Deck 2: Atoms and Molecules
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/92
Play
Full screen (f)
Deck 2: Atoms and Molecules
1
Isotopes differ from each other in what way?
A) They have different numbers of protons in the nucleus.
B) They have different numbers of neutrons in the nucleus.
C) They have different numbers of electrons outside the nucleus.
D) More than one response is correct.
A) They have different numbers of protons in the nucleus.
B) They have different numbers of neutrons in the nucleus.
C) They have different numbers of electrons outside the nucleus.
D) More than one response is correct.
They have different numbers of neutrons in the nucleus.
2
Which of the following particles is the smallest?
A) proton
B) electron
C) neutron
D) they are all the same size
A) proton
B) electron
C) neutron
D) they are all the same size
electron
3
Which of the following uses the unit of "u" or "amu"?
A) atomic weights of atoms
B) relative masses of atoms
C) molecular weights of molecules
D) more than one response is correct
A) atomic weights of atoms
B) relative masses of atoms
C) molecular weights of molecules
D) more than one response is correct
more than one response is correct
4
Which of the following carries a negative charge?
A) a proton
B) a neutron
C) an electron
D) both proton and neutron
A) a proton
B) a neutron
C) an electron
D) both proton and neutron

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
5
What is the reason that U-238 is different from U-235?
A) three more electrons
B) three more protons
C) three more neutrons
D) there is no difference
A) three more electrons
B) three more protons
C) three more neutrons
D) there is no difference

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
6
The average relative mass of an ozone molecule is 48.0 u.An ozone molecule contains only oxygen atoms.What does this molecular weight indicate about the formula of the ozone molecule?
A) It is monoatomic.
B) It is diatomic.
C) It is triatomic.
D) Impossible to determine
A) It is monoatomic.
B) It is diatomic.
C) It is triatomic.
D) Impossible to determine

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
7
Which of the following pairs are about equal in mass?
A) proton and electron
B) electron and neutron
C) proton and neutron
D) nucleus and surrounding electrons
A) proton and electron
B) electron and neutron
C) proton and neutron
D) nucleus and surrounding electrons

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
8
The symbols for elements with accepted names
A) consist of a single capital letter.
B) consist of a capital letter and a small letter.
C) consist of either a single capital letter or a capital letter and a small letter.
D) No answer is correct.
A) consist of a single capital letter.
B) consist of a capital letter and a small letter.
C) consist of either a single capital letter or a capital letter and a small letter.
D) No answer is correct.

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
9
Using whole numbers,determine the molecular weight of calcium hydroxide,Ca(OH)2.
A) 56
B) 57
C) 58
D) 74
A) 56
B) 57
C) 58
D) 74

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
10
How many electrons are in a neutral atom of carbon-13 (13C)?
A) 6
B) 18
C) 12
D) no way to tell
A) 6
B) 18
C) 12
D) no way to tell

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
11
Which of the following is located in the nucleus of an atom?
A) protons
B) neutrons
C) electrons
D) protons and neutrons
A) protons
B) neutrons
C) electrons
D) protons and neutrons

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
12
Determine the molecular weight of hydrogen peroxide,H2O2,in u (or amu).
A) 17.01
B) 18.02
C) 34.02
D) 33.01
A) 17.01
B) 18.02
C) 34.02
D) 33.01

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
13
Atoms are neutral.How can they have no charge?
A) equal numbers of protons and neutrons
B) equal numbers of protons and electrons
C) equal numbers of neutrons and electrons
D) any charge has been drained out of the atom
A) equal numbers of protons and neutrons
B) equal numbers of protons and electrons
C) equal numbers of neutrons and electrons
D) any charge has been drained out of the atom

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
14
Why is CaO the symbol for calcium oxide instead of CAO?
A) both can be the symbols for calcium oxide
B) both are incorrect;the symbol is cao
C) a capital letter means a new symbol
D) both are incorrect as the symbol should be CaOx
A) both can be the symbols for calcium oxide
B) both are incorrect;the symbol is cao
C) a capital letter means a new symbol
D) both are incorrect as the symbol should be CaOx

