Deck 20: Organic Chemistry II
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/94
Play
Full screen (f)
Deck 20: Organic Chemistry II
1
Which of the following is a secondary alcohol?
A)CH3CH2OH
B)C6H5CH2OH
C)C(CH3)3OH
D)CH3(CH2)3OH
E)CH(CH3)2OH
A)CH3CH2OH
B)C6H5CH2OH
C)C(CH3)3OH
D)CH3(CH2)3OH
E)CH(CH3)2OH
CH(CH3)2OH
2
How many primary alcohols are there with the formula C4H9OH?
A)4
B)3
C)5
D)1
E)2
A)4
B)3
C)5
D)1
E)2
2
3
The compound 3-bromo-3-ethylpentane likely undergoes nucleophilic substitution by an SN1 reaction.True or false?
True
4
Which of the following is the most volatile?
A)CH3CH(OH)CH2CH3
B)CH3(CH2)3OH
C)(CH3)2C(OH)CH3
D)(CH3)3COH
E)CH3CH2OCH2CH3
A)CH3CH(OH)CH2CH3
B)CH3(CH2)3OH
C)(CH3)2C(OH)CH3
D)(CH3)3COH
E)CH3CH2OCH2CH3

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
5
Where is the electrophilic site in the compound 2-bromo-2-methylbutane?

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
6
Ethers are less volatile than alcohols of the same molar mass.True or false?

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
7
If 2-bromobutane reacts with OH - by an SN1 mechanism,the product is optically active and has the reverse chirality compared to the reactant.True or false?

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
8
Both C6H5OH and C6H5CH2OH are weak acids with Ka values about 10 -10

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
9
Consider the reaction CH3(CH2)4CH2CH(Br)CH3 + NaOH CH3(CH2)4CH2CH(OH)CH3
(optically pure enantiomer)
The rate law for this reaction over a wide range of [OH - ] is R = k1[RX] + k2[RX][OH - ] where RX is 2-bromooctane and k2/k1 = 20.At very low hydroxide ion concentration,
A)the product of the reaction does not rotate the plane of polarized light.
B)the product formed is CH3(CH2)4CH=CHCH3 and not the alcohol.
C)the reaction proceeds by an SN2 mechanism.
D)the hydroxide ion attacks the face of the carbocation in a concerted mechanism on the face opposite the leaving group.
E)the product is optically active and rotates the plane of polarized light in the opposite direction with respect to 2-bromooctane.
(optically pure enantiomer)
The rate law for this reaction over a wide range of [OH - ] is R = k1[RX] + k2[RX][OH - ] where RX is 2-bromooctane and k2/k1 = 20.At very low hydroxide ion concentration,
A)the product of the reaction does not rotate the plane of polarized light.
B)the product formed is CH3(CH2)4CH=CHCH3 and not the alcohol.
C)the reaction proceeds by an SN2 mechanism.
D)the hydroxide ion attacks the face of the carbocation in a concerted mechanism on the face opposite the leaving group.
E)the product is optically active and rotates the plane of polarized light in the opposite direction with respect to 2-bromooctane.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
10
Consider the reaction CH3(CH2)4CH2CH(Br)CH3 + NaOH CH3(CH2)4CH2CH(OH)CH3
(optically pure enantiomer)
If 2-bromooctane rotates the plane of polarized light to the right while the product rotates the plane of polarized light to the left,which of the following statements is true?
A)This is an example of an elimination reaction.
B)The reaction occurs by an SN2 mechanism.
C)The reaction occurs by an SN1 mechanism.
D)This is an example of an addition reaction.
E)This is an example of an electrophilic substitution.
(optically pure enantiomer)
If 2-bromooctane rotates the plane of polarized light to the right while the product rotates the plane of polarized light to the left,which of the following statements is true?
A)This is an example of an elimination reaction.
B)The reaction occurs by an SN2 mechanism.
C)The reaction occurs by an SN1 mechanism.
D)This is an example of an addition reaction.
E)This is an example of an electrophilic substitution.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
11
Which of the following is a tertiary alcohol?
A)C6H5CH2OH
B)C(CH3)3OH
C)CH3CH2OH
D)CH(CH3)2OH
E)CH3(CH2)3OH
A)C6H5CH2OH
B)C(CH3)3OH
C)CH3CH2OH
D)CH(CH3)2OH
E)CH3(CH2)3OH

