Deck 13: The Chemistry of Solids
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/77
Play
Full screen (f)
Deck 13: The Chemistry of Solids
1
What is the distance,in atomic radii,along any edge of a body-centered cubic unit cell?
A)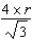
B) 2 r
C) 4 r
D)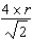
E) r
A)
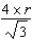
B) 2 r
C) 4 r
D)
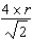
E) r
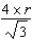
2
Gold (atomic mass 197.0 g/mol),with an atomic radius of 144.2 pm,crystallizes in a face-centered cubic lattice.What is the density of gold?
A) 9.65 g/cm3
B) 1.21 g/cm3
C) 4.82 g/cm3
D) 2.41 g/cm3
E) 19.3 g/cm3
A) 9.65 g/cm3
B) 1.21 g/cm3
C) 4.82 g/cm3
D) 2.41 g/cm3
E) 19.3 g/cm3
19.3 g/cm3
3
Which of the following statements is/are CORRECT?
1)Of the four unit cell structures in which metals crystallize,three are based on the cubic unit cell and the fourth is the hexagonal unit cell.
2)Transition metals with high densities,especially those in period 6,preferentially crystallize with a primitive cubic cell structure.
3)The preferred crystal structure of the Group 1A metals is face-centered cubic.
A) 1 only
B) 2 only
C) 3 only
D) 1 and 2
E) 1,2,and 3
1)Of the four unit cell structures in which metals crystallize,three are based on the cubic unit cell and the fourth is the hexagonal unit cell.
2)Transition metals with high densities,especially those in period 6,preferentially crystallize with a primitive cubic cell structure.
3)The preferred crystal structure of the Group 1A metals is face-centered cubic.
A) 1 only
B) 2 only
C) 3 only
D) 1 and 2
E) 1,2,and 3
1 only
4
Which one of the following statements is INCORRECT?
A) Polonium is the only metal that has a primitive cubic lattice.
B) The lattice structure of the alkali metals is body-centered cubic.
C) Many metals can crystallize in more than one type of crystal lattice.
D) Metals with a body-centered cubic lattice contain a net of four metal atoms per unit cell.
E) A hexagonal close packed structure is not an example of a cubic unit cell.
A) Polonium is the only metal that has a primitive cubic lattice.
B) The lattice structure of the alkali metals is body-centered cubic.
C) Many metals can crystallize in more than one type of crystal lattice.
D) Metals with a body-centered cubic lattice contain a net of four metal atoms per unit cell.
E) A hexagonal close packed structure is not an example of a cubic unit cell.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
5
Potassium crystallizes in the body-centered cubic system.If the edge of the unit cell is 524 pm,what is the radius of a potassium atom in picometers?
A) 227 pm
B) 908 pm
C) 1210 pm
D) 1050 pm
E) 98.3 pm
A) 227 pm
B) 908 pm
C) 1210 pm
D) 1050 pm
E) 98.3 pm

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
6
Nickel has a face-centered cubic cell,and its density is 8.90 g/cm3.What is the radius (in pm)of a nickel atom? (The molar mass of nickel is 58.69 g/mol)
A) 62.3 pm
B) 88.1 pm
C) 125 pm
D) 249 pm
E) 535 pm
A) 62.3 pm
B) 88.1 pm
C) 125 pm
D) 249 pm
E) 535 pm

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
7
For a metal that crystallizes in a body-centered cubic unit cell,what percentage of the space in the cell is occupied by the metal atoms?
A) 47%
B) 52%
C) 68%
D) 74%
E) 87%
A) 47%
B) 52%
C) 68%
D) 74%
E) 87%

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
8
Rhodium crystallizes with a face-centered cubic unit cell.If the edge length of the unit cell is 379 pm,what is the radius of a rhodium atom in picometers?
A) 134 pm
B) 536 pm
C) 1070 pm
D) 309 pm
E) 47.4 pm
A) 134 pm
B) 536 pm
C) 1070 pm
D) 309 pm
E) 47.4 pm

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
9
In any cubic lattice an atom lying at the face of a unit cell is shared equally by how many unit cells?
A) 2
B) 1
C) 4
D) 8
E) 6
A) 2
B) 1
C) 4
D) 8
E) 6

