)isopropyl.
B)n-propyl.
C)sec-propyl.
D)tert-propyl.
A)isopropyl.
B)n-propyl.
C)sec-propyl.
D)tert-propyl.

Unlock Deck
Unlock for access to all 290 flashcards in this deck.
Unlock Deck
k this deck
32
Which one of the following behaves like an acid?
A)CH3COCH3
B)(CH3)2NH
C)C2H5OH
D)C2H5COOH
A)CH3COCH3
B)(CH3)2NH
C)C2H5OH
D)C2H5COOH

Unlock Deck
Unlock for access to all 290 flashcards in this deck.
Unlock Deck
k this deck
33
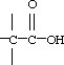
Which functional group below represents a carboxylic acid?
A)
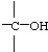
B)
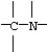
C)
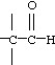
D)
A)
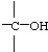
B)

C)
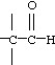
D)


Unlock Deck
Unlock for access to all 290 flashcards in this deck.
Unlock Deck
k this deck
34
Which functional group below represents an aldehyde?
A)
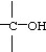
B)
C)
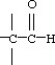
D)
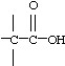
A)

B)

C)
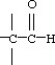
D)


Unlock Deck
Unlock for access to all 290 flashcards in this deck.
Unlock Deck
k this deck
35
The functional groups in the molecule below are H2NCH2CO2H
A)alkane,amide,and carboxylic acid.
B)alkane,amine,and carboxylic acid.
C)alkane,amide,ketone,and alcohol.
D)alkane,amine,ketone,and alcohol.
A)alkane,amide,and carboxylic acid.
B)alkane,amine,and carboxylic acid.
C)alkane,amide,ketone,and alcohol.
D)alkane,amine,ketone,and alcohol.

Unlock Deck
Unlock for access to all 290 flashcards in this deck.
Unlock Deck
k this deck
36
The C-C=O bond angle in acetic acid,shown below,is closest to O  CH3C-OH
CH3C-OH
A)90°.
B)109.5°.
C)120°.
D)180°.
 CH3C-OH
CH3C-OHA)90°.
B)109.5°.
C)120°.
D)180°.

Unlock Deck
Unlock for access to all 290 flashcards in this deck.
Unlock Deck
k this deck
37
Which of the following compounds is most soluble in water?
A)decanol
B)dimethyl ether
C)ethane
D)ethanol
A)decanol
B)dimethyl ether
C)ethane
D)ethanol

Unlock Deck
Unlock for access to all 290 flashcards in this deck.
Unlock Deck
k this deck
38
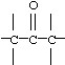
Which functional group below represents a ketone?
A)
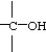
B)
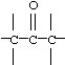
C)
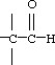
D)
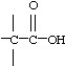
A)

B)

C)
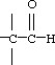
D)


Unlock Deck
Unlock for access to all 290 flashcards in this deck.
Unlock Deck
k this deck
39
What type of compound contains the -NH2 functional group?
A)alcohol
B)amine
C)carboxylic acid
D)ether
A)alcohol
B)amine
C)carboxylic acid
D)ether

Unlock Deck
Unlock for access to all 290 flashcards in this deck.
Unlock Deck
k this deck
40
The functional groups in the molecule below are O O 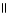
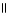 HCCH2COCH3
HCCH2COCH3
A)alkane,aldehyde,and ester.
B)alkane,aldehyde,ketone,and ether.
C)alkane,carboxylic acid,and ester.
D)alkane,ketone,and ester.
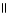
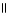 HCCH2COCH3
HCCH2COCH3A)alkane,aldehyde,and ester.
B)alkane,aldehyde,ketone,and ether.
C)alkane,carboxylic acid,and ester.
D)alkane,ketone,and ester.

Unlock Deck
Unlock for access to all 290 flashcards in this deck.
Unlock Deck
k this deck
41
What class of hydrocarbons has the general formula CnH2n?
A)alkanes
B)alkenes and cycloalkanes
C)alkynes
D)aromatics
A)alkanes
B)alkenes and cycloalkanes
C)alkynes
D)aromatics

Unlock Deck
Unlock for access to all 290 flashcards in this deck.
Unlock Deck
k this deck
42
The names of compounds with carbon-carbon double bonds contain the suffix
A)-ane.
B)-ene.
C)-yne.
D)-one.
A)-ane.
B)-ene.
C)-yne.
D)-one.

