Deck 38: Atomic Physics
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/72
Play
Full screen (f)
Deck 38: Atomic Physics
1
The energy contained in a length of a 3.00-mW laser beam in vacuum is 1.80 * 10-10 J.What is the length of the beam?
A)2.0 m
B)24 m
C)15 m
D)18 m
A)2.0 m
B)24 m
C)15 m
D)18 m
18 m
2
How much energy is contained in a 3.00-mW laser beam of length 2.00 m that moves through vacuum?
A)2.00 * 10-11 J
B)1.80 * 10-10 J
C)2.00 * 10-8 J
D)1.80 * 10-7 J
A)2.00 * 10-11 J
B)1.80 * 10-10 J
C)2.00 * 10-8 J
D)1.80 * 10-7 J
2.00 * 10-11 J
3
The energy contained in laser beam of length 2.00 m that moves through vacuum is 1.80 * 10-10 J.What is the power of the laser?
A)3.0 mW
B)12 mW
C)23 mW
D)27 mW
A)3.0 mW
B)12 mW
C)23 mW
D)27 mW
27 mW
4
The wavelength of the  line of the Lyman group for the hydrogen spectrum is
line of the Lyman group for the hydrogen spectrum is
A)93 nm.
B)106 nm.
C)250 nm.
D)397 nm.
 line of the Lyman group for the hydrogen spectrum is
line of the Lyman group for the hydrogen spectrum isA)93 nm.
B)106 nm.
C)250 nm.
D)397 nm.

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
5
The energy required to ionize hydrogen when the electron is in the second level is _______ the energy required to ionize hydrogen when electron is in the first level.
A)twice
B)half of
C)four times
D)one fourth of
E)equal to
A)twice
B)half of
C)four times
D)one fourth of
E)equal to

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
6
If the period of rotation of electron in the Bohr hydrogen atom is 4.10 fs,which Bohr orbit does the electron occupy?
A)first
B)second
C)third
D)fourth
E)fifth
A)first
B)second
C)third
D)fourth
E)fifth

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
7
The period of rotation of electron on the 6-th Bohr orbit is __________ the period of rotation of electron on the 3- rd Bohr orbit.
A)equal to
B)twice
C)four times
D)six times
E)eight times
A)equal to
B)twice
C)four times
D)six times
E)eight times

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
8
The electron in a singly ionized helium atom,which consists of two protons,two neutrons and only one electron,makes a transition from the n = 2 state to the n = 1 state,emitting a single photon in the process.What is the energy of this photon?
A)3.40 eV
B)10.2 eV
C)13.6 eV
D)40.8 eV
E)None are correct.
A)3.40 eV
B)10.2 eV
C)13.6 eV
D)40.8 eV
E)None are correct.

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
9
The wavelength of the 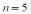 line of the Balmer group for the hydrogen spectrum is
line of the Balmer group for the hydrogen spectrum is
A)105 nm.
B)286 nm.
C)371 nm.
D)434 nm.
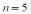 line of the Balmer group for the hydrogen spectrum is
line of the Balmer group for the hydrogen spectrum isA)105 nm.
B)286 nm.
C)371 nm.
D)434 nm.

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
10
A photon needed to excite an electron from the 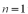 to the
to the 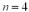 state.What is the energy of the photon?
state.What is the energy of the photon?
A)2.55 eV
B)6.61 eV
C)9.25 eV
D)12.8 eV
E)20.4 eV
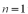 to the
to the 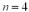 state.What is the energy of the photon?
state.What is the energy of the photon?A)2.55 eV
B)6.61 eV
C)9.25 eV
D)12.8 eV
E)20.4 eV

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
11
The frequency of the  line of the Lyman group for the hydrogen spectrum is
line of the Lyman group for the hydrogen spectrum is
A)9.88 * 1014 Hz
B)3.22 * 1015 Hz
C)1.21 * 1015 Hz
D)2.25 * 1015 Hz
 line of the Lyman group for the hydrogen spectrum is
line of the Lyman group for the hydrogen spectrum isA)9.88 * 1014 Hz
B)3.22 * 1015 Hz
C)1.21 * 1015 Hz
D)2.25 * 1015 Hz

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
12
Positronium consists of a bound state of an electron and a positron.A positron is exactly the same as an electron,except it has a charge of +e instead of -e.In the classical model,both particles orbit a point midway between them.The reduced mass of positronium is equal to
A)the mass of an electron.
B)the mass of an electron squared.
C)the square root of the mass of an electron.
D)None are correct.
A)the mass of an electron.
B)the mass of an electron squared.
C)the square root of the mass of an electron.
D)None are correct.