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
10
Niobium crystallizes in a body-centered cubic lattice.If the radius of niobium is 146 pm,what is the unit cell edge length?
A) 337 pm
B) 292 pm
C) 195 pm
D) 146 pm
E) 63.2 pm
A) 337 pm
B) 292 pm
C) 195 pm
D) 146 pm
E) 63.2 pm

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
11
Which of the following statements concerning the cubic unit cell is/are CORRECT?
1)For cubic unit cells,three cell symmetries occur: primitive cubic,face-centered cubic,and body-centered cubic.
2)The cell edges of a cubic unit cell are all equal in length.
3)The corner angles of a cubic cell are 90 .
A) 1 only
B) 2 only
C) 3 only
D) 2 and 3
E) 1,2,and 3
1)For cubic unit cells,three cell symmetries occur: primitive cubic,face-centered cubic,and body-centered cubic.
2)The cell edges of a cubic unit cell are all equal in length.
3)The corner angles of a cubic cell are 90 .
A) 1 only
B) 2 only
C) 3 only
D) 2 and 3
E) 1,2,and 3

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
12
Which of the following statements concerning a metal crystallized in a face-centered cubic cell is/are CORRECT?
1)One metal atom is located on each face of the unit cell,where it is shared equally between four unit cells.
2)One metal atom is located at the center of the unit cell.
3)A metal atom is located at each of the eight lattice points,where it is shared equally between eight unit cells.
A) 1 only
B) 2 only
C) 3 only
D) 1 and 3
E) 1,2 and 3
1)One metal atom is located on each face of the unit cell,where it is shared equally between four unit cells.
2)One metal atom is located at the center of the unit cell.
3)A metal atom is located at each of the eight lattice points,where it is shared equally between eight unit cells.
A) 1 only
B) 2 only
C) 3 only
D) 1 and 3
E) 1,2 and 3

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
13
What is the distance,in atomic radii,across a diagonal face of a face-centered unit cell?
A)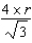
B) 2 r
C) 4 r
D)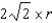
E) r
A)
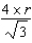
B) 2 r
C) 4 r
D)
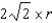
E) r

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
14
Polonium (atomic mass 209.0 g/mol)crystallizes in a primitive cubic unit cell.If the density of polonium is 9.15 g/cm3,what is the radius of a polonium atom (in pm)?
A) 168 pm
B) 238 pm
C) 336 pm
D) 475 pm
E) 672 pm
A) 168 pm
B) 238 pm
C) 336 pm
D) 475 pm
E) 672 pm

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
15
Gold crystallizes in a face-centered cubic lattice with an edge length of 407.8 pm.What is the density of gold?
A) 1.21 g/cm3
B) 4.82 g/cm3
C) 2.41 g/cm3
D) 9.65 g/cm3
E) 19.3 g/cm3
A) 1.21 g/cm3
B) 4.82 g/cm3
C) 2.41 g/cm3
D) 9.65 g/cm3
E) 19.3 g/cm3

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
16
If a metal crystallizes in a body-centered cubic lattice,each metal atom has ____ "nearest neighbors."
A) 3
B) 4
C) 6
D) 8
E) 12
A) 3
B) 4
C) 6
D) 8
E) 12

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
17
Arrange the three common unit cells in order from least dense to most dense packing.
A) face-centered cubic < body-centered cubic < primitive cubic
B) primitive cubic < body-centered cubic < face-centered cubic
C) primitive cubic < face-centered cubic < body-centered cubic
D) body-centered cubic < primitive cubic < face-centered cubic
E) body-centered cubic < face-centered cubic < primitive cubic
A) face-centered cubic < body-centered cubic < primitive cubic
B) primitive cubic < body-centered cubic < face-centered cubic
C) primitive cubic < face-centered cubic < body-centered cubic
D) body-centered cubic < primitive cubic < face-centered cubic
E) body-centered cubic < face-centered cubic < primitive cubic

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
18
Which of the following concerning the 2-D lattice provided below is/are correct?
1)One possible unit cell contains a single A and a single .
2)More than one unit cell which reproduces this lattice is possible.
3)One possible unit cell contains four A's and four 's.
A) 1 only
B) 2 only
C) 3 only
D) 1 and 2
E) 1,2,and 3
1)One possible unit cell contains a single A and a single .
2)More than one unit cell which reproduces this lattice is possible.
3)One possible unit cell contains four A's and four 's.