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
12
Which of the following compounds is most likely to undergo nucleophilic substitution by an SN2 reaction?
A)CH3CH2CH2Br
B)(CH3CH2)3CBr
C)CH3CH2CBr(CH3)CH3
D)(CH3)3CBr
A)CH3CH2CH2Br
B)(CH3CH2)3CBr
C)CH3CH2CBr(CH3)CH3
D)(CH3)3CBr

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
13
What is the product of the reaction
C5H9CH2CH2Br + NaCN
C5H9CH2CH2Br + NaCN

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
14
Which of the following is most volatile?
A)(CH3)2C(OH)CH3
B)(CH3)3COH
C)CH3CH(OH)CH2CH3
D)CH3(CH2)3OH
E)CH3CH2CH2CH3
A)(CH3)2C(OH)CH3
B)(CH3)3COH
C)CH3CH(OH)CH2CH3
D)CH3(CH2)3OH
E)CH3CH2CH2CH3

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
15
The major product of the reaction of (CH3)2CHCH(Br)CH3 with concentrated KOH in alcohol solvent is
A)2-methyl-2-butene.
B)3-methyl-1-butene.
C)2-hydroxy-3-methylbutane.
D)pentene.
E)3-methylbuatanol.
A)2-methyl-2-butene.
B)3-methyl-1-butene.
C)2-hydroxy-3-methylbutane.
D)pentene.
E)3-methylbuatanol.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
16
If 2-bromobutane reacts with OH - by an SN2 mechanism,the product is optically active and has the reverse chirality compared to the reactant.True or false?

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
17
In the SN1 mechanism for nucleophilic substitution,the carbocation produced is a Lewis acid and the nucleophile is a Lewis base.True or false?

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
18
The -OH group occurs in
A)aldehydes and alcohols.
B)alcohols only.
C)alcohols, phenols, and carboxylic acids.
D)phenols and ketones.
E)ketones and carboxylic acids.
A)aldehydes and alcohols.
B)alcohols only.
C)alcohols, phenols, and carboxylic acids.
D)phenols and ketones.
E)ketones and carboxylic acids.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
19
The compound CH3CH2OCH2CH3 is
A)an alcohol.
B)an ester.
C)a ketone.
D)an aldehyde.
E)an ether.
A)an alcohol.
B)an ester.
C)a ketone.
D)an aldehyde.
E)an ether.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
20
How many secondary alcohols are there with the formula C4H9OH?
A)4
B)2
C)3
D)1
E)5
A)4
B)2
C)3
D)1
E)5

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
21
All the following produce a silver mirror with Tollens reagent except
A)HCHO.
B)CH3CH2CH(CHO)CH3.
C)CH3CH2COCH3.
D)CH3(CH2)3CHO.
E)CH3CH2CHO.
A)HCHO.
B)CH3CH2CH(CHO)CH3.
C)CH3CH2COCH3.
D)CH3(CH2)3CHO.
E)CH3CH2CHO.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
22
When aldehydes react with Tollens reagent,
A)a red precipitate forms.
B)silver ions are produced.
C)a ketone is produced.
D)the aldehyde reduces silver ions.
E)an alcohol is produced.
A)a red precipitate forms.
B)silver ions are produced.
C)a ketone is produced.
D)the aldehyde reduces silver ions.
E)an alcohol is produced.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
23
The product of the reaction of CH3CH2CHO with excess KMnO4(aq)in acidic solution is
A)CH3CH2COOH.
B)CH3CH2COCH2CH3.
C)CH3COOH.
D)CH3CH2CH3.
E)CH3CH2OH.
A)CH3CH2COOH.
B)CH3CH2COCH2CH3.
C)CH3COOH.
D)CH3CH2CH3.
E)CH3CH2OH.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
24
Which of the following compounds is the strongest acid? 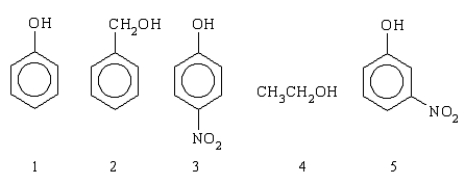
A)5
B)3
C)2
D)1
E)4