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
15
What is meant when the symbol C-12 (or 12C)is used?
A) the carbon atom weighs 12 grams
B) the carbon atom weighs 12 pounds
C) the carbon atom weighs 12 amu
D) the melting point of carbon is 12°C
A) the carbon atom weighs 12 grams
B) the carbon atom weighs 12 pounds
C) the carbon atom weighs 12 amu
D) the melting point of carbon is 12°C

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
16
How many protons are found in the nucleus of a boron-11 (11B)atom?
A) 11
B) 6
C) 5
D) 4
A) 11
B) 6
C) 5
D) 4

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
17
A molecular formula
A) is represented using the symbols of the elements in the formula.
B) is represented using a system of circles that contain different symbols.
C) cannot be represented conveniently using symbols for the elements.
D) is represented using words rather than symbols.
A) is represented using the symbols of the elements in the formula.
B) is represented using a system of circles that contain different symbols.
C) cannot be represented conveniently using symbols for the elements.
D) is represented using words rather than symbols.

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
18
What is the meaning of the two (2)in ethyl alcohol,C2H5OH?
A) all alcohol molecules contain two carbon atoms
B) there are two carbon atoms per molecule of ethyl alcohol
C) carbon is diatomic
D) all of these are correct statements
A) all alcohol molecules contain two carbon atoms
B) there are two carbon atoms per molecule of ethyl alcohol
C) carbon is diatomic
D) all of these are correct statements

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
19
How many neutrons are found in the nucleus of a boron-11 (11B)atom?
A) 11
B) 6
C) 5
D) 4
A) 11
B) 6
C) 5
D) 4

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
20
Refer to a periodic table and tell how many helium atoms (He)would be needed to get close to the same mass as an average oxygen atom (O).
A) six
B) four
C) twelve
D) one-fourth
A) six
B) four
C) twelve
D) one-fourth

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
21
Naturally occurring lithium (Li)consists of only two isotopes,Li-6 (6.02 u)and Li-7 (7.02 u),where the isotopic masses are given in parentheses.Use the periodic table and determine which isotope is present in the larger percentage in the natural element.
A) Li-6
B) Li-7
C) each is present at 50%
D) cannot be determined from the information available
A) Li-6
B) Li-7
C) each is present at 50%
D) cannot be determined from the information available

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
22
How many carbon atoms are contained in 5.50 g of ethane,C2H6?
A) 2.75 *10-22
B) 3.29 * 1024
C) 1.10 * 1023
D) 2.21 * 1023
A) 2.75 *10-22
B) 3.29 * 1024
C) 1.10 * 1023
D) 2.21 * 1023

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
23
The formula for dinitrogen monoxide is N2O.If a sample of the oxide was found to contain 0.0800 g of oxygen,how many grams of nitrogen would it contain?
A) 0.140
B) 0.280
C) 0.560
D) 0.0700
A) 0.140
B) 0.280
C) 0.560
D) 0.0700

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
24
How many silicon atoms (Si)are contained in a 12.5 g sample of silicon?
A) 2.68 * 1023
B) 5.83 *10-22
C) 1.35 *1024
D) 1.71 * 1021
A) 2.68 * 1023
B) 5.83 *10-22
C) 1.35 *1024
D) 1.71 * 1021

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
25
Naturally occurring neon (Ne)has the following isotopic composition (the mass of each isotope is given in parenthesis).Calculate the atomic weight of neon in u from these data: neon-20,90.92% (19.99 u);neon-21,0.257% (20.99 u);neon-22,8.82% (21.99 u)
A) 28.97
B) 37.62
C) 2017
D) 20.17
A) 28.97
B) 37.62
C) 2017
D) 20.17

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
26
How many atoms are contained in a sample of krypton,Kr,that weighs 8.38 g?
A) one Avogadro's number
B) one-tenth Avogadro's number
C) one
D) one-tenth
A) one Avogadro's number
B) one-tenth Avogadro's number
C) one
D) one-tenth