A) 1 only
B) 2 only
C) 3 only
D) 1 and 2
E) 1,2,and 3

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
19
Which equation represents the number of atoms in a body-centered cubic unit cell?
A)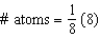
B)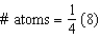
C)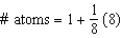
D)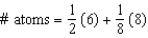
E)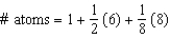
A)
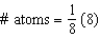
B)
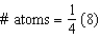
C)
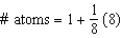
D)
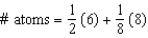
E)
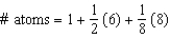

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
20
The space-filling representation provided below is an example of a _____ unit cell,which contains _____ atom(s). 
A) primitive cubic,1 atom
B) body centered cubic,2 atoms
C) face centered cubic,4 atoms
D) primitive cubic,8 atoms
E) body centered cubic,3 atoms

A) primitive cubic,1 atom
B) body centered cubic,2 atoms
C) face centered cubic,4 atoms
D) primitive cubic,8 atoms
E) body centered cubic,3 atoms

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
21
Copper crystallizes in a face-centered cubic lattice.The radius of a copper atom is 128 pm.What is the edge length of the unit cell?
A) 362 pm
B) 256 pm
C) 512 pm
D) 272 pm
E) 128 pm
A) 362 pm
B) 256 pm
C) 512 pm
D) 272 pm
E) 128 pm

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
22
If an ionic solid has a face-centered cubic lattice of anions (Xn-)and all the octahedral holes are occupied by metal cations (Mm+),what is the formula for the compound?
A) M2X
B) MX
C) MX2
D) M2X3
E) M3X2
A) M2X
B) MX
C) MX2
D) M2X3
E) M3X2

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
23
What is the formula of the compound represented by the unit cell provided below? 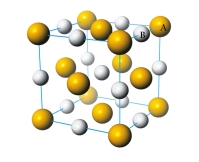
A) AB
B) AB2
C) AB3
D) A2B3
E) A2B4

A) AB
B) AB2
C) AB3
D) A2B3
E) A2B4

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
24
A metal crystallizes in a face-centered cubic lattice.The radius of the atom is 125 pm and the density of the element is 8.91 g/cm3.What is the identity of the metal?
A) Yb
B) Cu
C) Ca
D) Sr
E) Ni
A) Yb
B) Cu
C) Ca
D) Sr
E) Ni

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
25
Calcium crystallizes in a face-centered cubic lattice.The density of the calcium is 1.55 g/cm3.What is the volume of a single unit cell?
A)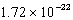 cm3
cm3
B)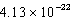 cm3
cm3
C)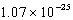 cm3
cm3
D)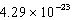 cm3
cm3
E)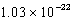 cm3
cm3
A)
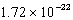 cm3
cm3B)
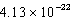 cm3
cm3C)
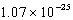 cm3
cm3D)
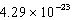 cm3
cm3E)
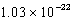 cm3
cm3
Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
26
Rubidium iodide (molar mass 212.4 g/mol)has a face-centered cubic unit cell with rubidium ions in octahedral holes.If the radius of iodide ion is 219 pm and the density of RbI is 3.55 g/cm3,what is the radius of the rubidium ion (in pm)?
A) 112 pm
B) 149 pm
C) 181 pm
D) 297 pm
E) 419 pm
A) 112 pm
B) 149 pm
C) 181 pm
D) 297 pm
E) 419 pm

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
27
The metal potassium crystallizes in a body-centered cubic lattice.If the density of potassium is 0.856 g/cm3,what is the unit cell volume?
A) 3.88 106 pm3
B) 3.64 104 pm3
C) 1.52 108 pm3
D) 7.59 107 pm3
E) 7.27 104 pm3
A) 3.88 106 pm3
B) 3.64 104 pm3
C) 1.52 108 pm3
D) 7.59 107 pm3
E) 7.27 104 pm3

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
28
Lithium chloride crystallizes in a face-centered cubic unit cell with chloride ions occupying the lattice points and lithium ions occupying octahedral holes.How many chloride ions surround each lithium ion in LiCl?
A) 1
B) 4
C) 6
D) 8
E) 12
A) 1
B) 4
C) 6
D) 8
E) 12

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
29
The metal barium crystallizes in a body-centered cubic lattice.If the density of barium is 3.51 g/cm3,what is the atomic radius of barium?
A) 15.1 pm
B) 174 pm
C) 42.5 pm
D) 19.0 pm
E) 219 pm
A) 15.1 pm
B) 174 pm
C) 42.5 pm
D) 19.0 pm
E) 219 pm