A)5
B)3
C)2
D)1
E)4

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
25
To produce butanone,which of the following should be reacted with Na2Cr2O7(aq),H2SO4(aq)?
A)CH3CH2CH(OH)CH3
B)CH3CH2CH(COOH)CH3
C)CH3CH2CH(CH2OH)CH3
D)(CH3)2CHCHO
E)CH3CH2CH2CH2OH
A)CH3CH2CH(OH)CH3
B)CH3CH2CH(COOH)CH3
C)CH3CH2CH(CH2OH)CH3
D)(CH3)2CHCHO
E)CH3CH2CH2CH2OH

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
26
Which of the following produces a silver mirror with Tollens reagent?
A)CH3COOH
B)CH3C(O)OCH3
C)CH3COCH3
D)CH3OCH3
E)CH3CHO
A)CH3COOH
B)CH3C(O)OCH3
C)CH3COCH3
D)CH3OCH3
E)CH3CHO

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
27
What is the product of the following reaction? 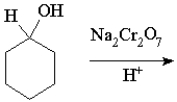


Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
28
Name the compound CH3COO(CH2)4CH3.
A)Methyl pentanoate
B)Hexanoic acid
C)2-hexanone
D)Pentyl acetate
E)Butyl acetate
A)Methyl pentanoate
B)Hexanoic acid
C)2-hexanone
D)Pentyl acetate
E)Butyl acetate

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
29
The ester CH3COO(CH2)4CH3,which is responsible for the odor of bananas,can be prepared from
A)CH3CH2OH and CH3(CH2)3COOH.
B)CH3CHO and CH3(CH2)3CH2OH.
C)CH3COOH and CH3(CH2)3CH2OH.
D)CH3CHO and CH3(CH2)3COOH.
E)CH3COOH and CH3(CH2)3CHO.
A)CH3CH2OH and CH3(CH2)3COOH.
B)CH3CHO and CH3(CH2)3CH2OH.
C)CH3COOH and CH3(CH2)3CH2OH.
D)CH3CHO and CH3(CH2)3COOH.
E)CH3COOH and CH3(CH2)3CHO.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
30
There are two isomeric butanals that differ in the position of the carbonyl group.True or false?

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
31
Oxidation of 2-propanol gives
A)acetaldehyde.
B)propanoic acid.
C)acetone.
D)dimethyl ether.
E)acetic acid.
A)acetaldehyde.
B)propanoic acid.
C)acetone.
D)dimethyl ether.
E)acetic acid.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
32
The carbonyl group occurs in all the following except
A)amides.
B)ketones.
C)carboxylic acids.
D)aldehydes.
E)phenols.
A)amides.
B)ketones.
C)carboxylic acids.
D)aldehydes.
E)phenols.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
33
Which of the following can produce esters?
A)A primary alcohol plus K2Cr2O7(aq), H2SO4
B)An alcohol plus an aldehyde
C)An aldehyde plus KMnO4(aq)in acidic solution
D)An acid plus an alcohol
E)An acid plus an aldehyde
A)A primary alcohol plus K2Cr2O7(aq), H2SO4
B)An alcohol plus an aldehyde
C)An aldehyde plus KMnO4(aq)in acidic solution
D)An acid plus an alcohol
E)An acid plus an aldehyde

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
34
Which of the following would be attacked by a nucleophilic reagent? 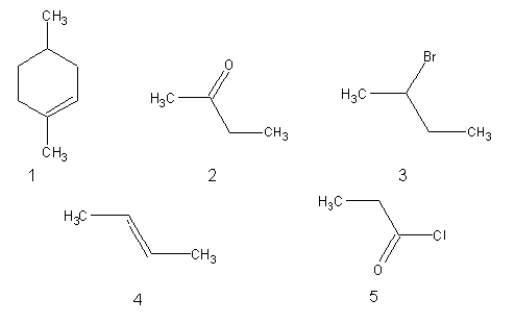
A)2, 3, and 5
B)1 and 4
C)3 and 5
D)2 and 5
E)3 only