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
13
The Bohr model of hydrogen is based on the quantization of
A)electrical charge.
B)orbital angular momentum.
C)electric force.
D)magnetic force.
A)electrical charge.
B)orbital angular momentum.
C)electric force.
D)magnetic force.

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
14
The minimum orbital angular momentum quantum number for the 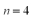 is
is
A)0.
B)1.
C)2.
D)3.
E)4.
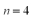 is
isA)0.
B)1.
C)2.
D)3.
E)4.

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
15
The frequency of the 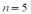 line of the Balmer group for the hydrogen spectrum is
line of the Balmer group for the hydrogen spectrum is
A)6.91 * 1014 Hz
B)7.33 * 1014 Hz
C)9.05 * 1014 Hz
D)8.54 * 1014 Hz
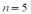 line of the Balmer group for the hydrogen spectrum is
line of the Balmer group for the hydrogen spectrum isA)6.91 * 1014 Hz
B)7.33 * 1014 Hz
C)9.05 * 1014 Hz
D)8.54 * 1014 Hz

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
16
The electron in a singly ionized helium atom,which consists of two protons,two neutrons and only one electron,makes a transition from the n = 2 state to the n = 1 state,emitting a single photon in the process.What is the energy of this photon?
A)3.40 eV
B)10.2 eV
C)13.6 eV
D)None are correct.
A)3.40 eV
B)10.2 eV
C)13.6 eV
D)None are correct.

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
17
An electron in the hydrogen atom makes a transition from the 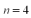 state to an nth state.The electron gains 0.50 eV of energy.What value of
state to an nth state.The electron gains 0.50 eV of energy.What value of 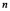 is the final state of the electron?
is the final state of the electron?
A)0
B)2
C)4
D)6
E)8
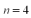 state to an nth state.The electron gains 0.50 eV of energy.What value of
state to an nth state.The electron gains 0.50 eV of energy.What value of 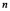 is the final state of the electron?
is the final state of the electron?A)0
B)2
C)4
D)6
E)8

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
18
The period of rotation of electron on the second Bohr orbit is
A)0.608 fs.
B)1.22 fs.
C)3.67 fs.
D)4.53 fs.
E)5.34 fs.
A)0.608 fs.
B)1.22 fs.
C)3.67 fs.
D)4.53 fs.
E)5.34 fs.

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
19
The maximum orbital angular momentum quantum number for the 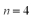 is
is
A)0.
B)1.
C)2.
D)3.
E)4.
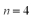 is
isA)0.
B)1.
C)2.
D)3.
E)4.

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
20
In the Bohr model of hydrogen,as the principle quantum number n increases,
A)the total energy increases and the kinetic energy decreases.
B)the potential energy increases and the speed decreases.
C)the orbital radius increases.
D)All are correct.
A)the total energy increases and the kinetic energy decreases.
B)the potential energy increases and the speed decreases.
C)the orbital radius increases.
D)All are correct.

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
21
A group of hydrogen-like atoms (atoms with only one electron)are in their ground state.Light of increasing intensity is shone at these atoms until the light becomes absorbed by the atoms.The longest wavelength of light when this occurs is 7.61 nm.What is the ground state energy of these atoms?
A)91.8 eV
B)13.6 eV
C)218 eV
D)122 eV
E)54 eV
A)91.8 eV
B)13.6 eV
C)218 eV
D)122 eV
E)54 eV

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
22
Find the wavelength of light emitted by a hydrogen atom when its electron makes the transition from the n = 5 state to the n = 2 state.
A)434 nm
B)652 nm
C)397 nm
D)486 nm
E)404 nm
A)434 nm
B)652 nm
C)397 nm
D)486 nm
E)404 nm

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
23
How many wave functions (different combinations of l and m)are possible in the n = 6 level of hydrogen?
A)6
B)26
C)30
D)36
E)720
A)6
B)26
C)30
D)36
E)720

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
24
What is the longest possible wavelength that a hydrogen atom will emit?
A)91 nm
B)122 nm
C)365 nm
D)1459 nm
E)There is no limit.
A)91 nm
B)122 nm
C)365 nm
D)1459 nm
E)There is no limit.