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
30
Chromium (atomic mass 52.00 g/mol)crystallizes in a body-centered cubic unit cell.If the length of an edge of the unit cell is 289 pm,what is the density (in g/cm3)of chromium?
A) 3.58 g/cm3
B) 7.15 g/cm3
C) 13.7 g/cm3
D) 14.3 g/cm3
E) 21.3 g/cm3
A) 3.58 g/cm3
B) 7.15 g/cm3
C) 13.7 g/cm3
D) 14.3 g/cm3
E) 21.3 g/cm3

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
31
Calcium sulfide has a face-centered cubic unit cell with calcium ions in octahedral holes.How many ions of each element are contained in each unit cell?
A) 1 calcium ions; 1 sulfide ions
B) 2 calcium ions; 2 sulfide ions
C) 2 calcium ions; 4 sulfide ions
D) 4 calcium ions; 2 sulfide ions
E) 4 calcium ions; 4 sulfide ions
A) 1 calcium ions; 1 sulfide ions
B) 2 calcium ions; 2 sulfide ions
C) 2 calcium ions; 4 sulfide ions
D) 4 calcium ions; 2 sulfide ions
E) 4 calcium ions; 4 sulfide ions

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
32
A metal crystallizes in a face-centered cubic lattice.The radius of the atom is 214 pm and the density of the element is 2.63 g/cm3.What is the volume of the unit cell?
A) 4.11 107 pm3
B) 3.80 109 pm3
C) 2.22 108 pm3
D) 9.80 106 pm3
E) 1.64 108 pm3
A) 4.11 107 pm3
B) 3.80 109 pm3
C) 2.22 108 pm3
D) 9.80 106 pm3
E) 1.64 108 pm3

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
33
Calcium oxide has a face centered cubic unit cell of oxide ions with the calcium in octahedral holes.If the radius of Ca2+ is 106 pm and the density of CaO is 3.34 g/cm3,what is the radius of the oxide ion? (100 cm = 1 1012 pm)
A) 160 pm
B) 120 pm
C) 106 pm
D) 135 pm
E) 269 pm
A) 160 pm
B) 120 pm
C) 106 pm
D) 135 pm
E) 269 pm

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
34
Magnesium sulfide (molar mass 56.37 g/mol)has a face-centered cubic unit cell with magnesium ions in octahedral holes.The ionic radii of magnesium ions and sulfide ions are 79 pm and 184 pm,respectively.What is the density of MgS (in g/cm3)?
A) 1.29 g/cm3
B) 2.57 g/cm3
C) 3.64 g/cm3
D) 5.15 g/cm3
E) 7.28 g/cm3
A) 1.29 g/cm3
B) 2.57 g/cm3
C) 3.64 g/cm3
D) 5.15 g/cm3
E) 7.28 g/cm3

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
35
In what type of unit cell are the "A" atoms arranged in the unit cell provided? 2 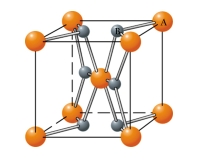
A) primitive cubic
B) body-centered cubic
C) face-centered cubic

A) primitive cubic
B) body-centered cubic
C) face-centered cubic

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
36
Which of the following statements is/are CORRECT? If an ionic compound with the formula MX forms a face-centered cubic unit cell with the anions (Xn-)at the lattice points,the cations (Mn+)may occupy
1)one fourth of the tetrahedral holes in each unit cell.
2)all of the octahedral holes in each unit cell.
3)the center of each face in each unit cell.
A) 1 only
B) 2 only
C) 3 only
D) 1 and 2
E) 1,2,and 3
1)one fourth of the tetrahedral holes in each unit cell.
2)all of the octahedral holes in each unit cell.
3)the center of each face in each unit cell.
A) 1 only
B) 2 only
C) 3 only
D) 1 and 2
E) 1,2,and 3