A)2, 3, and 5
B)1 and 4
C)3 and 5
D)2 and 5
E)3 only

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
35
Predict the product of the reaction of acetic acid with trimethylamine.
A)CH3CON(CH3)3+
B)CH3CONHCH3
C)CH3CON(CH3)2
D)CH3CONH2
E)No reaction occurs.
A)CH3CON(CH3)3+
B)CH3CONHCH3
C)CH3CON(CH3)2
D)CH3CONH2
E)No reaction occurs.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
36
The formation of an amide from a primary amine and a carboxylic acid is
A)a substitution reaction.
B)an oxidation reaction.
C)an addition reaction.
D)an elimination reaction.
E)a condensation reaction.
A)a substitution reaction.
B)an oxidation reaction.
C)an addition reaction.
D)an elimination reaction.
E)a condensation reaction.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
37
When aldehydes react with Tollens reagent,
A)a red precipitate forms.
B)a ketone is produced.
C)silver ions are produced.
D)an alcohol is produced.
E)a carboxylic acid is produced.
A)a red precipitate forms.
B)a ketone is produced.
C)silver ions are produced.
D)an alcohol is produced.
E)a carboxylic acid is produced.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
38
Oxidation of secondary alcohols produces
A)acids.
B)aldehydes.
C)ketones.
D)Secondary alcohols cannot be oxidized.
E)ethers.
A)acids.
B)aldehydes.
C)ketones.
D)Secondary alcohols cannot be oxidized.
E)ethers.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
39
Predict the product(s)of the reaction of acetic acid with methylamine at 200oC.
A)CH3COO --and CH3NH3+
B)No reaction occurs.
C)CH3C(O)OCH3
D)CH3CONHCH3
E)CH3CONH2
A)CH3COO --and CH3NH3+
B)No reaction occurs.
C)CH3C(O)OCH3
D)CH3CONHCH3
E)CH3CONH2

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
40
When an ester is formed via a condensation reaction with elimination of water,the oxygen atom in the water molecule comes from
A)the hydroxyl group of the acid.
B)the alcohol.
C)the aldehyde.
D)the carbonyl group of the acid.
E)the solvent.
A)the hydroxyl group of the acid.
B)the alcohol.
C)the aldehyde.
D)the carbonyl group of the acid.
E)the solvent.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
41
Which of the following would be attacked by an electrophilic reagent? 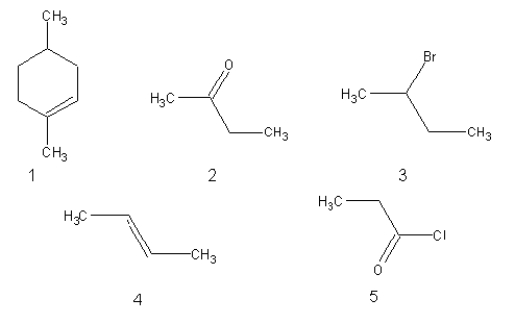
A)2 and 5 only
B)1 and 4 only
C)1, 2, 4, and 5
D)3 and 5

A)2 and 5 only
B)1 and 4 only
C)1, 2, 4, and 5
D)3 and 5

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
42
The following polymer is called 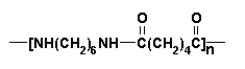
A)polyester.
B)dacron.
C)teflon.
D)kevlar.
E)nylon-66.
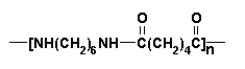
A)polyester.
B)dacron.
C)teflon.
D)kevlar.
E)nylon-66.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
43
What is the product of the reaction
CH3CH(NH2)CH3 + CH3CH2C(O)OCH3
CH3CH(NH2)CH3 + CH3CH2C(O)OCH3

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
44
The structure of rubber,a polymer,is 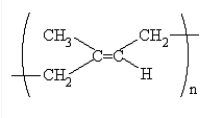 What is the formula of the monomer used to produce rubber?
What is the formula of the monomer used to produce rubber?
A)(CH3)2CCHCH3
B)CH3CCCH3
C)CH2CCCH2
D)CH2C(CH3)CHCH2
E)CH3CH(CH3)CH2CH3
 What is the formula of the monomer used to produce rubber?
What is the formula of the monomer used to produce rubber?A)(CH3)2CCHCH3
B)CH3CCCH3
C)CH2CCCH2
D)CH2C(CH3)CHCH2
E)CH3CH(CH3)CH2CH3

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
45
The following polymer is called 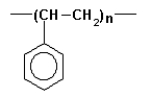
A)teflon.
B)PVC.
C)polypropylene.
D)polyethylene.
E)polystyrene.

