Deck 9: Bonding and Molecular Structure
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/97
Play
Full screen (f)
Deck 9: Bonding and Molecular Structure
1
An atom of Se has ____ valence electrons.
A) 2
B) 6
C) 4
D) 3
E) 8
A) 2
B) 6
C) 4
D) 3
E) 8
6
2
Which of the following is a correct Lewis structure for H2SO4?
A)
B)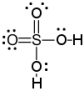
C)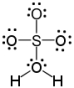
D)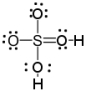
E)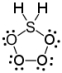
A)

B)

C)

D)

E)


3
Which of the following groups contains no ionic compounds?
A) HCN,NO2,Ca(NO3)2
B) PCl5,LiBr,Zn(OH)2
C) KOH,CCl4,SF4
D) NaH,CaF2,NaNH2
E) CH2O,H2S,NH3
A) HCN,NO2,Ca(NO3)2
B) PCl5,LiBr,Zn(OH)2
C) KOH,CCl4,SF4
D) NaH,CaF2,NaNH2
E) CH2O,H2S,NH3
CH2O,H2S,NH3
4
Which of the following statements is/are CORRECT?
1)Ionic bonds form when one or more valence electrons are transferred from one atom to another.
2)Covalent bonds involve sharing of electrons between atoms.
3)In most covalently bonded compounds,electrons are NOT shared equally between the atoms.
A) 1 only
B) 2 only
C) 3 only
D) 1 and 2
E) 1,2,and 3
1)Ionic bonds form when one or more valence electrons are transferred from one atom to another.
2)Covalent bonds involve sharing of electrons between atoms.
3)In most covalently bonded compounds,electrons are NOT shared equally between the atoms.
A) 1 only
B) 2 only
C) 3 only
D) 1 and 2
E) 1,2,and 3

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
5
In which pair do compounds exhibit predominantly ionic bonding?
A) KI and O3
B) NaF and H2O
C) PCl3 and HF
D) Na2SO3 and CH4
E) RbBr and CaO
A) KI and O3
B) NaF and H2O
C) PCl3 and HF
D) Na2SO3 and CH4
E) RbBr and CaO

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
6
Which of the following molecules or ions will have a Lewis structure most like that of phosphorus trichloride,PCl3?
A) ClO3-
B) SO3
C) CO32-
D) BF3
E) Cl2CO
A) ClO3-
B) SO3
C) CO32-
D) BF3
E) Cl2CO

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
7
Which of the following molecules or ions are isoelectronic: SO3,NF3,NO3-,CO32-?
A) SO3 and NF3
B) NF3 and CO32-
C) SO3,NF3 and CO32-
D) SO3,NO3- and CO32-
E) SO3,NF3,NO3-,and CO32-
A) SO3 and NF3
B) NF3 and CO32-
C) SO3,NF3 and CO32-
D) SO3,NO3- and CO32-
E) SO3,NF3,NO3-,and CO32-

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
8
Which of the following has a Lewis structure similar to CS2?
A) NH2-
B) O3
C) NO2-
D) SO2
E) ClO2-
A) NH2-
B) O3
C) NO2-
D) SO2
E) ClO2-

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
9
In the Lewis formula for sulfur dioxide,SO2,the number of lone pairs of electrons around the sulfur atom is
A) 0.
B) 1.
C) 2.
D) 3.
E) 4.
A) 0.
B) 1.
C) 2.
D) 3.
E) 4.