Unlock Deck
Unlock for access to all 290 flashcards in this deck.
Unlock Deck
k this deck
43
What is the hybridization of the carbon in (CH3)2C*=CHC  N indicated by the asterisk (*)?
N indicated by the asterisk (*)?
A)sp
B)sp2
C)sp3
D)dsp3
 N indicated by the asterisk (*)?
N indicated by the asterisk (*)?A)sp
B)sp2
C)sp3
D)dsp3

Unlock Deck
Unlock for access to all 290 flashcards in this deck.
Unlock Deck
k this deck
44
What is the IUPAC name for the following compound? 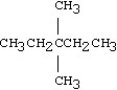
A)heptane
B)3,3-dimethylpentane
C)2,2-dimethyl-2-ethylpropane
D)2,2-diethylpropane

A)heptane
B)3,3-dimethylpentane
C)2,2-dimethyl-2-ethylpropane
D)2,2-diethylpropane

Unlock Deck
Unlock for access to all 290 flashcards in this deck.
Unlock Deck
k this deck
45
Name the product of hydrogenation of trans-2-pentene.
A)pentane
B)cis-2-pentene
C)trans-2-pentane
D)none of these
A)pentane
B)cis-2-pentene
C)trans-2-pentane
D)none of these

Unlock Deck
Unlock for access to all 290 flashcards in this deck.
Unlock Deck
k this deck
46
Which of the molecules shown below are unsaturated? (1)CH3CH2CH2CH(CH3)2
(2)CH3CH=CHCH(CH3)2
(3)HC CCH2CH(CH3)2
CCH2CH(CH3)2
A)All are unsaturated.
B)Only (2)and (3)are unsaturated.
C)Only (2)is unsaturated.
D)Only (3)is unsaturated.
(2)CH3CH=CHCH(CH3)2
(3)HC
 CCH2CH(CH3)2
CCH2CH(CH3)2A)All are unsaturated.
B)Only (2)and (3)are unsaturated.
C)Only (2)is unsaturated.
D)Only (3)is unsaturated.

Unlock Deck
Unlock for access to all 290 flashcards in this deck.
Unlock Deck
k this deck
47
The monosaccharide shown below is a(n) 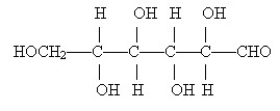
A)aldohexose.
B)aldopentose.
C)ketohexose.
D)ketopentose.

A)aldohexose.
B)aldopentose.
C)ketohexose.
D)ketopentose.

Unlock Deck
Unlock for access to all 290 flashcards in this deck.
Unlock Deck
k this deck
48
The term "carbohydrates" refers to a large class of polyhydroxylated
A)alcohols and carboxylic acids.
B)aldehydes and ketones.
C)amines and amides.
D)ethers and esters.
A)alcohols and carboxylic acids.
B)aldehydes and ketones.
C)amines and amides.
D)ethers and esters.

Unlock Deck
Unlock for access to all 290 flashcards in this deck.
Unlock Deck
k this deck
49
What is the IUPAC name for the following compound? 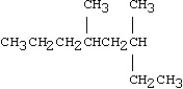
A)1,3-dimethyl-1-ethylhexane
B)4,6-dimethyl-6-ethylhexane
C)3,5-dimethyloctane
D)sec-hexylbutane

A)1,3-dimethyl-1-ethylhexane
B)4,6-dimethyl-6-ethylhexane
C)3,5-dimethyloctane
D)sec-hexylbutane

