Deck 6: Chemical Reactions: Mole and Mass Relationships
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/57
Play
Full screen (f)
Deck 6: Chemical Reactions: Mole and Mass Relationships
1
How many molecules are present in 3.25 mol of C2H6O?
A)3.25 molecules
B)46.0 molecules
C)1.85 × 1023 molecules
D)6.02 × 1023 molecules
E)1.96 × 1024 molecules
A)3.25 molecules
B)46.0 molecules
C)1.85 × 1023 molecules
D)6.02 × 1023 molecules
E)1.96 × 1024 molecules
1.96 × 1024 molecules
2
How many molecules are present in 4.25 mol of CCl4?
A)9.26 × 1024 molecules
B)2.56 × 1024 molecules
C)653.69 molecules
D)153.81 molecules
E)3.69 × 10-23 molecules
A)9.26 × 1024 molecules
B)2.56 × 1024 molecules
C)653.69 molecules
D)153.81 molecules
E)3.69 × 10-23 molecules
2.56 × 1024 molecules
3
42.0 g Cl2 contains ________ molecules Cl2.
A)1.18
B)3.57 × 1023
C)6.75 × 1023
D)1.78 × 1023
E)0.592
A)1.18
B)3.57 × 1023
C)6.75 × 1023
D)1.78 × 1023
E)0.592
3.57 × 1023
4
How many molecules are there in 3.00 moles of NH3?
A)1.00 molecules
B)3.00 molecules
C)17.00 molecules
D)1.20 × 1024 molecules
E)1.81 × 1024 molecules
A)1.00 molecules
B)3.00 molecules
C)17.00 molecules
D)1.20 × 1024 molecules
E)1.81 × 1024 molecules

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
5
The molecular weight of PCl3 is ________ amu.
A)66.42
B)136.00
C)137.33
D)139.00
E)199.26
A)66.42
B)136.00
C)137.33
D)139.00
E)199.26

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
6
The molar mass of Fe(OH)3 is ________ g.
A)72.86
B)74.88
C)89.87
D)104.86
E)106.87
A)72.86
B)74.88
C)89.87
D)104.86
E)106.87

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
7
The formula weight of Al2(SO4)3 is ________ grams.
A)214.14
B)278.02
C)315.14
D)342.14
E)450.14
A)214.14
B)278.02
C)315.14
D)342.14
E)450.14

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
8
A thimble of water contains 4.0 × 1021molecules.The number of moles of H2O is
A)2.4 × 1045.
B)6.6 × 10-3.
C)6.6 × 10-23.
D)2.4 × 1023.
E)2.4 × 10-23.
A)2.4 × 1045.
B)6.6 × 10-3.
C)6.6 × 10-23.
D)2.4 × 1023.
E)2.4 × 10-23.

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
9
The number of grams in 0.350 mol of Na is
A)0.350.
B)8.05.
C)11.0.
D)23.0.
E)65.7.
A)0.350.
B)8.05.
C)11.0.
D)23.0.
E)65.7.

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
10
Acid rain forms in the upper atmosphere by the reaction of sulfur trioxide with water forming sulfuric acid.Calculate the mass in grams of 5.65 × 1021 molecules of SO3.
A)2.72 × 1047 g
B)1.17 × 10-4 g
C)0.751 g
D)0.0751 g
E)1.17 g
A)2.72 × 1047 g
B)1.17 × 10-4 g
C)0.751 g
D)0.0751 g
E)1.17 g

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
11
The formula weight of ammonium carbonate, (NH4)2CO3 is ________ amu.
A)64.11
B)82.10
C)96.09
D)120.13
E)180.17
A)64.11
B)82.10
C)96.09
D)120.13
E)180.17

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
12
The molar mass of butane,C4H10,is ________ grams.
A)22.01
B)49.05
C)52.08
D)58.12
E)68.24
A)22.01
B)49.05
C)52.08
D)58.12
E)68.24

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
13
Which quantity contains Avogadro's number of molecules?
A)8.8 g CO2
B)34 g of NH3
C)9 g of H2O
D)98 g of H2SO4
E)73 g of HCl
A)8.8 g CO2
B)34 g of NH3
C)9 g of H2O
D)98 g of H2SO4
E)73 g of HCl