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
27
How many moles of N2O contain the same number of nitrogen atoms as 4.60 g of NO2?
A) 0.500
B) 0.0500
C) 0.100
D) 0.200
A) 0.500
B) 0.0500
C) 0.100
D) 0.200

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
28
How many moles of hydrogen molecules (H2)would be required to produce two moles of hydrogen peroxide (H2O2)?
A) 1
B) 2
C) 3
D) 4
A) 1
B) 2
C) 3
D) 4

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
29
What mass of arsenic (As)in grams contains the same number of atoms as 39.95 g of argon (Ar)?
A) 33.0
B) 74.92
C) 4.16
D) 149.84
A) 33.0
B) 74.92
C) 4.16
D) 149.84

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
30
Which element is approximately 65 percent of sulfuric acid by weight?
A) hydrogen
B) sulfur
C) oxygen
D) any of these
A) hydrogen
B) sulfur
C) oxygen
D) any of these

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
31
Which of the following has the largest mass?
A) 5.0 mol H2O
B) 3.5 mol NH3
C) 8.0 mol C
D) 6.0 mol C2H2
A) 5.0 mol H2O
B) 3.5 mol NH3
C) 8.0 mol C
D) 6.0 mol C2H2

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
32
The number of Cr atoms in a 26.0 g sample of chromium is x.How many atoms,expressed in terms of x,would be contained in 26.98 g of aluminum (Al)?
A) x
B) x/2
C) 2x
D) x+2
A) x
B) x/2
C) 2x
D) x+2

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
33
How many grams of iron (Fe)is contained in 15.8 g of Fe(OH)3?
A) 12.1
B) 8.26
C) 11.8
D) 5.21
A) 12.1
B) 8.26
C) 11.8
D) 5.21

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
34
How many hydrogen atoms are in 1.00 mole of NH3?
A) 3.00
B) 6.02 * 1023
C) 12.0 * 1023
D) 18.1 * 1023
A) 3.00
B) 6.02 * 1023
C) 12.0 * 1023
D) 18.1 * 1023

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
35
What is the mass number of a carbon-13 (13C)atom?
A) 13
B) 12
C) 6
D) 7
A) 13
B) 12
C) 6
D) 7

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
36
What is the number of hydrogen atoms in a 18.016 gram sample of water?
A) 2.000
B) 6.022 * 1023
C) 18.02
D) 1.204 * 1024
A) 2.000
B) 6.022 * 1023
C) 18.02
D) 1.204 * 1024

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
37
What is the weight percentage of nitrogen in urea,CN2H4O?
A) 46.7
B) 30.4
C) 32.6
D) 16.3
A) 46.7
B) 30.4
C) 32.6
D) 16.3

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
38
How many moles of oxygen atoms are in one mole of CO2?
A) 1
B) 2
C) 6.02 * 1023
D) 12.04 * 1023
A) 1
B) 2
C) 6.02 * 1023
D) 12.04 * 1023

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
39
The mass of mercury (Hg),a liquid at room temperature,is 200.6 amu/mol.A 200.6 gram sample of mercury is heated until it boils.What is the mass of one mole of mercury vapor (gas)?
A) <200.6 or it wouldn't be a gas
B) the same as Avogadro's number
C) the same as when it is a liquid
D) none of the answers are correct
A) <200.6 or it wouldn't be a gas
B) the same as Avogadro's number
C) the same as when it is a liquid
D) none of the answers are correct

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
40
Calculate the weight percentage of hydrogen in water.
A) 33.3
B) 66.7
C) 2.00
D) 11.1
A) 33.3
B) 66.7
C) 2.00
D) 11.1

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
41
The system of atomic mass units is based on
A) assigning 12C as weighing exactly 12 u & comparing other elements to it.
B) measuring the true mass of each subatomic particle.
C) comparing the differences in protons and electrons.
D) viewing how atoms are affected by electromagnetic fields.
A) assigning 12C as weighing exactly 12 u & comparing other elements to it.
B) measuring the true mass of each subatomic particle.
C) comparing the differences in protons and electrons.
D) viewing how atoms are affected by electromagnetic fields.