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
25
The muon has the same charge as an electron but has a mass that is 207 times greater.The negatively charged muon can bind to a proton to form a new type of hydrogen atom.How does the energy of a muon in the first excited state Em1 of a muonic hydrogen atom compare with the energy of an electron in the first excited state Ee1of a conventional hydrogen atom?
A)|Em1| |Ee1|
B)|Em1| 100|Ee1|
C)|Em1| |Ee1|/100
D)|Em1| 200|Ee1|
E)|Em1| |Ee1|/200
A)|Em1| |Ee1|
B)|Em1| 100|Ee1|
C)|Em1| |Ee1|/100
D)|Em1| 200|Ee1|
E)|Em1| |Ee1|/200

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
26
The Pfund series results from emission/absorption of photons due to transitions of electrons in a hydrogen to/from the n=5 energy level from/to higher energy levels.What is the shortest wavelength photon emitted in the Pfund series?
A)658 nm
B)2.29 µm
C)2.74 µm
D)3.68 µm
E)12.4 µm
A)658 nm
B)2.29 µm
C)2.74 µm
D)3.68 µm
E)12.4 µm

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
27
For an electron in the ground state of a hydrogen atom,what is the probability of finding that electron within a Bohr radius of the proton? The ground state wave function for hydrogen is 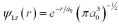 .
.
A)0.124
B)0.261
C)0.323
D)0.626
E)0.752
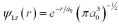 .
.A)0.124
B)0.261
C)0.323
D)0.626
E)0.752

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
28
The frequency of rotation of electron on the second Bohr orbit is
A)3.67 * 1014 Hz
B)8.20 * 1014 Hz
C)9.03 * 1014 Hz
D)6.44 * 1014 Hz
E)1.27 * 1015 Hz
A)3.67 * 1014 Hz
B)8.20 * 1014 Hz
C)9.03 * 1014 Hz
D)6.44 * 1014 Hz
E)1.27 * 1015 Hz

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
29
A hydrogen atom is in a quantum state with principal quantum number n = 3.The atom emits a photon with a wavelength of 660 nm.Determine the maximum possible orbital angular momentum of the electron after emission.
A)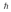
B)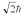
C)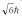
D)
E)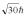
A)
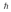
B)
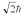
C)
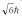
D)

E)
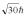

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
30
A group of hydrogen-like atoms (atoms with only one electron)are in their ground state.Light of increasing intensity is shone at these atoms until the light becomes absorbed by the atoms.The lowest frequency of light when this occurs is 2.22 * 1016 Hz.What is the ground state energy of these atoms?
A)91.8 eV
B)13.6 eV
C)218 eV
D)122 eV
E)54 eV
A)91.8 eV
B)13.6 eV
C)218 eV
D)122 eV
E)54 eV

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
31
In a "muonic" atom an electron is replaced by a muon of mass 105.66 MeV which orbits a proton.What is the ground-state energy of the muon in this type of atom?
A)-281 eV
B)-632 eV
C)-1264 eV
D)-2528 eV
E)-3254 eV
A)-281 eV
B)-632 eV
C)-1264 eV
D)-2528 eV
E)-3254 eV

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
32
The Pfund series results from emission/absorption of photons due to transitions of electrons in a hydrogen to/from the n=5 energy level from/to higher energy levels.What is the longest wavelength photon emitted in the Pfund series?
A)658 nm
B)2.29 µm
C)2.74 µm
D)7.47 µm
E)12.4 µm
A)658 nm
B)2.29 µm
C)2.74 µm
D)7.47 µm
E)12.4 µm

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
33
A group of hydrogen-like atoms (atoms with only one electron)are in their ground state.Light of increasing intensity is shone at these atoms until the light becomes absorbed by the atoms.The lowest energy of light when this occurs has energy of 40 eV.What is the ground state energy of these atoms?
A)10 eV
B)13.6 eV
C)30 eV
D)40 eV
E)53 eV
A)10 eV
B)13.6 eV
C)30 eV
D)40 eV
E)53 eV

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
34
A hydrogen atom is in a quantum state with principal quantum number n = 2.The atom absorbs a photon with a wavelength of 660 nm.Determine the maximum possible orbital angular momentum of the electron after absorption.
A)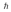
B)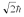
C)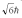
D)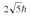
E)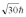
A)
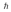
B)
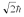
C)
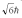
D)
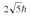
E)
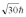

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
35
A hydrogen atom is in a quantum state with principal quantum number n = 9.The atom emits a photon and after emission its principal quantum number becomes 2.The wavelength of the emitted photon is
A)384 nm.
B)420 nm.
C)557 nm.
D)620 nm.
E)701 nm.
A)384 nm.
B)420 nm.
C)557 nm.
D)620 nm.
E)701 nm.