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
37
If an ionic compound with the formula MX2 forms a face-centered cubic unit cell with the cations (M2n+)at the lattice points,the anions (Xn-)will occupy
A) all of the tetrahedral holes in each unit cell.
B) half of the tetrahedral holes in each unit cell.
C) all of the octahedral holes in each unit cell.
D) the center of each face in each unit cell.
E) the cubic hole in the center of the each unit cell.
A) all of the tetrahedral holes in each unit cell.
B) half of the tetrahedral holes in each unit cell.
C) all of the octahedral holes in each unit cell.
D) the center of each face in each unit cell.
E) the cubic hole in the center of the each unit cell.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
38
The metal chromium crystallizes in a body-centered cubic lattice.If the density of chromium is 7.14 g/cm3,what is the unit cell edge length?
A) 289 pm
B) 77.0 pm
C) 77.5 pm
D) 61.1 pm
E) 230 pm
A) 289 pm
B) 77.0 pm
C) 77.5 pm
D) 61.1 pm
E) 230 pm

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
39
If an ionic compound with the formula MX forms a primitive cubic unit cell with the anions (Xn-)at the lattice points,the cations (M2n+)will occupy
A) all of the tetrahedral holes in each unit cell.
B) half of the tetrahedral holes in each unit cell.
C) the cubic hole in the center of the each unit cell.
D) the center of each face in each unit cell.
E) all of the octahedral holes in each unit cell.
A) all of the tetrahedral holes in each unit cell.
B) half of the tetrahedral holes in each unit cell.
C) the cubic hole in the center of the each unit cell.
D) the center of each face in each unit cell.
E) all of the octahedral holes in each unit cell.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
40
Cesium bromide crystallizes in a primitive cubic unit cell with bromide ions at the lattice points.The cesium ions occupy cubic holes.How many bromide ions surround each cesium ion in cesium bromide?
A) 1
B) 2
C) 4
D) 8
E) 12
A) 1
B) 2
C) 4
D) 8
E) 12

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
41
Which of the following is/are physical properties of amorphous solids?
1)Amorphous solids have well defined melting points.
2)At the particulate level,amorphous solids do not have long range order.
3)Polymeric materials never form amorphous solids.
A) 1 only
B) 2 only
C) 3 only
D) 1 and 3
E) 2 and 3
1)Amorphous solids have well defined melting points.
2)At the particulate level,amorphous solids do not have long range order.
3)Polymeric materials never form amorphous solids.
A) 1 only
B) 2 only
C) 3 only
D) 1 and 3
E) 2 and 3

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
42
In metals,there are not enough electrons to fill all of the electronic energy levels.At 0 K,the highest energy level filled is referred to as the ________.
A) valence band
B) conduction band
C) free energy
D) Fermi level
E) band gap
A) valence band
B) conduction band
C) free energy
D) Fermi level
E) band gap

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
43
Which of the following statements concerning semiconductors is/are CORRECT?
1)The conduction of electricity in p-type semiconductors occurs by the movement of electrons in the conduction band.
2)Doping an intrinsic semiconductor,such as silicon,with a Group 3A element will produce a p-type semiconductor.
3)An n-type semiconductor uses the movement of positive holes in the valence band to conduct electricity.
A) 1 only
B) 2 only
C) 3 only
D) 1 and 2
E) 1,2 and 3
1)The conduction of electricity in p-type semiconductors occurs by the movement of electrons in the conduction band.
2)Doping an intrinsic semiconductor,such as silicon,with a Group 3A element will produce a p-type semiconductor.
3)An n-type semiconductor uses the movement of positive holes in the valence band to conduct electricity.
A) 1 only
B) 2 only
C) 3 only
D) 1 and 2
E) 1,2 and 3

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
44
Which of the compounds below is not an example of a molecular solid?
A) I2(s)
B) SiO2(s)
C) CO2(s)
D) H2O(s)
E) C25H52(s)
A) I2(s)
B) SiO2(s)
C) CO2(s)
D) H2O(s)
E) C25H52(s)

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
45
The lattice energy of NaBr(s)is -751 kJmol.Use this value and the following thermochemical data to determine the electron attachment enthalpy of Br(g). IE is the enthalpy of ionization.