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
10
If two or more species have the same number of electrons,resulting in similar Lewis structures,they are said to be ____.
A) isoelectronic
B) resonant structures
C) ionic
D) neutral
E) covalent
A) isoelectronic
B) resonant structures
C) ionic
D) neutral
E) covalent

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
11
How many lone pairs of electrons are assigned to the iodine atom in ICl?
A) 0
B) 1
C) 2
D) 3
E) 4
A) 0
B) 1
C) 2
D) 3
E) 4

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
12
How many valence electrons are present in the Lewis formula for the hypochlorite ion, 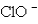 ?
?
A) 20
B) 12
C) 18
D) 14
E) 16
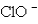 ?
?A) 20
B) 12
C) 18
D) 14
E) 16

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
13
Which of the following is a correct Lewis electron-dot formula for CO?
A)
B)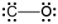
C)
D)
E)
A)

B)
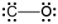
C)

D)

E)


Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
14
Which combination of atoms is most likely to produce a compound with ionic bonds?
A) B and C
B) S and O
C) N and F
D) Si and Cl
E) Mg and O
A) B and C
B) S and O
C) N and F
D) Si and Cl
E) Mg and O

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
15
The correct Lewis structure for Cl2CO is (the 2 Cl's and the O are bound to the C and not to each other):
A)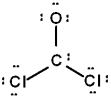
B)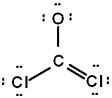
C)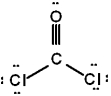
D)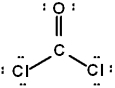
E) none of these
A)

B)

C)

D)

E) none of these

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
16
Which of the following is a correct Lewis structure for sulfate ion?
A)
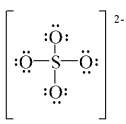
B)
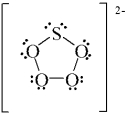
C)
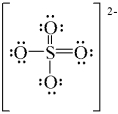
D)
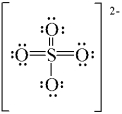
E)

A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
17
Which of the following statements is/are CORRECT?
1)Chemical reactions result in the gain,loss,or rearrangement of valence electrons.
2)For main group elements,the number of valence electrons equals eight minus the element's group number.
3)Core electrons are not involved in bonding or in chemical reactions.
A) 1 only
B) 2 only
C) 3 only
D) 1 and 3
E) 1,2,and 3
1)Chemical reactions result in the gain,loss,or rearrangement of valence electrons.
2)For main group elements,the number of valence electrons equals eight minus the element's group number.
3)Core electrons are not involved in bonding or in chemical reactions.
A) 1 only
B) 2 only
C) 3 only
D) 1 and 3
E) 1,2,and 3

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
18
What is the total number of electrons (both lone and bond pairs)in the Lewis structure of C2O42-?
A) 30
B) 34
C) 32
D) 46
E) 44
A) 30
B) 34
C) 32
D) 46
E) 44

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
19
Which combination of atoms is most likely to produce a compound with covalent bonds?
A) K and Br
B) Al and S
C) S and Cl
D) Sn and F
E) Li and I
A) K and Br
B) Al and S
C) S and Cl
D) Sn and F
E) Li and I

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
20
The following representation of an atom is called 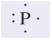
A) a Lewis dot structure.
B) an ion.
C) a structural formula.
D) an electrostatic potential map.
E) an ionic bond.
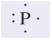
A) a Lewis dot structure.
B) an ion.
C) a structural formula.
D) an electrostatic potential map.
E) an ionic bond.

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
21
What is the formal charge on atom N in the following Lewis structure? 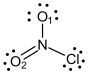
A) 2
B) 1
C) 0
D) -1
E) -2

A) 2
B) 1
C) 0
D) -1
E) -2

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
22
Which of the following exhibits resonance?
A) SCl6
B) H2O
C) SO2
D) NH3
E) none
A) SCl6
B) H2O
C) SO2
D) NH3
E) none

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
23
One resonance structure for OCN- ion is drawn below.What is the formal charge on each atom? 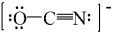
A) O atom = 0,C atom = 0,and N atom = 0
B) O atom = 0,C atom = 0,and N atom = -1
C) O atom = -1,C atom = 0,and N atom = 0
D) O atom = -1,C atom = -1,and N atom = +1
E) O atom = +1,C atom = 0,and N atom = -2
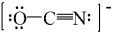
A) O atom = 0,C atom = 0,and N atom = 0
B) O atom = 0,C atom = 0,and N atom = -1
C) O atom = -1,C atom = 0,and N atom = 0
D) O atom = -1,C atom = -1,and N atom = +1
E) O atom = +1,C atom = 0,and N atom = -2