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
14
Which statement based on the mole concept is not correct?
A)The molar mass of a diatomic element is its atomic weight in grams times two.
B)The number of atoms in one mole of platinum is the same as the number of atoms in one mole of uranium.
C)One mole of sodium chloride,NaCl,contains the same number of ions as one mole of calcium sulfate,CaSO4.
D)One mole of methane,CH4,contains the same number of atoms as one mole of carbon dioxide,CO2.
E)One mole of bromine,Br2,contains the same number of molecules as one mole of propane,C3H8.
A)The molar mass of a diatomic element is its atomic weight in grams times two.
B)The number of atoms in one mole of platinum is the same as the number of atoms in one mole of uranium.
C)One mole of sodium chloride,NaCl,contains the same number of ions as one mole of calcium sulfate,CaSO4.
D)One mole of methane,CH4,contains the same number of atoms as one mole of carbon dioxide,CO2.
E)One mole of bromine,Br2,contains the same number of molecules as one mole of propane,C3H8.

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
15
Which statement concerning the mole is not correct?
A)The molar mass of any compound is equal to its molecular weight in grams.
B)A mole of metal contains NA atoms of that metal.
C)A mole of any compound contains one mole of each kind of atom found in that compound.
D)A mole of a diatomic element contains 2 × NA moles of atoms of the element.
E)All of these statements are correct.
A)The molar mass of any compound is equal to its molecular weight in grams.
B)A mole of metal contains NA atoms of that metal.
C)A mole of any compound contains one mole of each kind of atom found in that compound.
D)A mole of a diatomic element contains 2 × NA moles of atoms of the element.
E)All of these statements are correct.

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
16
If a tablet of acetaminophen,C8H9NO2,weighs 324 mg,how many moles are there in the tablet?
A)2.15 × 10-3
B)48.9
C)4.89 × 104
D) 2.15
E)466
A)2.15 × 10-3
B)48.9
C)4.89 × 104
D) 2.15
E)466

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
17
Calculate the number of moles of aspirin,C9H8O4,in 4.0 gram sample.
A)2.2 × 10-4 moles
B)2.2 moles
C)4.6 × 10-3 moles
D)0.022 moles
E)180 moles
A)2.2 × 10-4 moles
B)2.2 moles
C)4.6 × 10-3 moles
D)0.022 moles
E)180 moles

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
18
Ammonium sulfate is a common component of fertilizer.Calculate it's formula weight.
A)63.07 amu
B)118.13 amu
C)132.13 amu
D)128.11 amu
E)114.10 amu
A)63.07 amu
B)118.13 amu
C)132.13 amu
D)128.11 amu
E)114.10 amu

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
19
The formula mass of copper(II)chloride is ________ g.
A)98.90
B)133.00
C)134.45
D)162.53
E)197.80
A)98.90
B)133.00
C)134.45
D)162.53
E)197.80

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
20
Which statement concerning the mole concept is not true?
A)The molar mass of a metal is its atomic weight expressed in grams.
B)The mole concept makes a connection between the mass of a substance and the number of particles or units of that substance.
C)One mole of any compound contains one mole of atoms.
D)One mole of sodium contains the same number of atoms as one mole of carbon.
E)One mole of water contains the same number of molecules as one mole of ammonia.
A)The molar mass of a metal is its atomic weight expressed in grams.
B)The mole concept makes a connection between the mass of a substance and the number of particles or units of that substance.
C)One mole of any compound contains one mole of atoms.
D)One mole of sodium contains the same number of atoms as one mole of carbon.
E)One mole of water contains the same number of molecules as one mole of ammonia.

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
21
The number of grams in 2.65 mol of SO2 is
A)2.65.
B)64.1.
C)24.2.
D)170.
E)1.60 × 1024.
A)2.65.
B)64.1.
C)24.2.
D)170.
E)1.60 × 1024.

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
22
In the balanced reaction shown,the mole ratio of Fe2S3 to O2 is
Fe2S3 + 4 O2 → 2 FeO + 3 SO2
A)5 to 5.
B)1 to 4.
C)4 to 1.
D)1 to 2.
E)4 to 3.
Fe2S3 + 4 O2 → 2 FeO + 3 SO2
A)5 to 5.
B)1 to 4.
C)4 to 1.
D)1 to 2.
E)4 to 3.