A) -1827 kJ/mol
B) -397 kJ/mol
C) -325 kJ/mol
D) +325 kJ/mol
E) +397 kJ/mol

A) -1827 kJ/mol
B) -397 kJ/mol
C) -325 kJ/mol
D) +325 kJ/mol
E) +397 kJ/mol

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
46
Strontium oxide has a face centered cubic unit cell of oxide ions with the strontium in octahedral holes.The radius of Sr2+ is 127 pm and the radius of O2- is 135 pm.What is the density of strontium oxide? (100 cm = 1 1012 pm)
A) 0.209 g/cm3
B) 1.20 g/cm3
C) 2.39 g/cm3
D) 3.59 g/cm3
E) 4.78 g/cm3
A) 0.209 g/cm3
B) 1.20 g/cm3
C) 2.39 g/cm3
D) 3.59 g/cm3
E) 4.78 g/cm3

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
47
Which one of the following elements is considered a semiconductor?
A) Li
B) Fe
C) Cl
D) Ni
E) Si
A) Li
B) Fe
C) Cl
D) Ni
E) Si

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
48
The bandgap of Si is 107.1 kJ/mol.What is the maximum wavelength of light that can excite an electron transition across the band gap? (h = 6.626 10-34 J.s; c = 3.000 108 m/s)
A) 551.4 nm
B) 1118 nm
C) 549.0 nm
D) 1852 nm
E) 516.9 nm
A) 551.4 nm
B) 1118 nm
C) 549.0 nm
D) 1852 nm
E) 516.9 nm

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
49
Which of the following might be used as a dopant in a silicon host to create a p-type semiconductor?
A) Al
B) P
C) As
D) S
E) Ge
A) Al
B) P
C) As
D) S
E) Ge

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
50
Which of the following compounds is expected to have the strongest ionic bonds?
A) MgO
B) KBr
C) NaI
D) BaO
E) SrS
A) MgO
B) KBr
C) NaI
D) BaO
E) SrS

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
51
Which of the following compounds is expected to have the strongest ionic bonds?
A) RbI
B) KCl
C) NaBr
D) CsF
E) LiF
A) RbI
B) KCl
C) NaBr
D) CsF
E) LiF

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
52
Silver chloride crystallizes with the sodium chloride (rock salt)structure.The length of a unit cell edge is 555 pm.What is the density of AgCl?
A) 5.57 g/cm3
B) 4.19 g/cm3
C) 2.79 g/cm3
D) 2.10 g/cm3
E) 1.39 g/cm3
A) 5.57 g/cm3
B) 4.19 g/cm3
C) 2.79 g/cm3
D) 2.10 g/cm3
E) 1.39 g/cm3

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
53
Calculate the lattice energy of NaBr(s),given the following thermochemical equations,where IE and EA are enthalpy of ionization and electron attachment enthalpy,respectively.

A) -1401 kJ
B) -751 kJ
C) -241 kJ
D) +241 kJ
E) +751 kJ

A) -1401 kJ
B) -751 kJ
C) -241 kJ
D) +241 kJ
E) +751 kJ

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
54
Lattice enthalpy may be calculated using the thermodynamic relationship known as
A) the Clausius-Clapeyron equation.
B) the Born-Haber cycle.
C) the dynamic equilibrium expression.
D) Avogadro's hypothesis.
E) cubic cell enthalpy of formation equation.
A) the Clausius-Clapeyron equation.
B) the Born-Haber cycle.
C) the dynamic equilibrium expression.
D) Avogadro's hypothesis.
E) cubic cell enthalpy of formation equation.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
55
Iron(II)sulfide has a primitive cubic unit cell with sulfide ions at the lattice points.The ionic radii of iron(II)ions and sulfide ions are 88 pm and 184 pm,respectively.What is the density of FeS (in g/cm3)?
A) 2.56 g/cm3
B) 4.71 g/cm3
C) 5.25 g/cm3
D) 6.66 g/cm3
E) 8.97 g/cm3
A) 2.56 g/cm3
B) 4.71 g/cm3
C) 5.25 g/cm3
D) 6.66 g/cm3
E) 8.97 g/cm3

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
56
Using the thermodynamic data below,and a value of -717 kJ/mole for the lattice enthalpy for KCl,calculate the ionization energy of K.
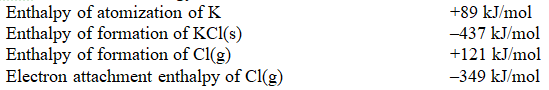
A) -576 kJ/mol
B) +141 kJ/mol
C) +419 kJ/mol
D) +576 kJ/mol
E) +597 kJ/mol
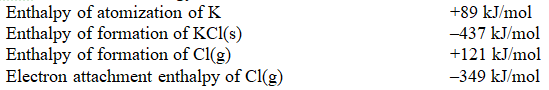
A) -576 kJ/mol
B) +141 kJ/mol
C) +419 kJ/mol
D) +576 kJ/mol
E) +597 kJ/mol