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
24
Which of the following is a correct Lewis structure for oxygen?
A)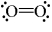
B)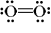
C)
D)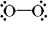
E)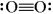
A)
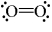
B)
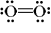
C)

D)
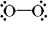
E)
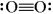

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
25
The molecule H2S has
A) 2 bonding pairs and 3 lone pairs
B) 2 bonding pairs and 2 lone pairs
C) 3 bonding pairs and 1 lone pair
D) 3 bonding pairs and 3 lone pairs
E) none of these
A) 2 bonding pairs and 3 lone pairs
B) 2 bonding pairs and 2 lone pairs
C) 3 bonding pairs and 1 lone pair
D) 3 bonding pairs and 3 lone pairs
E) none of these

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
26
All of the following Lewis structures of nitrogen oxides are possible EXCEPT 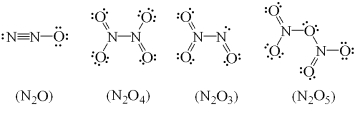
A) N2O.
B) N2O4.
C) N2O3.
D) N2O5.
E) All of the above are correct structures.

A) N2O.
B) N2O4.
C) N2O3.
D) N2O5.
E) All of the above are correct structures.

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
27
Which of the following molecules or ions does not have one or more resonance structures?
A) O3
B) OCN-
C) SO2
D) H2CO
E) NO3-
A) O3
B) OCN-
C) SO2
D) H2CO
E) NO3-

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
28
What is the formal charge on carbon in CH2Cl2?
A) +2
B) +1
C) 0
D) -1
E) -2
A) +2
B) +1
C) 0
D) -1
E) -2

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
29
The central atom in N2O is a nitrogen atom.This nitrogen atom is surrounded by
A) two single bonds and two lone pairs of electrons.
B) two single bonds and one lone pair of electrons.
C) one single bond,one double bond,and one lone pairs of electrons.
D) one single bond,one double bond,and one lone pair of electrons.
E) two double bonds and no lone pairs of electrons.
A) two single bonds and two lone pairs of electrons.
B) two single bonds and one lone pair of electrons.
C) one single bond,one double bond,and one lone pairs of electrons.
D) one single bond,one double bond,and one lone pair of electrons.
E) two double bonds and no lone pairs of electrons.

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
30
Which is the best Lewis structure of CH2FCO2H (connectivity correct as given)?
A)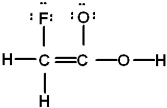
B)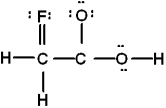
C)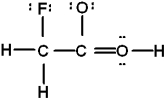
D)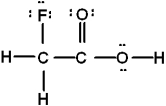
E) none of these is acceptable
A)
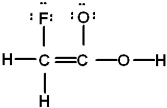
B)

C)

D)

E) none of these is acceptable

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
31
How many different molecules have the molecular formula C6H14?
A) 1
B) 2
C) 3
D) 4
E) 5
A) 1
B) 2
C) 3
D) 4
E) 5

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
32
Which of the following are possible Lewis structures for C2H6O? 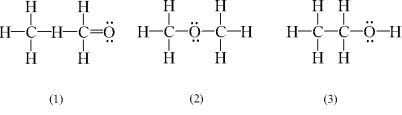
A) 1
B) 2
C) 3
D) 2 and 3
E) 1,2,and 3

A) 1
B) 2
C) 3
D) 2 and 3
E) 1,2,and 3

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
33
Which of the following is/are true concerning formal charge?
1)The formal charge of each individual atom in a molecule or ion is an actual atomic charge that can be experimentally determined.
2)The formal charge of each individual atom is always the same for each possible resonance form.
3)The sum of the formal charges of each atom in a molecule or ion equal the overall charge of the molecule or ion.
A) 1 only
B) 2 only
C) 3 only
D) 1 and 2
E) 1 and 3
1)The formal charge of each individual atom in a molecule or ion is an actual atomic charge that can be experimentally determined.
2)The formal charge of each individual atom is always the same for each possible resonance form.
3)The sum of the formal charges of each atom in a molecule or ion equal the overall charge of the molecule or ion.
A) 1 only
B) 2 only
C) 3 only
D) 1 and 2
E) 1 and 3