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
42
Approximately,how many atoms of beryllium would be required to equal the mass of 10 atoms of aluminum?
A) 3 atoms of beryllium
B) 10 atoms of beryllium
C) 30 atoms of beryllium
D) 4 atoms of beryllium
A) 3 atoms of beryllium
B) 10 atoms of beryllium
C) 30 atoms of beryllium
D) 4 atoms of beryllium

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
43
What is wrong with the following molecular formula: SOO (sulfur dioxide)?
A) OSO is the correct form
B) SO should be So
C) OO should be written as O2
D) OO should be written as O2
A) OSO is the correct form
B) SO should be So
C) OO should be written as O2
D) OO should be written as O2

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
44
An isotope of a given element has a mass number equal to twice the atomic number.This neutral isotope contains twelve electrons.This isotope is _____ .
A) magnesium-12.
B) magnesium-24.
C) chromium-24.
D) chromium-12.
A) magnesium-12.
B) magnesium-24.
C) chromium-24.
D) chromium-12.

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
45
How many electrons are found around the species below? 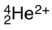
A) -2
B) 0
C) 2
D) 4
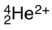
A) -2
B) 0
C) 2
D) 4

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
46
Consider the representation shown below.It should be classified as 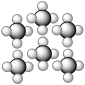
A) an element consisting of 6 atoms.
B) a compound containing atoms of two elements.
C) a homogenous mixture of two elements.
D) a homogenous mixture of two compounds.

A) an element consisting of 6 atoms.
B) a compound containing atoms of two elements.
C) a homogenous mixture of two elements.
D) a homogenous mixture of two compounds.

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
47
The weight % of S in K2SO4 is _____ .
A) 14.2%
B) 18.4%
C) 54.4%
D) 22.4%
A) 14.2%
B) 18.4%
C) 54.4%
D) 22.4%

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
48
Determine the number of electrons and protons in the element Tc.
A) 43 protons,43 electrons
B) 43 protons,56 electrons
C) 56 protons,43 electrons
D) 99 protons,43 electrons
A) 43 protons,43 electrons
B) 43 protons,56 electrons
C) 56 protons,43 electrons
D) 99 protons,43 electrons

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
49
A neutral isotope of an element contains 21 electrons and 24 neutrons.What is the following representation for this nucleus?
A)
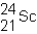
B)
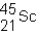
C)

D)
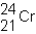
A)
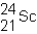
B)
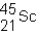
C)

D)
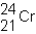

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
50
Write the formula for a compound consisting of 3 sodium atoms,1 phosphorus atom,and 4 oxygen atoms.
A) S3PO4
B) 3NaP4O
C) Na3P2(O2)
D) Na3PO4
A) S3PO4
B) 3NaP4O
C) Na3P2(O2)
D) Na3PO4

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
51
What is the number of moles of water in one liter of water if one gram of water takes up one milliliter of space?
A) 1
B) 18
C) 55.6
D) 1000
A) 1
B) 18
C) 55.6
D) 1000

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
52
Oxalic acid is found in many plants,like spinach and black tea.What is the mass of the carbon found in one mole of oxalic acid,H2C2O4?
A) 12.01 g of C
B) 24.02 g of Cl
C) 45.01 g of C
D) 90.02 g of C
A) 12.01 g of C
B) 24.02 g of Cl
C) 45.01 g of C
D) 90.02 g of C

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
53
Atoms that have the same atomic number but differ by mass number are called?
A) protons
B) neutrons
C) isotopes
D) positrons
A) protons
B) neutrons
C) isotopes
D) positrons

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
54
What is the molar mass of Ba3(PO4)2?
A) 232.30 g/mol
B) 327.27 g/mol
C) 369.63 g/mol
D) 601.92 g/mol
A) 232.30 g/mol
B) 327.27 g/mol
C) 369.63 g/mol
D) 601.92 g/mol