Unlock Deck
Unlock for access to all 290 flashcards in this deck.
Unlock Deck
k this deck
50
Which of the following compounds is 5-ethyl-4-isopropyl-2-methylnonane?
A)
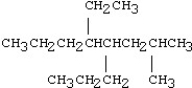
B)
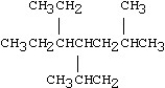
C)
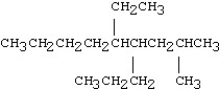
D)
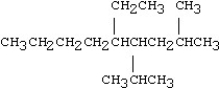
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 290 flashcards in this deck.
Unlock Deck
k this deck
51
The following structure represents a butyl group. 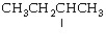
A)normal
B)secondary
C)tertiary
D)quaternary
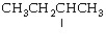
A)normal
B)secondary
C)tertiary
D)quaternary

Unlock Deck
Unlock for access to all 290 flashcards in this deck.
Unlock Deck
k this deck
52
Which name is not correct?
A)1,2-dichloropentane
B)2,3-dimethylbutane
C)2-ethylbutane
D)4-propylheptane
A)1,2-dichloropentane
B)2,3-dimethylbutane
C)2-ethylbutane
D)4-propylheptane

Unlock Deck
Unlock for access to all 290 flashcards in this deck.
Unlock Deck
k this deck
53
How many carbons are found in a molecule that contains the prefix but-?
A)2
B)4
C)3
D)7
A)2
B)4
C)3
D)7

Unlock Deck
Unlock for access to all 290 flashcards in this deck.
Unlock Deck
k this deck
54
What is the molecular geometry around the carbon in (CH3)2C=CHC*  N indicated by the asterisk (*)?
N indicated by the asterisk (*)?
A)linear
B)trigonal planar
C)trigonal pyramidal
D)tetrahedral
 N indicated by the asterisk (*)?
N indicated by the asterisk (*)?A)linear
B)trigonal planar
C)trigonal pyramidal
D)tetrahedral

Unlock Deck
Unlock for access to all 290 flashcards in this deck.
Unlock Deck
k this deck
55
The most important synthetic chemical reactions that alkenes and alkynes undergo are called
A)addition reactions.
B)combustion reactions.
C)photochemical halogenation reactions.
D)substitution reactions.
A)addition reactions.
B)combustion reactions.
C)photochemical halogenation reactions.
D)substitution reactions.

Unlock Deck
Unlock for access to all 290 flashcards in this deck.
Unlock Deck
k this deck
56
How many geometrical and structural isomers are there of butene?
A)3
B)4
C)5
D)6
A)3
B)4
C)5
D)6

Unlock Deck
Unlock for access to all 290 flashcards in this deck.
Unlock Deck
k this deck
57
What is the name of the following structure? 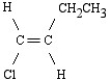
A)cis-1-ethyl-2-chloroethane
B)cis-1-chloro-1-butene
C)trans-1-chloro-1-butene
D)trans-1-chloro-2-ethylethane

A)cis-1-ethyl-2-chloroethane
B)cis-1-chloro-1-butene
C)trans-1-chloro-1-butene
D)trans-1-chloro-2-ethylethane

Unlock Deck
Unlock for access to all 290 flashcards in this deck.
Unlock Deck
k this deck
58
Which of the following is trans-2-butene? (Only bonds are shown in the skeletal structures below;the carbon and hydrogen atoms are deleted. ) 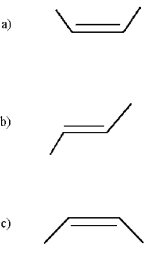
A)structure a
B)structure b
C)structure c
D)none of these
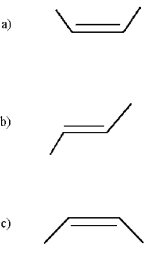
A)structure a
B)structure b
C)structure c
D)none of these

Unlock Deck
Unlock for access to all 290 flashcards in this deck.
Unlock Deck
k this deck
59
"Wood alcohol" is the common name for
A)methanol.
B)ethanol.
C)2-propanol.
D)1,2-ethanediol.
A)methanol.
B)ethanol.
C)2-propanol.
D)1,2-ethanediol.

