Deck 11: Chemical Bonds: the Formation of Compounds From Atoms
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/102
Play
Full screen (f)
Deck 11: Chemical Bonds: the Formation of Compounds From Atoms
1
How many valence electrons are present in an atom of lithium in the ground state?
A)1
B)2
C)3
D)7
A)1
B)2
C)3
D)7
1
2
Atoms of the metallic elements generally form ions by
A)gaining electrons,forming positive ions.
B)gaining electrons,forming negative ions.
C)losing electrons,forming positive ions.
D)losing electrons,forming negative ions.
A)gaining electrons,forming positive ions.
B)gaining electrons,forming negative ions.
C)losing electrons,forming positive ions.
D)losing electrons,forming negative ions.
losing electrons,forming positive ions.
3
What is the total number of electrons present in a 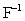 ion?
ion?
A)10
B)8
C)6
D)9
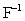 ion?
ion?A)10
B)8
C)6
D)9
10
4
Which of the following statements is true for most atoms?
A)In general,as the atomic radius increases,the first ionization energy increases.
B)In general,as the atomic radius increases,the first ionization energy decreases.
C)In general,as the first ionization energy decreases,the electronegativity increases.
D)No correct answer is given.
A)In general,as the atomic radius increases,the first ionization energy increases.
B)In general,as the atomic radius increases,the first ionization energy decreases.
C)In general,as the first ionization energy decreases,the electronegativity increases.
D)No correct answer is given.

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
5
As one progresses left to right across a period on the periodic table,first ionization energy generally
A)increases.
B)decreases.
C)remains the same.
A)increases.
B)decreases.
C)remains the same.

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
6
Which of the following pairs is incorrectly matched? 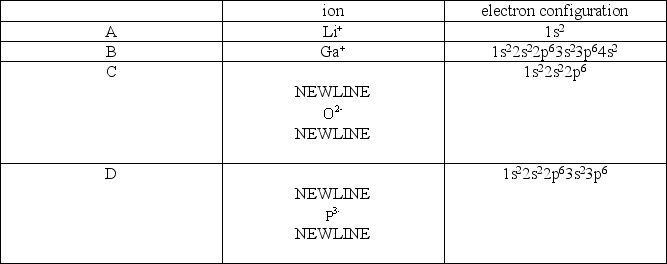


Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
7
As one descends a group on the periodic table,atomic radius generally
A)increases.
B)decreases.
C)remains the same.
A)increases.
B)decreases.
C)remains the same.

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
8
What is the total number of electrons present in a Ca+2 ion?
A)2
B)18
C)20
D)22
A)2
B)18
C)20
D)22

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
9
Which structure represents the best Lewis structure for O2?
A)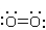
B)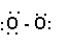
C)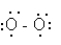
D)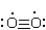
A)
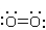
B)
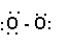
C)
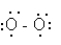
D)
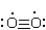

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
10
As one descends a group on the periodic table,first ionization energy generally
A)increases.
B)decreases.
C)remains the same.
A)increases.
B)decreases.
C)remains the same.

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
11
When potassium fluoride forms from a potassium atom and a fluorine atom
A)a proton is transferred from the potassium atom to the fluorine atom.
B)a proton is transferred from the fluorine atom to the potassium atom.
C)an electron is transferred from the potassium atom to the fluorine atom.
D)an electron is transferred from the fluorine atom to the potassium atom.
A)a proton is transferred from the potassium atom to the fluorine atom.
B)a proton is transferred from the fluorine atom to the potassium atom.
C)an electron is transferred from the potassium atom to the fluorine atom.
D)an electron is transferred from the fluorine atom to the potassium atom.