A)teflon.
B)PVC.
C)polypropylene.
D)polyethylene.
E)polystyrene.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
46
The systematic name of CH3CH2CH(CHO)CH3 is
A)2-ethylpropanal.
B)2-pentanal.
C)3-methylbutanal.
D)2-pentanone.
E)2-methylbutanal.
A)2-ethylpropanal.
B)2-pentanal.
C)3-methylbutanal.
D)2-pentanone.
E)2-methylbutanal.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
47
Which of the following reactants can be used to produce propanamide? 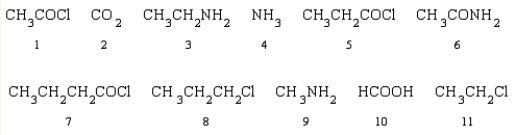
A)5 and 6
B)4 and 7
C)2, 4, and 11
D)4 and 5
E)2, 4, and 8

A)5 and 6
B)4 and 7
C)2, 4, and 11
D)4 and 5
E)2, 4, and 8

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
48
Fill in the missing compound in the following reaction.
CH3CH2Br + __________ CH3CH2CN + NaI
CH3CH2Br + __________ CH3CH2CN + NaI

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
49
What reactants could be used to synthesize CH3CONH2?

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
50
The systematic name of CH3CH2COCH3 is
A)2-butanone.
B)3-butanal.
C)methypropylether.
D)2-butanal.
E)3-butanone.
A)2-butanone.
B)3-butanal.
C)methypropylether.
D)2-butanal.
E)3-butanone.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
51
All the following polymers are produced by addition polymerization except
A)polyethylene.
B)Dacron.
C)PVC.
D)polypropylene.
E)Teflon.
A)polyethylene.
B)Dacron.
C)PVC.
D)polypropylene.
E)Teflon.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
52
Which of the following monomers is used to produce Teflon?
A)CHClCH2
B)CF2CF2
C)CH2CH2
D)CH(CH3)CH2
E)CH(CN)CH2
A)CHClCH2
B)CF2CF2
C)CH2CH2
D)CH(CH3)CH2
E)CH(CN)CH2

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
53
Fill in the missing compounds or reagents in the following reaction scheme.
C6H5CHCH2 + ______ C6H5CH(Br)CH3 + CH3CH2O - Na+ ________
C6H5CHCH2 + ______ C6H5CH(Br)CH3 + CH3CH2O - Na+ ________

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
54
Condensation polymerization involves the use of
A)one or two monomers, each with a nitrile group.
B)one or two monomers with reactive groups at each end of the molecules.
C)a monomer with a double bond.
D)a monomer with a triple bond.
E)one or two monomers, each with one reactive group.
A)one or two monomers, each with a nitrile group.
B)one or two monomers with reactive groups at each end of the molecules.
C)a monomer with a double bond.
D)a monomer with a triple bond.
E)one or two monomers, each with one reactive group.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
55
What is the product of the reaction
C6H5COCl + CH3NH2
C6H5COCl + CH3NH2

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
56
Which of the following monomers is used to produce polystyrene?
A)CH(CH3)CH2
B)CHClCH2
C)CH2CH2
D)CH(CN)CH2
E)CH(C6H5)CH2
A)CH(CH3)CH2
B)CHClCH2
C)CH2CH2
D)CH(CN)CH2
E)CH(C6H5)CH2

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
57
What is the product of the reaction
CH3CH2CH2Br + NaHS
CH3CH2CH2Br + NaHS

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
58
The polymer that is formed from acrylonitrile is
A)teflon.
B)orlon.
C)cucite.
D)polystyrene.
E)PVC.
A)teflon.
B)orlon.
C)cucite.
D)polystyrene.
E)PVC.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
59
Which of the following reactants can be used to produce butanamide? 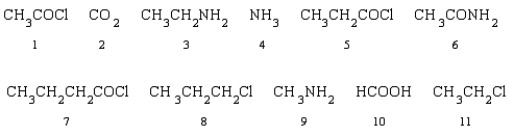
A)1 and 6
B)1 and 3
C)4 and 7
D)8 and9
E)6 and 11