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
23
How many moles of CO2 are produced when 2.5 moles of O2 react according to the following equation?
C3H5 + 5 O2 → 3 CO2 + 4 H2O
A)1.5
B)3.0
C)5.0
D)6.0
E)18
C3H5 + 5 O2 → 3 CO2 + 4 H2O
A)1.5
B)3.0
C)5.0
D)6.0
E)18

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
24
50.0 g of Cl2 contains ________ mol Cl2.
A)50.0
B)1775
C)0.705
D)1.41
E)4.24 × 1023
A)50.0
B)1775
C)0.705
D)1.41
E)4.24 × 1023

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
25
105 g of MgCl2 contains ________ mol MgCl2.
A)105
B)6.62 × 1023
C)1.10
D)1.76
E)1.06 × 1024
A)105
B)6.62 × 1023
C)1.10
D)1.76
E)1.06 × 1024

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
26
Determine the number of moles of water produced when one mole of NH3 reacts according to the balanced reaction shown.
4 NH3 + 5 O2 → 4 NO + 6 H2O
A)1.00
B)1.25
C)1.50
D)0.67
E)1.33
4 NH3 + 5 O2 → 4 NO + 6 H2O
A)1.00
B)1.25
C)1.50
D)0.67
E)1.33

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
27
How many moles of NaHCO3 are present in a 2.00 g sample?
A)2.38 × 10-2 mol
B)1.27 × 10-2 mol
C)2.00 mol
D)85.0 mol
E)168 mol
A)2.38 × 10-2 mol
B)1.27 × 10-2 mol
C)2.00 mol
D)85.0 mol
E)168 mol

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
28
Interpret in words the equation shown.
P4O10(s)+ 6 H2O(l)→ 4 H3PO4(aq)
A)One mole of solid tetraphosphorus decaoxide reacts with six moles of liquid water to produce four moles of phosphoric acid solution.
B)Zero moles of solid phosphoric oxide dissolve in six moles of water to produce four moles of phosphorous acid.
C)One mole of phosphorus(X)oxide combines with six moles of water to produce four moles of solution of hydrogen phosphate.
D)Four moles of solid phosphorus,five moles of diatomic oxygen gas,and six moles of liquid water react together to produce four moles of phosphoric acid in solution.
E)Four atoms of phosphorus,16 atoms of oxygen,and 12 atoms of hydrogen rearrange to produce four molecules of phosphoric acid.
P4O10(s)+ 6 H2O(l)→ 4 H3PO4(aq)
A)One mole of solid tetraphosphorus decaoxide reacts with six moles of liquid water to produce four moles of phosphoric acid solution.
B)Zero moles of solid phosphoric oxide dissolve in six moles of water to produce four moles of phosphorous acid.
C)One mole of phosphorus(X)oxide combines with six moles of water to produce four moles of solution of hydrogen phosphate.
D)Four moles of solid phosphorus,five moles of diatomic oxygen gas,and six moles of liquid water react together to produce four moles of phosphoric acid in solution.
E)Four atoms of phosphorus,16 atoms of oxygen,and 12 atoms of hydrogen rearrange to produce four molecules of phosphoric acid.

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
29
In the reaction shown,what is the mole ratio that would be used to determine the number of moles of oxygen needed to react with 3.2 moles of C4H10?
2 C4H10+ 13O2→ 8 CO2+ 10 H2O
A)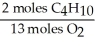
B)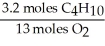
C)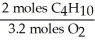
D)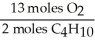
E)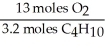
2 C4H10+ 13O2→ 8 CO2+ 10 H2O
A)
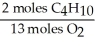
B)
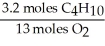
C)
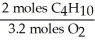
D)
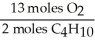
E)
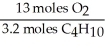