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
34
Which of the following are resonance structures for formate ion,HCO2-? 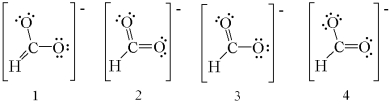
A) 1 and 2
B) 2 and 3
C) 3 and 4
D) 1,3,and 4
E) 2,3,and 4

A) 1 and 2
B) 2 and 3
C) 3 and 4
D) 1,3,and 4
E) 2,3,and 4

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
35
Which of the following is the correct Lewis dash formula for carbon diselenide?
A)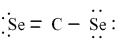
B)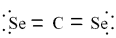
C)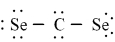
D)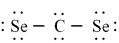
E)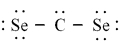
A)
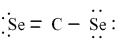
B)
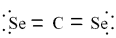
C)
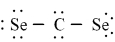
D)
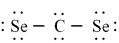
E)
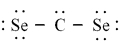

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
36
The nitrogen atom in cyanide ion,CN-,is surrounded by
A) one single bond and three lone pairs of electrons.
B) one double bond and one lone pair of electrons.
C) one double bond and two lone pairs of electrons.
D) one triple bond and one lone pair of electrons.
E) one triple bond and no lone pairs of electrons.
A) one single bond and three lone pairs of electrons.
B) one double bond and one lone pair of electrons.
C) one double bond and two lone pairs of electrons.
D) one triple bond and one lone pair of electrons.
E) one triple bond and no lone pairs of electrons.

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
37
What is the formal charge on each atom in dichloromethane,CH2Cl2?
A) C atom = 0,each H atom = 0,and each Cl atom = 0
B) C atom = -2,each H atom = +1,and two Cl atoms = 0
C) C atom = +4,each H = -1,and each Cl atom = -1
D) C atom = +4,each H = +1,and each Cl atom = -1
E) C atom = -4,each H = +1,and each Cl atom = +1
A) C atom = 0,each H atom = 0,and each Cl atom = 0
B) C atom = -2,each H atom = +1,and two Cl atoms = 0
C) C atom = +4,each H = -1,and each Cl atom = -1
D) C atom = +4,each H = +1,and each Cl atom = -1
E) C atom = -4,each H = +1,and each Cl atom = +1

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
38
What is the formal charge of the atom in the Lewis structure for isocyanate shown below? 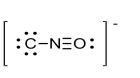
A) 0
B) (-2)
C) +1
D) (-1)
E) -3
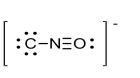
A) 0
B) (-2)
C) +1
D) (-1)
E) -3

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
39
How many hydrogen atoms are needed to complete the following hydrocarbon structure? 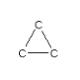
A) 4
B) 6
C) 2
D) 8
E) 3

A) 4
B) 6
C) 2
D) 8
E) 3

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
40
Which of the following is not a valid resonance structure for N3-?
A)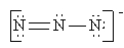
B)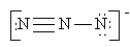
C)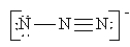
D)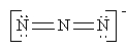
E) all are correct
A)
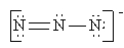
B)
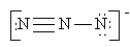
C)
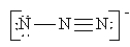
D)
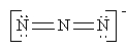
E) all are correct

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
41
What is the molecular geometry around an atom in a molecule or ion which is surrounded by zero lone pairs of electrons and four single bonds.
A) tetrahedral
B) linear
C) bent
D) trigonal pyramidal
E) trigonal planar
A) tetrahedral
B) linear
C) bent
D) trigonal pyramidal
E) trigonal planar

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
42
What is the molecular geometry around carbon atom C3? 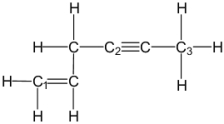
A) linear
B) tetrahedral
C) trigonal planar
D) trigonal pyramidal
E) square planar