Unlock Deck
Unlock for access to all 290 flashcards in this deck.
Unlock Deck
k this deck
60
What is the name of the following structure? 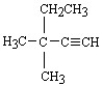
A)tert-butylethyne
B)3,3-dimethyl-1-pentyne
C)3-ethyl-3-methyl-1-butyne
D)trans-ethylmethylbutyne

A)tert-butylethyne
B)3,3-dimethyl-1-pentyne
C)3-ethyl-3-methyl-1-butyne
D)trans-ethylmethylbutyne

Unlock Deck
Unlock for access to all 290 flashcards in this deck.
Unlock Deck
k this deck
61
In addition to an amine,which functional group do all amino acids contain?
A)an alcohol
B)an amide
C)a carboxylic acid
D)an oxyacid
A)an alcohol
B)an amide
C)a carboxylic acid
D)an oxyacid

Unlock Deck
Unlock for access to all 290 flashcards in this deck.
Unlock Deck
k this deck
62
In water,which form of glucose predominates?
A)cyclic α
B)cyclic β
C)open chain
D)All three are equal in solution.
A)cyclic α
B)cyclic β
C)open chain
D)All three are equal in solution.

Unlock Deck
Unlock for access to all 290 flashcards in this deck.
Unlock Deck
k this deck
63
Which compound will have the largest dipole moment?
A)cis-1,2-dichloroethylene
B)trans-1,2-dichloroethylene
C)tetrabromoethylene
D)tetrachloroethylene
A)cis-1,2-dichloroethylene
B)trans-1,2-dichloroethylene
C)tetrabromoethylene
D)tetrachloroethylene

Unlock Deck
Unlock for access to all 290 flashcards in this deck.
Unlock Deck
k this deck
64
How many distinct isomers can be drawn for a molecule of C4H8?
A)1
B)2
C)3
D)4
A)1
B)2
C)3
D)4

Unlock Deck
Unlock for access to all 290 flashcards in this deck.
Unlock Deck
k this deck
65
Which of the following statements best describes the difference between vegetable oils and animal fats?
A)Animals fats have more carboxylic acid groups.
B)Vegetable oils have carbon chains with more unsaturated bonds.
C)Vegetable oils have more alcohol groups.
D)Animal fats have more unsaturated bonds.
A)Animals fats have more carboxylic acid groups.
B)Vegetable oils have carbon chains with more unsaturated bonds.
C)Vegetable oils have more alcohol groups.
D)Animal fats have more unsaturated bonds.

Unlock Deck
Unlock for access to all 290 flashcards in this deck.
Unlock Deck
k this deck
66
Which of the following statements about cis-trans isomers is not correct?
A)Conversion between cis- and trans-isomers occurs easily by rotation around the double bond.
B)In the trans- isomer,the groups of interest are on opposite sides across the double bond.
C)In the cis- isomer,the groups of interest are on the same side of the double bond.
D)There are no cis-trans isomers in alkynes.
A)Conversion between cis- and trans-isomers occurs easily by rotation around the double bond.
B)In the trans- isomer,the groups of interest are on opposite sides across the double bond.
C)In the cis- isomer,the groups of interest are on the same side of the double bond.
D)There are no cis-trans isomers in alkynes.

Unlock Deck
Unlock for access to all 290 flashcards in this deck.
Unlock Deck
k this deck
67
All of the fatty acids below contain eighteen carbons and range from saturated to three double bonds.Which has the highest melting point?
A)stearic acid (saturated)
B)oleic acid (one double bond)
C)linoleic acid (two double bonds)
D)linolenic acid (three double bonds)
A)stearic acid (saturated)
B)oleic acid (one double bond)
C)linoleic acid (two double bonds)
D)linolenic acid (three double bonds)

Unlock Deck
Unlock for access to all 290 flashcards in this deck.
Unlock Deck
k this deck
68
Which substance is a monosaccharide?
A)cellulose
B)glucose
C)glycogen
D)starch
A)cellulose
B)glucose
C)glycogen
D)starch

Unlock Deck
Unlock for access to all 290 flashcards in this deck.
Unlock Deck
k this deck
69
The monosaccharide shown below is a(n) 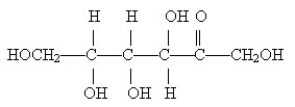
A)aldohexose.
B)aldopentose.
C)ketohexose.
D)ketopentose.