A)1 and 6
B)1 and 3
C)4 and 7
D)8 and9
E)6 and 11

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
60
What are the formulas of the monomers used to produce the nylon polymer shown below? 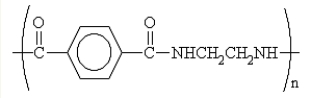
A)NH2CH2CH2NH2 and (COCl)-C6H4-(COCl)(substituents are para)
B)NH2CH2CH2Cl and (COCl)-C6H4-(COOH)
C)HOOC-CH2CH2-COOH and NH2-C6H4-NH2 (substituents are para)
D)ClCH2CH2Cl and (COOH)-C6H4-(COOH)(substituents are para)
E)ClCH2CH2Cl and NH2-C6H4-NH2 (substituents are para)

A)NH2CH2CH2NH2 and (COCl)-C6H4-(COCl)(substituents are para)
B)NH2CH2CH2Cl and (COCl)-C6H4-(COOH)
C)HOOC-CH2CH2-COOH and NH2-C6H4-NH2 (substituents are para)
D)ClCH2CH2Cl and (COOH)-C6H4-(COOH)(substituents are para)
E)ClCH2CH2Cl and NH2-C6H4-NH2 (substituents are para)

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
61
Which of the following refers to the secondary structure of a protein?
A)Denaturation
B)( helix)
C)Amino acid sequence
D)Disulfide linkages
E)Base pairs
A)Denaturation
B)( helix)
C)Amino acid sequence
D)Disulfide linkages
E)Base pairs

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
62
Which of the following polymers are addition polymers? 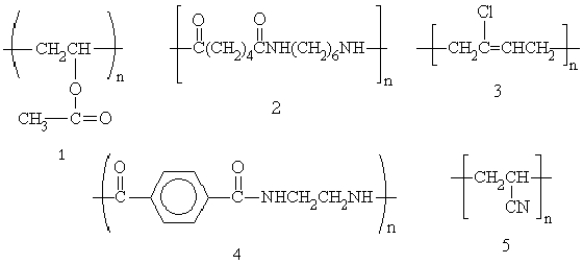
A)1, 3, and 5
B)2 and 3
C)2 and 4
D)4 and 5
E)3 only

A)1, 3, and 5
B)2 and 3
C)2 and 4
D)4 and 5
E)3 only

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
63
The helix results from the pairing of specific bases by hydrogen bonding.Which pairs form hydrogen bonds?
A)AT and GC
B)AC and GC
C)GT
D)AT, GC, AC, and GT
E)AC
A)AT and GC
B)AC and GC
C)GT
D)AT, GC, AC, and GT
E)AC

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
64
Amines react with both carboxylic acids and acid chlorides to produce amides.True or false?

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
65
The typical amino acid,NH2CHRCOOH,has pKa1 and pKa2 of 2.4 and 9.8,respectively.What is(are)the major species in an aqueous solution whose pH is 10?

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
66
If the base sequence along a portion of one strand of a double helix is CTACACG,the corresponding sequence on the other strand is
A)GATGTGC.
B)CUACACG.
C)TCGTGTC.
D)CTUCUCG.
E)CTACACG.
A)GATGTGC.
B)CUACACG.
C)TCGTGTC.
D)CTUCUCG.
E)CTACACG.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
67
Esters can be produced by a condensation reaction between acid halides and alcohols.If the acid halide CH3COCl reacts with ethanol,which of the following is eliminated?
A)HCl
B)H2O
C)OH -
D)H3O+
A)HCl
B)H2O
C)OH -
D)H3O+

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
68
In the reaction of CH3CH2COOH with CH3NH2,what molecule is eliminated?

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
69
What reactants could be used to synthesize CH3CH2CH2C(O)OCH3?

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
70
What reactants would be used to synthesize the following compound? 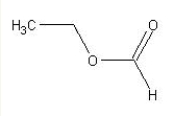 Choose from the following.
Choose from the following. 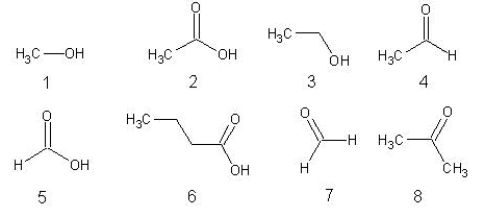
 Choose from the following.
Choose from the following. 