A) linear
B) tetrahedral
C) trigonal planar
D) trigonal pyramidal
E) square planar

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
43
Use VSEPR theory to predict the electron-pair geometry and the molecular geometry of sulfur dioxide,SO2.
A) The electron-pair geometry is trigonal-planar,the molecular geometry is trigonal-planar.
B) The electron-pair geometry is trigonal-planar,the molecular geometry is bent.
C) The electron-pair geometry is tetrahedral,the molecular geometry is bent.
D) The electron-pair geometry is tetrahedral,the molecular geometry is linear.
E) The electron-pair geometry is trigonal-bipyramidal,the molecular geometry is linear.
A) The electron-pair geometry is trigonal-planar,the molecular geometry is trigonal-planar.
B) The electron-pair geometry is trigonal-planar,the molecular geometry is bent.
C) The electron-pair geometry is tetrahedral,the molecular geometry is bent.
D) The electron-pair geometry is tetrahedral,the molecular geometry is linear.
E) The electron-pair geometry is trigonal-bipyramidal,the molecular geometry is linear.

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
44
Use VSEPR theory to predict the electron-pair geometry and the molecular geometry of nitrogen trichloride,NCl3.
A) The electron-pair geometry is linear,the molecular geometry is linear.
B) The electron-pair geometry is trigonal-planar,the molecular geometry is trigonal-planar.
C) The electron-pair geometry is trigonal-planar,the molecular geometry is bent.
D) The electron-pair geometry is tetrahedral,the molecular geometry is tetrahedral.
E) The electron-pair geometry is tetrahedral,the molecular geometry is trigonal-pyramidal.
A) The electron-pair geometry is linear,the molecular geometry is linear.
B) The electron-pair geometry is trigonal-planar,the molecular geometry is trigonal-planar.
C) The electron-pair geometry is trigonal-planar,the molecular geometry is bent.
D) The electron-pair geometry is tetrahedral,the molecular geometry is tetrahedral.
E) The electron-pair geometry is tetrahedral,the molecular geometry is trigonal-pyramidal.

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
45
The Lewis structure of which of the following formula violates the octet rule?
A) SF6
B) NF3
C) OF2
D) HF
E) SiF4
A) SF6
B) NF3
C) OF2
D) HF
E) SiF4

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
46
What is the bond angle in a trigonal planar molecule or ion?
A) 109°
B) 180°
C) 90°
D) 72°
E) 120°
A) 109°
B) 180°
C) 90°
D) 72°
E) 120°

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
47
Which of the following elements is most likely to form a molecular structure that disobeys the octet rule?
A) B
B) C
C) N
D) O
E) F
A) B
B) C
C) N
D) O
E) F

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
48
Use VSEPR theory to predict the electron-pair geometry and the molecular geometry of boron tribromide,BBr3.
A) The electron-pair geometry is trigonal-pyramidal,the molecular geometry is trigonal-pyramidal.
B) The electron-pair geometry is trigonal-planar,the molecular geometry is trigonal-planar.
C) The electron-pair geometry is trigonal-planar,the molecular geometry is bent.
D) The electron-pair geometry is tetrahedral,the molecular geometry is trigonal-pyramidal.
E) The electron-pair geometry is trigonal-pyramidal,the molecular geometry is t-shaped.
A) The electron-pair geometry is trigonal-pyramidal,the molecular geometry is trigonal-pyramidal.
B) The electron-pair geometry is trigonal-planar,the molecular geometry is trigonal-planar.
C) The electron-pair geometry is trigonal-planar,the molecular geometry is bent.
D) The electron-pair geometry is tetrahedral,the molecular geometry is trigonal-pyramidal.
E) The electron-pair geometry is trigonal-pyramidal,the molecular geometry is t-shaped.