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
30
The combustion of propane gas (C3H8)is used to fuel barbeque grills.In order to produce 5.65 moles of water how many moles of oxygen gas are needed?
C3H8(g)+ 5 O2(g)→ 3 CO2(g)+ 4 H2O(l)
A)3.39 mol
B)4.52 mol
C)7.06 mol
D)1.13 mol
E)1.41 mol
C3H8(g)+ 5 O2(g)→ 3 CO2(g)+ 4 H2O(l)
A)3.39 mol
B)4.52 mol
C)7.06 mol
D)1.13 mol
E)1.41 mol

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
31
How much Ca(NO3)2 should be weighed out to have 0.650 mol?
A)66.4 g
B)97.6 g
C)107 g
D)133 g
E)165 g
A)66.4 g
B)97.6 g
C)107 g
D)133 g
E)165 g

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
32
The combustion of propane gas is used to fuel barbeque grills.If 4.65 moles of propane,C3H8,are burned in a grilling session,how many moles of carbon dioxide gas are formed?
C3H8(g)+ 5 O2(g)→ 3 CO2(g)+ 4 H2O(l)
A)1.55 mol
B)14.0 mol
C)18.6 mol
D)1.16 mol
E)3.49 mol
C3H8(g)+ 5 O2(g)→ 3 CO2(g)+ 4 H2O(l)
A)1.55 mol
B)14.0 mol
C)18.6 mol
D)1.16 mol
E)3.49 mol

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
33
The Haber process is used to make ammonia,which is an important source of nitrogen that can be metabolized by plants.Using the equation below,determine how many moles of ammonia will be formed from 8.55 mole of nitrogen.
N2(g)+ 3 H2(g)→ 2 NH3(g)
A)4.28 mol
B)0.240 mol
C)5.7 mol
D)17.1 mol
E)12.8 mol
N2(g)+ 3 H2(g)→ 2 NH3(g)
A)4.28 mol
B)0.240 mol
C)5.7 mol
D)17.1 mol
E)12.8 mol

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
34
Consider the reaction N2(g)+ O2(g)→ 2 NO(g).
A)How many g NO can be produced when 25.0 g of nitrogen reacts?
B)How many g NO can be produced when 25.0 g of oxygen reacts?
C)Based on your answers in a and b,predict the amount of NO that can be produced when 25.0 g nitrogen is reacted with 25.0 g of oxygen.
D)Explain the reasoning used in part c.
A)How many g NO can be produced when 25.0 g of nitrogen reacts?
B)How many g NO can be produced when 25.0 g of oxygen reacts?
C)Based on your answers in a and b,predict the amount of NO that can be produced when 25.0 g nitrogen is reacted with 25.0 g of oxygen.
D)Explain the reasoning used in part c.

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
35
In the reaction shown,how many moles of HCl are needed to react with 2.4 moles of Al?
2 Al + 6 HCl → 2 AlCl3 + 3 H2
A)0.8
B)1.3
C)4.8
D)6.4
E)7.2
2 Al + 6 HCl → 2 AlCl3 + 3 H2
A)0.8
B)1.3
C)4.8
D)6.4
E)7.2

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
36
In the reaction shown,what is the mole ratio that would be used to determine the number of moles of 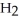 That would be produced when 3.5 moles of AlC
That would be produced when 3.5 moles of AlC  Are produced?
Are produced?
2 Al + 6 HCl → 2 AlC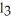 + 3
+ 3 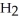
A)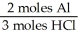
B)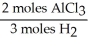
C)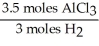
D)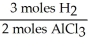
E)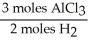
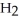 That would be produced when 3.5 moles of AlC
That would be produced when 3.5 moles of AlC 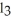 Are produced?
Are produced?2 Al + 6 HCl → 2 AlC
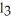 + 3
+ 3 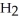
A)
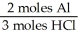
B)
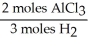
C)
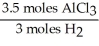
D)
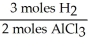
E)
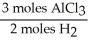

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
37
A student weighed 0.550 g of lithium chloride,LiCl,to use in a reaction.How many moles is this?
A)77.08
B)42.39
C)23.31
D)5.11
E)0.0130
A)77.08
B)42.39
C)23.31
D)5.11
E)0.0130