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
55
How many neutrons are in an atom that has a mass number of 75 and contains 35 protons?
A) 40
B) 35
C) 75
D) can't tell
A) 40
B) 35
C) 75
D) can't tell

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
55
PPP

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
56
If you have 3.011 *1023 atoms of carbon,what would you expect its mass to be?
A) 12.01 g
B) 6.005 g
C) 3.003 g
D) 1.000 g
A) 12.01 g
B) 6.005 g
C) 3.003 g
D) 1.000 g

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
56
PP

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
57
If calcium carbonate found in limestone is 40.0% calcium,how many grams of calcium are in 485 g of calcium carbonate?
A) 12.1 g of calcium
B) 291 g of calcium
C) 19,400 g of calcium
D) 194 g of calcium
A) 12.1 g of calcium
B) 291 g of calcium
C) 19,400 g of calcium
D) 194 g of calcium

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
57
PP

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
58
Which of the following correctly describes subatomic particles?
Mass: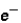
<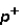
= n
Magnitude of charge: n <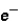
=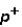
Location: outside nucleus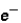
,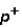
,inside nucleus n
A) 1 only
B) 2 only
C) 3 only
D) 1 and 2
Mass:
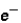
<
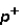
= n
Magnitude of charge: n <
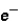
=
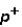
Location: outside nucleus
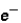
,
,inside nucleus n
A) 1 only
B) 2 only
C) 3 only
D) 1 and 2

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
59
How many moles of Na2Cr2O7 contain 14 moles of oxygen atoms?
A) 2 mol Na2Cr2O7
B) 14 mol Na2Cr2O7
C) 7 mol Na2Cr2O7
D) 1 mol Na2Cr2O7
A) 2 mol Na2Cr2O7
B) 14 mol Na2Cr2O7
C) 7 mol Na2Cr2O7
D) 1 mol Na2Cr2O7

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
60
The symbol for bromine is _____ .
A) B
B) Br
C) Be
D) none of these
A) B
B) Br
C) Be
D) none of these

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
61
The charge of the nucleus depends only on the atomic number.

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
62
Isotopes of the same element always have the same number of neutrons.

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
63
One mole of O3 weighs 16 grams.

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
64
One mole of an element would weigh the same as a mole of an isotope of the same element.

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
65
A mole of copper contains the same number of atoms as a mole of zinc.

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
66
The symbols for all of the elements are derived from the Latin names.

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
67
Individuals,ages 19-70,should include at least 1000 mg of calcium in their daily diet.What is the minimum number of one-gram calcium supplement tablets you would need to take each day to meet this requirement if a tablet is 40% by mass calcium?
A) 1
B) 2
C) 3
D) 4
A) 1
B) 2
C) 3
D) 4

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
68
Isotopes of the same element always have the same atomic mass.

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
69
One mole of an element would weigh the same as a mole of an isotope of the same element.

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
70
The most accurate way to determine atomic mass is with a mass spectrometer.

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
71
You discover a new element which consists of two isotopes.The first isotope,243X (mass = 242.45 u)comprises 40.000% of the total.The second isotope,248X (mass = 247.11 u)accounts for the rest.What would be the average atomic mass for your new element?
A) 242.45 u
B) 244.32 u
C) 245.25
D) 247.11
A) 242.45 u
B) 244.32 u
C) 245.25
D) 247.11

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
72
H2O2 contains equal parts by weight of hydrogen and oxygen.

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
73
The first letter of the symbol for each of the elements is the first letter of its English name.

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
74
Electrons do not make an important contribution to the mass of an atom.

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
75
One mole of H2O contains 2.0 grams of hydrogen.

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
76
The symbols for all of the elements always begin with a capital letter.

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
77
A diet is planned for a trip on a space ship and is lacking in milk,but is rich in turnips and broccoli.Such a diet could provide a sufficient amount of calcium for adults.

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck