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
49
Which of the following species will have a Lewis structure most like that of IF4-?
A) XeF4
B) SO42-
C) PF4+
D) SF4
E) IO4-
A) XeF4
B) SO42-
C) PF4+
D) SF4
E) IO4-

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
50
What is the correct Lewis structure for IF3?
A)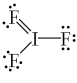
B)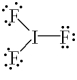
C)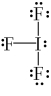
D)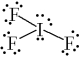
E)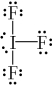
A)

B)

C)

D)

E)
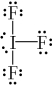

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
51
Which of the following elements is able to form a molecular structure that exceeds the octet rule?
A) F
B) N
C) C
D) B
E) Sb
A) F
B) N
C) C
D) B
E) Sb

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
52
Which of the following has an incomplete octet in its Lewis structure?
A) SO2
B) F2
C) NO2
D) ICl
E) CO2
A) SO2
B) F2
C) NO2
D) ICl
E) CO2

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
53
What is the correct Lewis structure for SF4?
A)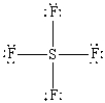
B)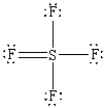
C)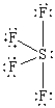
D)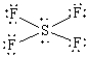
E)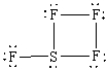
A)

B)

C)

D)
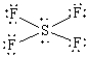
E)


Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
54
When you draw the Lewis structure for PF2Cl3,how many single bonds,double bonds,and lone pair electrons reside on the central phosphorus atom?
A) 5 single bonds,0 double bonds,0 lone pairs
B) 4 single bonds,1 double bond,0 lone pairs
C) 5 single bonds,0 double bonds,1 lone pair
D) 4 single bonds,1 double bond,1 lone pair
E) 3 single bonds,2 double bonds,0 lone pairs
A) 5 single bonds,0 double bonds,0 lone pairs
B) 4 single bonds,1 double bond,0 lone pairs
C) 5 single bonds,0 double bonds,1 lone pair
D) 4 single bonds,1 double bond,1 lone pair
E) 3 single bonds,2 double bonds,0 lone pairs

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
55
What is the electron-pair geometry around an atom in a molecule or ion which is surrounded by zero lone pairs of electrons and five single bonds.
A) trigonal bipyramidal
B) see-saw or distorted tetrahedron
C) T-shaped
D) linear
E) trigonal planar
A) trigonal bipyramidal
B) see-saw or distorted tetrahedron
C) T-shaped
D) linear
E) trigonal planar

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
56
What is the molecular geometry around oxygen atom O2? 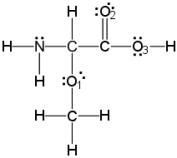
A) linear
B) trigonal pyramidal
C) trigonal planar
D) T-shaped
E) tetrahedral

A) linear
B) trigonal pyramidal
C) trigonal planar
D) T-shaped
E) tetrahedral

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
57
The central atom in SF6 is surrounded by
A) six single bonds and no lone pairs of electrons.
B) six single bonds and one lone pair of electrons.
C) six single bonds and two lone pairs of electrons.
D) five single bonds,one double bond,and one lone pair of electrons.
E) four single bonds,two double bonds,and no lone pairs of electrons.
A) six single bonds and no lone pairs of electrons.
B) six single bonds and one lone pair of electrons.
C) six single bonds and two lone pairs of electrons.
D) five single bonds,one double bond,and one lone pair of electrons.
E) four single bonds,two double bonds,and no lone pairs of electrons.

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
58
When both of the electrons in a molecular bond originate from the same atom,the bond is called a(n)
A) sigma bond.
B) coordinate covalent bond.
C) pi bond.
D) metallic bond.
E) ionic bond.
A) sigma bond.
B) coordinate covalent bond.
C) pi bond.
D) metallic bond.
E) ionic bond.