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
36
A hydrogen atom relaxes to a lower energy state by emitting a 2.55 eV photon.What is the lower energy state?
A)n = 1
B)n = 2
C)n = 3
D)n = 4
E)n = 5
A)n = 1
B)n = 2
C)n = 3
D)n = 4
E)n = 5

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
37
He+ is a helium atom with one electron missing.Treating this like a hydrogen atom,what is the first excited state of this atom?
A)-6.40 eV
B)-13.6 eV
C)-54.4 eV
D)-76.8 eV
E)-152.2 eV
A)-6.40 eV
B)-13.6 eV
C)-54.4 eV
D)-76.8 eV
E)-152.2 eV

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
38
A laser (with wavelength of 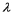 )excites the electron in a hydrogen atom from its ground state to its first excited state.What laser wavelength is required to raise the electron from the first excited state to its next lowest excited state?
)excites the electron in a hydrogen atom from its ground state to its first excited state.What laser wavelength is required to raise the electron from the first excited state to its next lowest excited state?
A)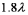
B)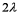
C)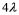
D)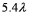
E)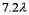
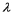 )excites the electron in a hydrogen atom from its ground state to its first excited state.What laser wavelength is required to raise the electron from the first excited state to its next lowest excited state?
)excites the electron in a hydrogen atom from its ground state to its first excited state.What laser wavelength is required to raise the electron from the first excited state to its next lowest excited state?A)
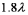
B)
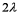
C)
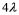
D)
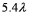
E)
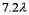

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
39
The angular frequency of rotation of electron on the second Bohr orbit is
A)3.67 * 1015 rad/sec
B)4.98 * 1015 rad/sec
C)5.34 * 1015 rad/sec
D)5.17 * 1015 rad/sec
E)5.71 * 1015 rad/sec
A)3.67 * 1015 rad/sec
B)4.98 * 1015 rad/sec
C)5.34 * 1015 rad/sec
D)5.17 * 1015 rad/sec
E)5.71 * 1015 rad/sec

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
40
Find the frequency of light emitted by a hydrogen atom when its electron makes the transition from the n = 5 state to the n = 2 state.
A)2.3 * 106 Hz
B)1.1 * 107 Hz
C)6.9 * 1014 Hz
D)3.3 * 1015 Hz
E)3.8 * 1015 Hz
A)2.3 * 106 Hz
B)1.1 * 107 Hz
C)6.9 * 1014 Hz
D)3.3 * 1015 Hz
E)3.8 * 1015 Hz

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
41
What is the energy of the orbiting electron in a hydrogen atom with a quantum number of 45?
A)+3.8 * 10-3 eV
B)-4.2 * 10-3 eV
C)-6.7 * 10-3 eV
D)+4.5 * 10-3 eV
A)+3.8 * 10-3 eV
B)-4.2 * 10-3 eV
C)-6.7 * 10-3 eV
D)+4.5 * 10-3 eV

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
42
If the energy of the orbiting electron in a hydrogen atom is -9.93 * 10-3 eV,what is its quantum number?
A)37
B)45
C)98
D)1370
A)37
B)45
C)98
D)1370

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
43
A photon of wavelength 1.17 m is emitted by a He+ when the electron jumps from the n = 7 state.What is the final state of the electron?
A)1
B)2
C)3
D)4
E)5
A)1
B)2
C)3
D)4
E)5

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
44
A UV laser emits light that has a wavelength 350 nm.If 1.23 * 1018 photons are emitted in 10 ns,what is the power of the laser?
A)77 MW
B)92 MW
C)83 MW
D)70 MW
E)5.0 MW
A)77 MW
B)92 MW
C)83 MW
D)70 MW
E)5.0 MW

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
45
What is the angular momentum of the orbiting electron in a hydrogen atom with quantum number of 45?
A)0.4 * 10-31 kg m2/s
B)1.6 * 10-31 kg m2/s
C)2.3 * 10-33 kg m2/s
D)4.7 * 10-33 kg m2/s
A)0.4 * 10-31 kg m2/s
B)1.6 * 10-31 kg m2/s
C)2.3 * 10-33 kg m2/s
D)4.7 * 10-33 kg m2/s