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
38
What does the balanced equation given below mean?
CH4 + 2 O2 → CO2 + 2H2O
A)One mole of methane reacts with two moles of oxygen to produce one mole of carbon dioxide and two moles of water.
B)One gram of methane reacts with two grams of oxygen to produce one gram of carbon dioxide and two grams of water.
C)One molecule of methane reacts with two molecules of oxygen to produce one molecule of carbon dioxide and two molecules of water.
D)A,B,and C are correct.
E)A and C are correct.
CH4 + 2 O2 → CO2 + 2H2O
A)One mole of methane reacts with two moles of oxygen to produce one mole of carbon dioxide and two moles of water.
B)One gram of methane reacts with two grams of oxygen to produce one gram of carbon dioxide and two grams of water.
C)One molecule of methane reacts with two molecules of oxygen to produce one molecule of carbon dioxide and two molecules of water.
D)A,B,and C are correct.
E)A and C are correct.

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
39
The active ingredient in many antacid tablets is calcium carbonate,CaCO3.How many moles of calcium carbonate are contained in a tablet weighing 550.mg?
A)5.51
B)55.1
C)5.51 × 10-3
D)5.51 × 10-4
E)5.51 × 104
A)5.51
B)55.1
C)5.51 × 10-3
D)5.51 × 10-4
E)5.51 × 104

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
40
The number of grams in 7.00 moles of N2 is
A)7 × (6.02 × 1023).
B)14.0.
C)28.0.
D)98.0.
E)196.
A)7 × (6.02 × 1023).
B)14.0.
C)28.0.
D)98.0.
E)196.

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
41
Which is the limiting reactant when 3.00 g of calcium are reacted with 8.00 g of water to produce hydrogen gas in the following equation?
Ca(s)+ 2 H2O(l)→ Ca(OH)2(aq)+ H2(g)
A)Ca
B)H2O
C)Ca(OH)2
D)H2
E)not enough information is given
Ca(s)+ 2 H2O(l)→ Ca(OH)2(aq)+ H2(g)
A)Ca
B)H2O
C)Ca(OH)2
D)H2
E)not enough information is given

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
42
Match the following.
The molar mass of

A)212.4 g
B)219 g
C)225 g
D)208.2 g
The molar mass of


A)212.4 g
B)219 g
C)225 g
D)208.2 g

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
43
How many grams of C will be consumed when 5.00 grams of Na2SO4 react according to the balanced reaction shown?
Na2SO4 + 2 C → Na2S + 2 CO2
A)0.038 g
B)0.211 g
C)0.844 g
D)1.69 g
E)17.1 g
Na2SO4 + 2 C → Na2S + 2 CO2
A)0.038 g
B)0.211 g
C)0.844 g
D)1.69 g
E)17.1 g

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
44
The fuel for your car is a mixture of octane and several other hydrocarbons.What mass of CO2 will be produced when 125 g of C8H18 react completely in the following equation?
2 C8H18 + 25 O2 → 16 CO2 + 18 H2O
A)6.03 g
B)386 g
C)259 g
D)40.5 g
E)0.199 g
2 C8H18 + 25 O2 → 16 CO2 + 18 H2O
A)6.03 g
B)386 g
C)259 g
D)40.5 g
E)0.199 g

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
45
Match the following.
The molar mass of BaC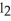
A)212.4 g
B)219 g
C)225 g
D)208.2 g
The molar mass of BaC
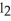
A)212.4 g
B)219 g
C)225 g
D)208.2 g

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
46
Match the following.
The mass of 1.22 moles of iron(II)nitrate
A)212.4 g
B)219 g
C)225 g
D)208.2 g
The mass of 1.22 moles of iron(II)nitrate
A)212.4 g
B)219 g
C)225 g
D)208.2 g

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
47
The percentage yield of a reaction can best be described as the ________ times 100%.
A)amount of product you could make if the reaction went to completion
B)total amount of materials involved in a reaction
C)amount of desired product divided by the amount of unwanted product
D)amount of product obtained divided by the maximum possible amount of product
E)amount of reactants left over divided by the original amounts
A)amount of product you could make if the reaction went to completion
B)total amount of materials involved in a reaction
C)amount of desired product divided by the amount of unwanted product
D)amount of product obtained divided by the maximum possible amount of product
E)amount of reactants left over divided by the original amounts

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
48
The reaction N2 + 3 H2 → 2 NH3 is used to produce ammonia.When 450.g of hydrogen was reacted with nitrogen,1575 g of ammonia were produced.What is the percent yield of this reaction?
A)62.1%
B)41.5%
C)30.8%
D)20.7%
E)More information is needed to solve this problem.
A)62.1%
B)41.5%
C)30.8%
D)20.7%
E)More information is needed to solve this problem.