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
59
Which of the following molecules or ions are likely to be free radicals: N2O,NO,and NO2?
A) N2O only
B) NO only
C) NO2 only
D) N2O and NO
E) NO and NO2
A) N2O only
B) NO only
C) NO2 only
D) N2O and NO
E) NO and NO2

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
60
In the Lewis structure of SF6,how many lone pairs are around the central atom?
A) 0
B) 1
C) 2
D) 3
E) 4
A) 0
B) 1
C) 2
D) 3
E) 4

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
61
Place the following molecules in order from smallest to largest H-N-H bond angles: NH4+,NH3,and NH2-.
A) NH4+ < NH3 < NH2-
B) NH4+ < NH2- < NH3
C) NH2- < NH3 < NH4+
D) NH2- < NH4+ < NH3
E) NH3 < NH2- < NH4+
A) NH4+ < NH3 < NH2-
B) NH4+ < NH2- < NH3
C) NH2- < NH3 < NH4+
D) NH2- < NH4+ < NH3
E) NH3 < NH2- < NH4+

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
62
Which of the following bonds would be the most polar?
A) N-N
B) N-P
C) N-C
D) N-As
E) N-Ge
A) N-N
B) N-P
C) N-C
D) N-As
E) N-Ge

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
63
Based on electron geometries,which of the following molecules has the smallest bond angle between any two adjacent hydrogen atoms?
A) CH4
B) H2O
C) BH3
D) PH3
E) SF6
A) CH4
B) H2O
C) BH3
D) PH3
E) SF6

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
64
Choose which central atom in the following molecules is most electronegative.
A) PH3
B) CH4
C) H2S
D) H2O
E) NH3
A) PH3
B) CH4
C) H2S
D) H2O
E) NH3

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
65
Use VSEPR theory to predict the molecular geometry of BrO3-.
A) trigonal-pyramidal
B) trigonal-planar
C) bent
D) T-shaped
E) linear
A) trigonal-pyramidal
B) trigonal-planar
C) bent
D) T-shaped
E) linear

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
66
Rank the following covalent bonds in order of decreasing polarity:
C-H,N-H,O-H,F-H.
A) F-H,O-H,N-H,C-H
B) O-H,F-H,N-H,C-H
C) N-H,F-H,O-H,C-H
D) C-H,N-H,O-H,F-H
E) C-H,F-H,O-H,N-H
C-H,N-H,O-H,F-H.
A) F-H,O-H,N-H,C-H
B) O-H,F-H,N-H,C-H
C) N-H,F-H,O-H,C-H
D) C-H,N-H,O-H,F-H
E) C-H,F-H,O-H,N-H

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
67
What is the O-C-N bond angle in OCN-?
A) 90
B) 107
C) 109.5
D) 120
E) 180
A) 90
B) 107
C) 109.5
D) 120
E) 180

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
68
Which of the following compounds has polar covalent bonds: CCl4,Cl2,HCl,and KCl?
A) CCl4 only
B) Cl2 only
C) HCl and KCl
D) Cl2 and KCl
E) CCl4 and HCl
A) CCl4 only
B) Cl2 only
C) HCl and KCl
D) Cl2 and KCl
E) CCl4 and HCl

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
69
Use VSEPR theory to predict the molecular geometry of BrF5.
A) tetrahedral
B) see-saw
C) trigonal-bipyramidal
D) square-pyramidal
E) octahedral
A) tetrahedral
B) see-saw
C) trigonal-bipyramidal
D) square-pyramidal
E) octahedral

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
70
What are the approximate F-Br-F bond angles in BrF5?
A) 90 and 180
B) 109.5
C) 90 and 120
D) 120
E) 180
A) 90 and 180
B) 109.5
C) 90 and 120
D) 120
E) 180

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
71
Which of the following molecules is nonpolar?
A) sulfur dioxide,SO2
B) phosphorus trifluoride,PF3
C) hydrogen fluoride,HF
D) phosphorus pentafluoride,PF5
E) nitrogen trifluoride,NF3
A) sulfur dioxide,SO2
B) phosphorus trifluoride,PF3
C) hydrogen fluoride,HF
D) phosphorus pentafluoride,PF5
E) nitrogen trifluoride,NF3