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
46
Find the energy difference between an excited state and the ground state of a system where the population ratio of the excited state to the ground state is 2.9 * 10-4.The temperature of the system is 300 K.
A)0.200 eV
B)0.191 eV
C)0.211 eV
D)0.223 eV
A)0.200 eV
B)0.191 eV
C)0.211 eV
D)0.223 eV

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
47
What is the energy of the photon required to knock the electron out of a singly ionized Helium atom where the electron was in the n = 4 state?
A)3.4 eV
B)1.7 eV
C)10.2 eV
D)51 eV
A)3.4 eV
B)1.7 eV
C)10.2 eV
D)51 eV

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
48
What is the wavelength of the photon emitted by a He+ when the electron jumps from the n = 7 to the n = 4 state?
A)8.8 m
B)3.4 m
C)4.4 m
D)896 nm
E)542 nm
A)8.8 m
B)3.4 m
C)4.4 m
D)896 nm
E)542 nm

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
49
The energy difference between an excited state and the ground state of a system is 0.2 eV.If the population ratio of the excited state to the ground state is 2.9 * 10-4,what is the temperature of the system?
A)300 K
B)330 K
C)285 K
D)315 K
A)300 K
B)330 K
C)285 K
D)315 K

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
50
A 5.0 MW UV laser emits light that has a wavelength 350 nm.If the duration of the pulse is 10 ns,how many photons are there in each pulse?
A)8.8 * 1016
B)12.3 * 1018
C)62.5 * 1020
D)89.1 * 1020
A)8.8 * 1016
B)12.3 * 1018
C)62.5 * 1020
D)89.1 * 1020

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
51
What is the longest wavelength in the Lyman series of hydrogen?
A)122 nm
B)312 nm
C)415 nm
D)535 nm
A)122 nm
B)312 nm
C)415 nm
D)535 nm

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
52
In the Bohr hydrogen atom,how many antinodes are there in the electron standing wave with a quantum number of 6?
A)12
B)6
C)3
D)5
A)12
B)6
C)3
D)5

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
53
A photon of wavelength 155 nm is absorbed by the electron in a hydrogen atom that was in the n = 2 state.What is the energy of the electron as it leaves the atom?
A)4.6 eV
B)1.2 eV
C)5.6 eV
D)2.2 eV
A)4.6 eV
B)1.2 eV
C)5.6 eV
D)2.2 eV

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
54
Identify the element that has the configuration 1s22s22p63s23p63d84s2.
A)Fe
B)Ni
C)Co
D)Au
A)Fe
B)Ni
C)Co
D)Au

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
55
Krypton has 36 electrons.What is the expected orbital angular momentum quantum number of the outermost electron for Krypton in its first excited state?
A)l = 0
B)l = 1
C)l = 2
D)l = 3
E)l = 4
A)l = 0
B)l = 1
C)l = 2
D)l = 3
E)l = 4

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
56
The radial probability density of a hydrogen atom tells the relative likelihood of finding the electron at a distance r from the nucleus.If ao is the Bohr radius,where is the electron in the 2p state most likely to be found?
A)r = ao
B)r = 2ao
C)r = 3ao
D)r = 4ao
A)r = ao
B)r = 2ao
C)r = 3ao
D)r = 4ao

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
57
A 5.0 UV laser emits light that has a wavelength 350 nm.If 1.23 * 1018 photons are emitted in a pulse,what is the duration of the pulse?
A)140 ns
B)89 ns
C)54 ns
D)110 ns
E)10 ns
A)140 ns
B)89 ns
C)54 ns
D)110 ns
E)10 ns

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
58
What are the quantum numbers n and l for a hydrogen atom with E = - (13.6/16)eV and L = 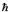 ?
?
A)n = 4,l = 2
B)n = 4,l = 6
C)n = 3,l = 3
D)n = 3,l = 6
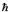 ?
?A)n = 4,l = 2
B)n = 4,l = 6
C)n = 3,l = 3
D)n = 3,l = 6

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
59
In a system where the energy difference between the excited state and the ground state is 0.2 eV,what is the population ratio of the excited state relative to the ground state at a temperature of 300 K?
A)4.4 * 10-4
B)2.9 * 10-4
C)1.02
D)0.001
A)4.4 * 10-4
B)2.9 * 10-4
C)1.02
D)0.001