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
12
What is the total number of electrons present in a 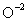 ion?
ion?
A)8
B)10
C)2
D)6
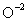 ion?
ion?A)8
B)10
C)2
D)6

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
13
What is the total number of electrons present in a Na+1 ion?
A)6
B)11
C)12
D)10
A)6
B)11
C)12
D)10

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
14
Atoms of the nonmetallic elements generally form ions by
A)gaining electrons,forming positive ions.
B)gaining electrons,forming negative ions.
C)losing electrons,forming positive ions.
D)losing electrons,forming negative ions.
A)gaining electrons,forming positive ions.
B)gaining electrons,forming negative ions.
C)losing electrons,forming positive ions.
D)losing electrons,forming negative ions.

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
15
As one progresses left to right across a period on the periodic table,atomic radius generally
A)increases.
B)decreases.
C)remains the same.
A)increases.
B)decreases.
C)remains the same.

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
16
Which of the following statements is false?
A)In order for a covalent bond to form,the atomic orbitals of the atoms must overlap.
B)The most common ions for the representative elements can be predicted based on their tendency to attain the same electron configuration of the closest noble gas.
C)Ionization energies show a large decrease when electrons are removed from a noble gas-like configuration.
D)Ionic compounds exist as large aggregates of positive and negative ions.
A)In order for a covalent bond to form,the atomic orbitals of the atoms must overlap.
B)The most common ions for the representative elements can be predicted based on their tendency to attain the same electron configuration of the closest noble gas.
C)Ionization energies show a large decrease when electrons are removed from a noble gas-like configuration.
D)Ionic compounds exist as large aggregates of positive and negative ions.

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
17
Choose the best Lewis structure for ![<strong>Choose the best Lewis structure for .</strong> A)[ ]<sup>-</sup> B)[ ]<sup>-</sup> C)[ ]<sup>-</sup> D)[ ]<sup>-</sup>](https://d2lvgg3v3hfg70.cloudfront.net/TB4036/11ea7f51_1e95_97c3_9ecd_4bb848a0ca83_TB4036_11.jpg) .
.
A)[![<strong>Choose the best Lewis structure for .</strong> A)[ ]<sup>-</sup> B)[ ]<sup>-</sup> C)[ ]<sup>-</sup> D)[ ]<sup>-</sup>](https://d2lvgg3v3hfg70.cloudfront.net/TB4036/11ea7f51_1e95_97c4_9ecd_893c63dbdfaa_TB4036_11.jpg) ]-
]-
B)[![<strong>Choose the best Lewis structure for .</strong> A)[ ]<sup>-</sup> B)[ ]<sup>-</sup> C)[ ]<sup>-</sup> D)[ ]<sup>-</sup>](https://d2lvgg3v3hfg70.cloudfront.net/TB4036/11ea7f51_1e95_97c5_9ecd_b365c728133c_TB4036_11.jpg) ]-
]-
C)[![<strong>Choose the best Lewis structure for .</strong> A)[ ]<sup>-</sup> B)[ ]<sup>-</sup> C)[ ]<sup>-</sup> D)[ ]<sup>-</sup>](https://d2lvgg3v3hfg70.cloudfront.net/TB4036/11ea7f51_1e95_97c6_9ecd_1b827668769e_TB4036_11.jpg) ]-
]-
D)[![<strong>Choose the best Lewis structure for .</strong> A)[ ]<sup>-</sup> B)[ ]<sup>-</sup> C)[ ]<sup>-</sup> D)[ ]<sup>-</sup>](https://d2lvgg3v3hfg70.cloudfront.net/TB4036/11ea7f51_1e95_97c7_9ecd_7509ba0e121a_TB4036_11.jpg) ]-
]-
![<strong>Choose the best Lewis structure for .</strong> A)[ ]<sup>-</sup> B)[ ]<sup>-</sup> C)[ ]<sup>-</sup> D)[ ]<sup>-</sup>](https://d2lvgg3v3hfg70.cloudfront.net/TB4036/11ea7f51_1e95_97c3_9ecd_4bb848a0ca83_TB4036_11.jpg) .
.A)[
![<strong>Choose the best Lewis structure for .</strong> A)[ ]<sup>-</sup> B)[ ]<sup>-</sup> C)[ ]<sup>-</sup> D)[ ]<sup>-</sup>](https://d2lvgg3v3hfg70.cloudfront.net/TB4036/11ea7f51_1e95_97c4_9ecd_893c63dbdfaa_TB4036_11.jpg) ]-
]-B)[
![<strong>Choose the best Lewis structure for .</strong> A)[ ]<sup>-</sup> B)[ ]<sup>-</sup> C)[ ]<sup>-</sup> D)[ ]<sup>-</sup>](https://d2lvgg3v3hfg70.cloudfront.net/TB4036/11ea7f51_1e95_97c5_9ecd_b365c728133c_TB4036_11.jpg) ]-
]-C)[
![<strong>Choose the best Lewis structure for .</strong> A)[ ]<sup>-</sup> B)[ ]<sup>-</sup> C)[ ]<sup>-</sup> D)[ ]<sup>-</sup>](https://d2lvgg3v3hfg70.cloudfront.net/TB4036/11ea7f51_1e95_97c6_9ecd_1b827668769e_TB4036_11.jpg) ]-
]-D)[
![<strong>Choose the best Lewis structure for .</strong> A)[ ]<sup>-</sup> B)[ ]<sup>-</sup> C)[ ]<sup>-</sup> D)[ ]<sup>-</sup>](https://d2lvgg3v3hfg70.cloudfront.net/TB4036/11ea7f51_1e95_97c7_9ecd_7509ba0e121a_TB4036_11.jpg) ]-
]-
Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
18
How many valence electrons are present in an atom of bromine in the ground state?
A)1
B)2
C)3
D)7
A)1
B)2
C)3
D)7