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
49
How many grams of fluorine are required to produce 20.0 grams of FeF3 from the reaction shown?
2 Fe + 3F2 → 2 FeF3
A)4.49 g
B)5.05 g
C)6.74 g
D)10.1 g
E)20.2 g
2 Fe + 3F2 → 2 FeF3
A)4.49 g
B)5.05 g
C)6.74 g
D)10.1 g
E)20.2 g

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
50
The Haber process is used to make ammonia,which is an important source of nitrogen that can be metabolized by plants.Using the equation below,determine how many kilograms of ammonia will be formed from 89.5 kg of hydrogen.
N2(g)+ 3H2(g)→ 2NH3(g)
A)5.02 × 105 kg
B)1130 kg
C)1.74 kg
D)2050 kg
E)502 kg
N2(g)+ 3H2(g)→ 2NH3(g)
A)5.02 × 105 kg
B)1130 kg
C)1.74 kg
D)2050 kg
E)502 kg

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
51
The following equation represents the formation of nitrogen dioxide,a major component of smog,
2 NO + O2 → 2 NO2
If 0.68 mole of NO is reacted with 0.79 mole of O2 to produce NO2,the limiting reactant is
A)both NO and O2.
B)NO.
C)O2.
D)NO2.
2 NO + O2 → 2 NO2
If 0.68 mole of NO is reacted with 0.79 mole of O2 to produce NO2,the limiting reactant is
A)both NO and O2.
B)NO.
C)O2.
D)NO2.

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
52
In a reaction to produce chlorine gas,the theoretical yield is 25.6g.What is the actual yield of the reaction if the percent yield is 83.5%?
A)214 g
B)21.4 g
C)3.26 g
D)0.0326 g
E)2.56 g
A)214 g
B)21.4 g
C)3.26 g
D)0.0326 g
E)2.56 g

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
53
Given the following balanced equation,answer the following:
C2H5OH + 3 O2 → 2 CO2 + 3 H2O
A)What is the theoretical yield of carbon dioxide in this reaction if 39.6 g of ethanol is burned in the presence of 54.8 g of oxygen?
B)What is the percent yield for this reaction if the actual amount of carbon dioxide formed is 45.5 grams?
C2H5OH + 3 O2 → 2 CO2 + 3 H2O
A)What is the theoretical yield of carbon dioxide in this reaction if 39.6 g of ethanol is burned in the presence of 54.8 g of oxygen?
B)What is the percent yield for this reaction if the actual amount of carbon dioxide formed is 45.5 grams?

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
54
In the reaction 2 C + O2 → 2 CO,how many moles of carbon are needed to produce 66.0 g of carbon monoxide?
A)0.424
B)1.18
C)2.36
D)4.71
E)28.3
A)0.424
B)1.18
C)2.36
D)4.71
E)28.3

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
55
Match the following.
The mass of 2.25 moles of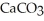
A)212.4 g
B)219 g
C)225 g
D)208.2 g
The mass of 2.25 moles of
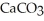
A)212.4 g
B)219 g
C)225 g
D)208.2 g

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
56
In a reaction to produce ammonia,the theoretical yield is 420.g.What is the percent yield if the actual yield is 350.g?
A)20.0%
B)83.3%
C)16.7%
D)120%
E)105%
A)20.0%
B)83.3%
C)16.7%
D)120%
E)105%

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
57
A solution containing 145.0 g AgNO3 is mixed with a CaCl2 solution and 98.4 of AgCl is recovered.Calculate the % yield of the reaction.
A)40.16%
B)61.11%
C)61.99%
D)80.44%
E)161.3%
A)40.16%
B)61.11%
C)61.99%
D)80.44%
E)161.3%

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck