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
60
A photon of wavelength 1.17 m is emitted by a He+ when the electron jumps to the n = 5 state.What was the initial state of the electron,prior to the jump?
A)6
B)7
C)8
D)9
E)10
A)6
B)7
C)8
D)9
E)10

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
61
What is the shortest wavelength photon that can be emitted by singly ionized helium (He+)?
A)23 nm
B)46 nm
C)54 nm
D)91 nm
E)365 nm
A)23 nm
B)46 nm
C)54 nm
D)91 nm
E)365 nm

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
62
About how many photons are emitted per second by a yellow (600 nm)60-mW laser?
A)2 * 1015
B)3 * 1019
C)3 * 1018
D)2 * 1012
E)2 * 1017
A)2 * 1015
B)3 * 1019
C)3 * 1018
D)2 * 1012
E)2 * 1017

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
63
How many electrons are allowed to inhabit the M-shell of an atom?
A)18
B)10
C)8
D)32
E)14
A)18
B)10
C)8
D)32
E)14

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
64
What is the angular momentum of an electron in an l = 4 level of the correct quantum mechanical hydrogen atom?
A)1/2 Js
B)0.5 * 105 Js
C)0.1 * 1025 Js
D)6.6 * 10-23 Js
E)4.6 * 10-34 Js
A)1/2 Js
B)0.5 * 105 Js
C)0.1 * 1025 Js
D)6.6 * 10-23 Js
E)4.6 * 10-34 Js

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
65
How much energy does a photon need to lift an electron from the n = 2 to the n = 8 level in the hydrogen atom?
A)1.0 eV
B)3.19 eV
C)7.2 eV
D)13.6 eV
E)22.4 eV
A)1.0 eV
B)3.19 eV
C)7.2 eV
D)13.6 eV
E)22.4 eV

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
66
An excited hydrogen atom emits a photon with an energy of 0.661 eV.What was the initial state of the hydrogen atom before emitting the photon?
A)n = 1
B)n = 2
C)n = 3
D)n = 4
E)n = 5
A)n = 1
B)n = 2
C)n = 3
D)n = 4
E)n = 5

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
67
What is the magnitude (in N)of the electric force between a proton and a (lowest energy)n = 1 electron in a hydrogen atom?
A)8.2 * 10-8
B)8.2 * 108
C)8.2 * 10-12
D)8.2 * 10-4
E)8.2 * 104
A)8.2 * 10-8
B)8.2 * 108
C)8.2 * 10-12
D)8.2 * 10-4
E)8.2 * 104

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
68
A hydrogen atom in its ground state absorbs a photon of energy 12.75 eV.What is its new value of n,the main quantum number in the Bohr model?
A)1
B)2
C)3
D)4
E)5
A)1
B)2
C)3
D)4
E)5

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
69
A collection of hydrogen atoms has been placed into the n = 4 excited state.Which of the following wavelengths of photons will NOT be emitted by the hydrogen atoms as they decay back to the ground state?
A)97 nm
B)490 nm
C)970 nm
D)1900 nm
A)97 nm
B)490 nm
C)970 nm
D)1900 nm

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
70
What is the radius of the n = 7 level of the Bohr hydrogen atom?
A)2.6 nm
B)9.3 nm
C)0.53 nm
D)0.053 nm
E)0.37 nm
A)2.6 nm
B)9.3 nm
C)0.53 nm
D)0.053 nm
E)0.37 nm

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
71
An excited hydrogen atom emits a photon with an energy of 0.661 eV.What was the final state of the hydrogen atom after emitting the photon?
A)n = 1
B)n = 2
C)n = 3
D)n = 4
E)n = 5
A)n = 1
B)n = 2
C)n = 3
D)n = 4
E)n = 5

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
72
For 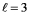 ,which value of m corresponds to a wave function that has its maximum probability in the x-y plane?
,which value of m corresponds to a wave function that has its maximum probability in the x-y plane?
A)l = 3,m = 0
B)l = 3,m = 1
C)l = 3,m = 2
D)l = 3,m = 3
E)l = 3,m = 4
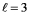 ,which value of m corresponds to a wave function that has its maximum probability in the x-y plane?
,which value of m corresponds to a wave function that has its maximum probability in the x-y plane?A)l = 3,m = 0
B)l = 3,m = 1
C)l = 3,m = 2
D)l = 3,m = 3
E)l = 3,m = 4

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck