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
19
Which element forms an ion that is larger than its atom?
A)Lithium
B)Calcium
C)Chromium
D)Cluorine
A)Lithium
B)Calcium
C)Chromium
D)Cluorine

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
20
How many valence electrons are present in an atom of magnesium in the ground state?
A)1
B)2
C)7
D)8
A)1
B)2
C)7
D)8

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
21
Which type of chemical bond involves the unequal sharing of electrons?
A)Ionic
B)Polar covalent
C)Nonpolar covalent
A)Ionic
B)Polar covalent
C)Nonpolar covalent

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
22
Which type of chemical bond involves the transfer of electrons?
A)Ionic
B)Polar covalent
C)Nonpolar covalent
A)Ionic
B)Polar covalent
C)Nonpolar covalent

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
23
Elements of Group 6A generally form ions with a charge of
A)+1
B)+2
C)-1
D)-2
A)+1
B)+2
C)-1
D)-2

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
24
What is the empirical formula of sodium oxide?
A)NaO
B)NaO2
C)Na2O
D)Na2O2
A)NaO
B)NaO2
C)Na2O
D)Na2O2

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
25
As one descends a group on the periodic table,electronegativity generally
A)increases.
B)decreases.
C)remains the same.
A)increases.
B)decreases.
C)remains the same.

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
26
Which element forms an ion that is smaller than its atom?
A)Strontium
B)Sulfur
C)Oxygen
D)Fluorine
A)Strontium
B)Sulfur
C)Oxygen
D)Fluorine

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
27
Halogens generally form ions with a charge of
A)+1
B)+2
C)-1
D)-2
A)+1
B)+2
C)-1
D)-2

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
28
What is the empirical formula of calcium oxide?
A)CaO
B)Ca2O
C)CaO2
D)CaO3
A)CaO
B)Ca2O
C)CaO2
D)CaO3

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
29
What is the empirical formula of barium nitride?
A)BaN
B)Ba3N
C)Ba2N3
D)Ba3N2
A)BaN
B)Ba3N
C)Ba2N3
D)Ba3N2

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
30
A Cl-1 ion has an electron configuration similar to that of
A)neon.
B)argon.
C)krypton.
D)xenon.
A)neon.
B)argon.
C)krypton.
D)xenon.

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
31
As one progresses left to right across a period on the periodic table,electronegativity generally
A)increases.
B)decreases.
C)remains the same.
A)increases.
B)decreases.
C)remains the same.