A)aldohexose.
B)aldopentose.
C)ketohexose.
D)ketopentose.

Unlock Deck
Unlock for access to all 290 flashcards in this deck.
Unlock Deck
k this deck
70
What functional group is commonly found in fats?
A)-NH2
B)-OH
C)-C-O-C
D)O
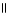
-O-C-C-
A)-NH2
B)-OH
C)-C-O-C
D)O
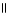
-O-C-C-

Unlock Deck
Unlock for access to all 290 flashcards in this deck.
Unlock Deck
k this deck
71
What class of compounds are cyclic?
A)amino acids
B)lipids
C)monosaccharides
D)proteins
A)amino acids
B)lipids
C)monosaccharides
D)proteins

Unlock Deck
Unlock for access to all 290 flashcards in this deck.
Unlock Deck
k this deck
72
Which statement below regarding fatty acids is not correct? Fatty acids
A)are always liquids.
B)are long chain carboxylic acids.
C)are usually unbranched chains.
D)usually have an even number of carbon atoms.
A)are always liquids.
B)are long chain carboxylic acids.
C)are usually unbranched chains.
D)usually have an even number of carbon atoms.

Unlock Deck
Unlock for access to all 290 flashcards in this deck.
Unlock Deck
k this deck
73
Starch is a polymer consisting of thousands of
A)α-glucose molecules.
B)β-glucose molecules.
C)long chain fatty acids.
D)amino acids.
A)α-glucose molecules.
B)β-glucose molecules.
C)long chain fatty acids.
D)amino acids.

Unlock Deck
Unlock for access to all 290 flashcards in this deck.
Unlock Deck
k this deck
74
Cellulose is a polymer consisting of thousands of
A)α-glucose molecules.
B)β-glucose molecules.
C)long chain fatty acids.
D)amino acids.
A)α-glucose molecules.
B)β-glucose molecules.
C)long chain fatty acids.
D)amino acids.

Unlock Deck
Unlock for access to all 290 flashcards in this deck.
Unlock Deck
k this deck
75
"Glycerol" is the common name for
A)ethanol.
B)2-propanol.
C)1,2-ethanediol.
D)1,2,3-propanetriol.
A)ethanol.
B)2-propanol.
C)1,2-ethanediol.
D)1,2,3-propanetriol.

Unlock Deck
Unlock for access to all 290 flashcards in this deck.
Unlock Deck
k this deck
76
When crystallized,which form of glucose predominates?
A)cyclic α
B)cyclic β
C)open chain
D)All three are equal in crystals.
A)cyclic α
B)cyclic β
C)open chain
D)All three are equal in crystals.

Unlock Deck
Unlock for access to all 290 flashcards in this deck.
Unlock Deck
k this deck
77
Which compound will exhibit cis-trans isomerism?
A)1,2-dichloroethane
B)1,2-dichloroethene
C)dichloroethyne
D)ethylene
A)1,2-dichloroethane
B)1,2-dichloroethene
C)dichloroethyne
D)ethylene

Unlock Deck
Unlock for access to all 290 flashcards in this deck.
Unlock Deck
k this deck
78
Hydrogenation of vegetable oils converts them into what type of molecule?
A)esters
B)ethers
C)polymers
D)saturated fats
A)esters
B)ethers
C)polymers
D)saturated fats

Unlock Deck
Unlock for access to all 290 flashcards in this deck.
Unlock Deck
k this deck
79
Sucrose,common table sugar,when hydrolyzed will form
A)amylose and glycogen.
B)cellulose and starch.
C)glucose and fructose.
D)lactose and maltose.
A)amylose and glycogen.
B)cellulose and starch.
C)glucose and fructose.
D)lactose and maltose.

Unlock Deck
Unlock for access to all 290 flashcards in this deck.
Unlock Deck
k this deck
80
Lipids are characterized by what property?
A)chemical reactivity
B)chemical structure
C)refractive index
D)solubility
A)chemical reactivity
B)chemical structure
C)refractive index
D)solubility

Unlock Deck
Unlock for access to all 290 flashcards in this deck.
Unlock Deck
k this deck