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
72
Three non-equivalent Lewis structures for carbonyl sulfide,SCO,are given below.Use the concepts of formal charge and electronegativity to choose the structure that is the best representation.
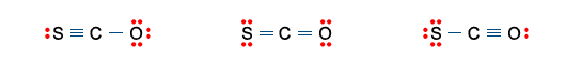
A B C
A) Structure A,because all the formal charges equal 0.
B) Structure B,because all the formal charges equal 0.
C) Structure C,because all the formal charges equal 0.
D) Structure A,because the negative formal charge resides on the most electronegative atom.
E) Structure C,because the negative formal charge resides on the most electronegative atom.
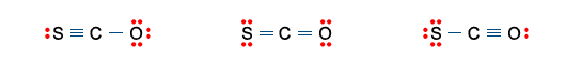
A B C
A) Structure A,because all the formal charges equal 0.
B) Structure B,because all the formal charges equal 0.
C) Structure C,because all the formal charges equal 0.
D) Structure A,because the negative formal charge resides on the most electronegative atom.
E) Structure C,because the negative formal charge resides on the most electronegative atom.

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
73
Use VSEPR theory to predict the molecular geometry around the carbon atom in formaldehyde,H2CO.
A) linear
B) bent
C) trigonal-planar
D) tetrahedral
E) octahedral
A) linear
B) bent
C) trigonal-planar
D) tetrahedral
E) octahedral

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
74
What are the approximate H-N-H bond angles in NH4+?
A) 109.5
B) 120
C) 109.5 and 120
D) 90
E) 90 and 120
A) 109.5
B) 120
C) 109.5 and 120
D) 90
E) 90 and 120

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
75
Use VSEPR theory to predict the electron-pair geometry and the molecular geometry of sulfur dioxide,XeF4.
A) The electron-pair geometry is tetrahedral,the molecular geometry is tetrahedral.
B) The electron-pair geometry is octahedral,the molecular geometry is tetrahedral.
C) The electron-pair geometry is tetrahedral,the molecular geometry is octahedral.
D) The electron-pair geometry is octahedral,the molecular geometry is square-planar.
E) The electron-pair geometry is square-planar,the molecular geometry is tetrahedral.
A) The electron-pair geometry is tetrahedral,the molecular geometry is tetrahedral.
B) The electron-pair geometry is octahedral,the molecular geometry is tetrahedral.
C) The electron-pair geometry is tetrahedral,the molecular geometry is octahedral.
D) The electron-pair geometry is octahedral,the molecular geometry is square-planar.
E) The electron-pair geometry is square-planar,the molecular geometry is tetrahedral.

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
76
Atoms having equal or nearly equal electronegativities are expected to form
A) no bonds
B) polar covalent bonds
C) nonpolar covalent bonds
D) ionic bonds
E) covalent bonds
A) no bonds
B) polar covalent bonds
C) nonpolar covalent bonds
D) ionic bonds
E) covalent bonds

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
77
Electronegativity is a measure of
A) the ability of a substance to conduct electricity.
B) the charge on a polyatomic cation.
C) the charge on a polyatomic anion.
D) the ability of an atom in a molecule to attract electrons to itself.
E) the oxidation number of an atom in a molecule or polyatomic anion.
A) the ability of a substance to conduct electricity.
B) the charge on a polyatomic cation.
C) the charge on a polyatomic anion.
D) the ability of an atom in a molecule to attract electrons to itself.
E) the oxidation number of an atom in a molecule or polyatomic anion.

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
78
Use VSEPR theory to predict the molecular geometry around the nitrogen atom in nitrite ion,NO2-.
A) trigonal-planar
B) tetrahedral
C) trigonal-pyramidal
D) T-shaped
E) bent
A) trigonal-planar
B) tetrahedral
C) trigonal-pyramidal
D) T-shaped
E) bent

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
79
Use VSEPR theory to predict the molecular geometry around either carbon atom in acetylene,C2H2.
A) linear
B) bent
C) trigonal-planar
D) tetrahedral
E) octahedral
A) linear
B) bent
C) trigonal-planar
D) tetrahedral
E) octahedral

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
80
What are the approximate O-S-O bond angles in SO32-?
A) 90
B) 109.5
C) 120
D) 180
E) 90 and 180
A) 90
B) 109.5
C) 120
D) 180
E) 90 and 180

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck