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
32
A bond that is principally ionic will form between
A)sulfur and hydrogen.
B)phosphorus and sulfur.
C)chlorine and iodine.
D)potassium and fluorine.
A)sulfur and hydrogen.
B)phosphorus and sulfur.
C)chlorine and iodine.
D)potassium and fluorine.

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
33
A bond that is principally covalent will form between
A)potassium and oxygen.
B)fluorine and oxygen.
C)sodium and chlorine.
D)aluminum and fluorine.
A)potassium and oxygen.
B)fluorine and oxygen.
C)sodium and chlorine.
D)aluminum and fluorine.

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
34
What is the empirical formula of lithium nitride?
A)LiN
B)Li3N
C)LiN3
D)Li2N
A)LiN
B)Li3N
C)LiN3
D)Li2N

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
35
A Ca+2 ion has an electron configuration similar to that of
A)neon.
B)argon.
C)krypton.
D)xenon.
A)neon.
B)argon.
C)krypton.
D)xenon.

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
36
Alkaline earth metals generally form ions with a charge of
A)+1
B)+2
C)-1
D)-2
A)+1
B)+2
C)-1
D)-2

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
37
Alkali metals generally form ions with a charge of
A)+1
B)+2
C)-1
D)-2
A)+1
B)+2
C)-1
D)-2

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
38
What is the empirical formula of aluminum oxide?
A)AlO
B)Al2O3
C)Al3O2
D)AlO3
A)AlO
B)Al2O3
C)Al3O2
D)AlO3

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
39
A bond that is principally ionic will form between
A)magnesium and chlorine.
B)silicon and phosphorus.
C)selenium and oxygen.
D)oxygen and nitrogen.
A)magnesium and chlorine.
B)silicon and phosphorus.
C)selenium and oxygen.
D)oxygen and nitrogen.

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
40
Which type of chemical bond involves the equal sharing of electrons?
A)Ionic
B)Polar covalent
C)Nonpolar covalent
A)Ionic
B)Polar covalent
C)Nonpolar covalent

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
41
In any period,the highest electronegativity is found in a
A)metal.
B)nonmetal.
C)metalloid.
A)metal.
B)nonmetal.
C)metalloid.

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
42
What is the number of valence electrons in a molecule of ammonia?
A)3
B)24
C)6
D)8
A)3
B)24
C)6
D)8

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
43
The shape of a water molecule is
A)bent.
B)pyramidal.
C)tetrahedral.
D)linear.
A)bent.
B)pyramidal.
C)tetrahedral.
D)linear.

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
44
A bond that is principally covalent will form between
A)calcium and oxygen.
B)rubidium and chlorine.
C)lithium and chlorine.
D)sulfur and oxygen.
A)calcium and oxygen.
B)rubidium and chlorine.
C)lithium and chlorine.
D)sulfur and oxygen.

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
45
As the difference in electronegativity between two atoms increases,the percent of ionic character of a bond between those two atoms
A)increases.
B)decreases.
C)remains the same.
A)increases.
B)decreases.
C)remains the same.

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
46
An ionic bond will form between which two atoms?
A)Potassium and chlorine
B)Hydrogen and oxygen
C)Chlorine and chlorine
D)Carbon and oxygen
A)Potassium and chlorine
B)Hydrogen and oxygen
C)Chlorine and chlorine
D)Carbon and oxygen

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
47
The atomic radius generally decreases as we move from left to right across a period because
A)the number of electrons in the atoms decreases from left to right.
B)the number of protons in the nucleus increases from left to right.
C)the sizes of the orbitals in the energy level decreases as we move from left to right.
D)the number of neutrons in the nucleus remains almost constant as we move from left.to right
A)the number of electrons in the atoms decreases from left to right.
B)the number of protons in the nucleus increases from left to right.
C)the sizes of the orbitals in the energy level decreases as we move from left to right.
D)the number of neutrons in the nucleus remains almost constant as we move from left.to right

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
48
A Lewis electron dot symbol shows
A)all the electrons in the ground-state of an atom.
B)all the core electrons in the ground-state of an atom.
C)all the valence electrons in the ground-state of an atom.
D)all the electrons in the outermost sublevel in an atom.
A)all the electrons in the ground-state of an atom.
B)all the core electrons in the ground-state of an atom.
C)all the valence electrons in the ground-state of an atom.
D)all the electrons in the outermost sublevel in an atom.