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
71
The primary structure of aspartame is 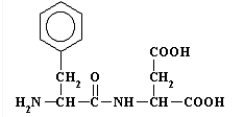 This dipeptide is
This dipeptide is
A)Phe-Asp.
B)Trp-Glu.
C)Phe-Glu.
D)Tyr-Glu.
E)Tyr-Asn.
 This dipeptide is
This dipeptide isA)Phe-Asp.
B)Trp-Glu.
C)Phe-Glu.
D)Tyr-Glu.
E)Tyr-Asn.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
72
Which of the following is a peptide bond?
A)(-C(O)O-)
B)(-CONH-)
C)(-C-NH-)
D)(-C=N-)
E)(-C(O)-)
A)(-C(O)O-)
B)(-CONH-)
C)(-C-NH-)
D)(-C=N-)
E)(-C(O)-)

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
73
The primary structure of a peptide is 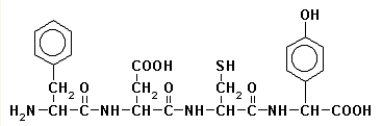 This peptide is
This peptide is
A)Tyr-Asn-Cys-Phe.
B)Tyr-Glu-Cys-Phe.
C)Phe-Asp-Cys-Tyr.
D)Phe-Glu-Met-Tyr.
E)Tyr-Asp-Met-Phe.
 This peptide is
This peptide isA)Tyr-Asn-Cys-Phe.
B)Tyr-Glu-Cys-Phe.
C)Phe-Asp-Cys-Tyr.
D)Phe-Glu-Met-Tyr.
E)Tyr-Asp-Met-Phe.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
74
Complete hydrolysis of a hexapeptide yielded Arg,Tyr,Val,Phe,Cys.After partial hydrolysis,the fragments Tyr-Cys,Arg-Phe,Cys,Val-Arg,and Phe-Tyr were obtained.What are the possible sequences in the hexapeptide?

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
75
Fill in the missing reactants and products. 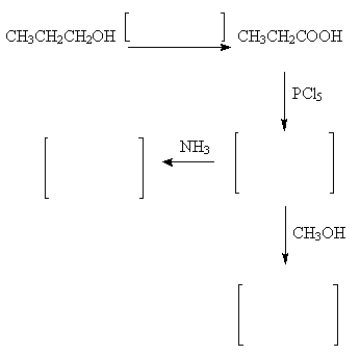


Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
76
What reactants would be used to synthesize the following compound? 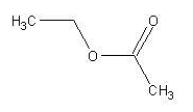 Choose from the following.
Choose from the following. 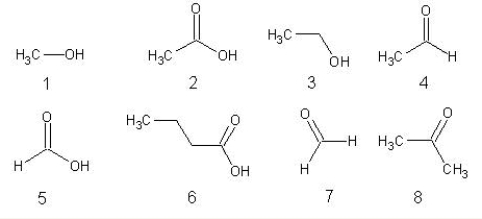
 Choose from the following.
Choose from the following. 

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
77
Which of the following polymers are condensation polymers? 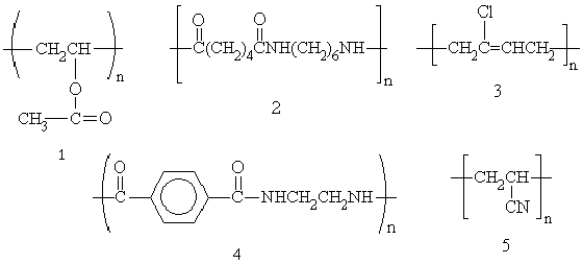
A)3 only
B)2 and 4
C)2 and 3
D)4 and 5
E)1, 3, and 5

A)3 only
B)2 and 4
C)2 and 3
D)4 and 5
E)1, 3, and 5

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
78
When amines condense with carboxylic acids,what molecule is eliminated?

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
79
How many hydrogen bonds are formed between T and A?
A)2
B)1
C)3
D)4
A)2
B)1
C)3
D)4

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
80
Consider the reactions
CH3CH2CH3 + Br2/light A
A + NaCN B
Identify A and B.
CH3CH2CH3 + Br2/light A
A + NaCN B
Identify A and B.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck