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
49
The shape of a carbon dioxide molecule is
A)bent.
B)linear.
C)trigonal planar.
D)tetrahedral.
A)bent.
B)linear.
C)trigonal planar.
D)tetrahedral.

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
50
What is the number of valence electrons in a chlorine atom in the ground state?
A)1
B)2
C)5
D)7
A)1
B)2
C)5
D)7

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
51
A nonpolar covalent bond will form between which two atoms?
A)Potassium and chlorine
B)Hydrogen and chlorine
C)Sodium and bromine
D)Chlorine and chlorine
A)Potassium and chlorine
B)Hydrogen and chlorine
C)Sodium and bromine
D)Chlorine and chlorine

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
52
Which is a nonpolar molecule?
A)CH4
B)KCl
C)HCl
D)H2O
A)CH4
B)KCl
C)HCl
D)H2O

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
53
What is the number of valence electrons in a molecule of carbon dioxide?
A)24
B)16
C)10
D)12
A)24
B)16
C)10
D)12

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
54
As the difference in electronegativity between two atoms increases,the polarity of a bond between those two atoms
A)increases.
B)decreases.
C)remains the same.
A)increases.
B)decreases.
C)remains the same.

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
55
What is the number of valence electrons in an aluminum atom in the ground state?
A)1
B)2
C)3
D)4
A)1
B)2
C)3
D)4

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
56
Which is a nonpolar molecule?
A)H2S
B)CO2
C)HF
D)NH3
A)H2S
B)CO2
C)HF
D)NH3

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
57
The shape of an ammonia molecule is
A)bent.
B)trigonal planar.
C)pyramidal.
D)linear.
A)bent.
B)trigonal planar.
C)pyramidal.
D)linear.

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
58
Which contains both ionic and covalent bonds?
A)(KCl)
B)(NH3 )
C)(KNO3)
D)([NO2]-1)
A)(KCl)
B)(NH3 )
C)(KNO3)
D)([NO2]-1)

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
59
The shape of a boron trifluoride molecule is
A)pyramidal.
B)trigonal planar.
C)tetrahedral.
D)linear.
A)pyramidal.
B)trigonal planar.
C)tetrahedral.
D)linear.

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
60
A polar covalent bond will form between which two atoms?
A)Beryllium and fluorine
B)Hydrogen and chlorine
C)Sodium and oxygen
D)Fluorine and fluorine
A)Beryllium and fluorine
B)Hydrogen and chlorine
C)Sodium and oxygen
D)Fluorine and fluorine

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
61
Covalent bonds will most likely form between
A)two atoms with a high ionization energy.
B)two atoms with a low ionization energy.
C)an atom with a high ionization energy and an atom with a low ionization energy.
D)no correct answer is given.
A)two atoms with a high ionization energy.
B)two atoms with a low ionization energy.
C)an atom with a high ionization energy and an atom with a low ionization energy.
D)no correct answer is given.

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
62
Which of the following does not have a noble gas electron configuration?
A)S-2
B)Al+3
C)Sb+5
D)Ar
A)S-2
B)Al+3
C)Sb+5
D)Ar

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
63
Which of the following characterizes covalent bonding?
A)The formation of ions
B)The type of bond normally found between metals and nonmetals
C)The loss of electrons
D)The formation of true,discrete molecules
A)The formation of ions
B)The type of bond normally found between metals and nonmetals
C)The loss of electrons
D)The formation of true,discrete molecules

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
64
The molecular shape shown in the following figure is 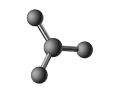
A)tetrahedral.
B)trigonal pyramidal.
C)bent.
D)trigonal planar.

A)tetrahedral.
B)trigonal pyramidal.
C)bent.
D)trigonal planar.

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
65
Which does not represent the correct formula for a compound of magnesium?
A)MgCl2
B)Mg2S
C)MgO
D)Mg3N2
A)MgCl2
B)Mg2S
C)MgO
D)Mg3N2

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
66
Which atom has the largest radius?
A)Se
B)Te
C)O
D)S
A)Se
B)Te
C)O
D)S

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
67
The shape of a carbon tetrachloride molecule is
A)pyramidal.
B)trigonal planar.
C)bent.
D)tetrahedral.
A)pyramidal.
B)trigonal planar.
C)bent.
D)tetrahedral.

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
68
In the following molecular shape,the angle is 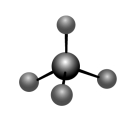
A)180°
B)120°
C)90°
D)109.5°

A)180°
B)120°
C)90°
D)109.5°

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
69
Which atom has four valence electrons?
A)Be
B)F
C)Ge
D)Te
A)Be
B)F
C)Ge
D)Te

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
70
Which compound contains a triple bond in its Lewis structure?
A)CO
B)SO2
C)H2S
D)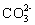
A)CO
B)SO2
C)H2S
D)
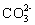

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
71
Which does not have a noble gas electron configuration?
A)Sc+3
B)Ar
C)O-2
D)K
A)Sc+3
B)Ar
C)O-2
D)K

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
72
The total number of valence electrons in a nitrate ion is
A)22
B)23
C)24
D)56
A)22
B)23
C)24
D)56

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
73
Carbon dioxide is a nonpolar molecule because
A)oxygen is more electronegative than carbon.
B)the two oxygen atoms are bonded to the carbon atom.
C)the individual dipoles of the carbon-oxygen bonds are oriented 180° to each other.
D)the carbon-oxygen bonds are polar covalent.
A)oxygen is more electronegative than carbon.
B)the two oxygen atoms are bonded to the carbon atom.
C)the individual dipoles of the carbon-oxygen bonds are oriented 180° to each other.
D)the carbon-oxygen bonds are polar covalent.

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
74
Ionic bonds will most likely form between
A)two atoms with a high ionization energy.
B)two atoms with a low ionization energy.
C)an atom with a high ionization energy and an atom with a low ionization energy.
D)no correct answer is given.
A)two atoms with a high ionization energy.
B)two atoms with a low ionization energy.
C)an atom with a high ionization energy and an atom with a low ionization energy.
D)no correct answer is given.

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
75
The molecular shape shown in the following figure is 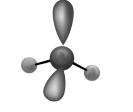
A)tetrahedral.
B)trigonal pyramidal.
C)bent.
D)trigonal planar.

A)tetrahedral.
B)trigonal pyramidal.
C)bent.
D)trigonal planar.

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
76
Which series is ranked in order of increasing electronegativity?
A)O,S,Se,Te
B)Cl,S,P,Si
C)Sr,Sn,N,O
D)C,Si,P,Se
A)O,S,Se,Te
B)Cl,S,P,Si
C)Sr,Sn,N,O
D)C,Si,P,Se

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
77
To break a covalent bond,energy must be
A)released.
B)absorbed.
C)both,absorbed and released.
D)neither,absorbed nor released.
A)released.
B)absorbed.
C)both,absorbed and released.
D)neither,absorbed nor released.

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
78
Which compound has double covalent bonds within its molecular structure?
A)NaCl
B)CO2
C)CH4
D)H2O
A)NaCl
B)CO2
C)CH4
D)H2O

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
79
Which atom has the largest radius?
A)Mg
B)Si
C)S
D)Cl
A)Mg
B)Si
C)S
D)Cl

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
80
Which of the following does not contain a polar covalent bond?
A)CH4
B)H2O
C)CH3OH
D)Cl2
A)CH4
B)H2O
C)CH3OH
D)Cl2

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck