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
57
Elements that have their highest energy electrons in a band of molecular orbitals that is separated from the lowest empty band by an energy difference much too large for electrons to jump between bands are called ____.
A) semiconductors
B) metals
C) conductors
D) insulators
E) isomorphs
A) semiconductors
B) metals
C) conductors
D) insulators
E) isomorphs

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
58
Which two of the following materials are most likely to be amorphous solids: water,nylon,glass,potassium nitrate?
A) water and glass
B) nylon and aspirin
C) water and nylon
D) water and aspirin
E) nylon and glass
A) water and glass
B) nylon and aspirin
C) water and nylon
D) water and aspirin
E) nylon and glass

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
59
Which of the following is expected to have the most negative lattice enthalpy?
A) LiCl
B) NaCl
C) KCl
D) RbCl
E) CsCl
A) LiCl
B) NaCl
C) KCl
D) RbCl
E) CsCl

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
60
The lattice energy of NaBr is -752 kJ/mol.This energy corresponds to which reaction below?
A) Na(s)+ 1/2 Br2(g) NaBr(s)
B) Na(g)+ Br(g) NaBr(s)
C) Na(g) + Br(g) NaBr(s)
D) Na+(g)+ Br-(g) NaBr(s)
E) Na+(aq)+ Br-(aq) NaBr(s)
A) Na(s)+ 1/2 Br2(g) NaBr(s)
B) Na(g)+ Br(g) NaBr(s)
C) Na(g) + Br(g) NaBr(s)
D) Na+(g)+ Br-(g) NaBr(s)
E) Na+(aq)+ Br-(aq) NaBr(s)

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
61
Which of the statements concerning the phase diagram is/are CORRECT?
1)Only the solid phase exists at point A.
2)At point C,the solid and liquid phases are in equilibrium.
3)At point D,the critical point,the substance exists as a supercritical fluid.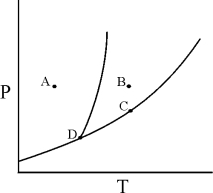
A) 1 only
B) 2 only
C) 3 only
D) 1 and 3
E) 1,2,and 3
1)Only the solid phase exists at point A.
2)At point C,the solid and liquid phases are in equilibrium.
3)At point D,the critical point,the substance exists as a supercritical fluid.

A) 1 only
B) 2 only
C) 3 only
D) 1 and 3
E) 1,2,and 3

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
62
A salt with a 1:1 ratio of anions to cations may pack in a face-centered cubic unit cell with the anions at the lattice points and the cations occupying one-half of the ________ holes.Zinc sulfide is an example of this structure.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
63
Given the accompanying phase diagram,under what conditions will liquid be found in equilibrium with either solid or gas? 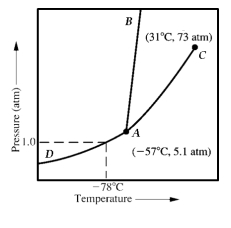
A) Anywhere along curve AB.
B) Anywhere along curve AC.
C) Anywhere along curve AD.
D) Anywhere along curve AB and AC.
E) Anywhere along curve AB and AD.

A) Anywhere along curve AB.
B) Anywhere along curve AC.
C) Anywhere along curve AD.
D) Anywhere along curve AB and AC.
E) Anywhere along curve AB and AD.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
64
What process occurs when the temperature of a substance at Point A is increased (at constant pressure)until the substance is at Point B? 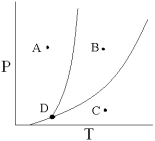
A) condensation
B) vaporization
C) sublimation
D) melting
E) freezing

A) condensation
B) vaporization
C) sublimation
D) melting
E) freezing

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
65
An unknown white solid was found to have a melting point of 150oC.It is soluble in
Water,but it is a poor conductor in aqueous solution.Which of the following substances
Is the most likely?
A) C6H12O6 (glucose)
B) KCl
C) Rb
D) C (diamond)
E) Si
Water,but it is a poor conductor in aqueous solution.Which of the following substances
Is the most likely?
A) C6H12O6 (glucose)
B) KCl
C) Rb
D) C (diamond)
E) Si

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
66
A low melting solid readily dissolves in water to give a nonconducting solution.The solid is most likely a ___.
A) molecular solid
B) ionic solid
C) covalent network solid
D) weak base
E) metallic solid
A) molecular solid
B) ionic solid
C) covalent network solid
D) weak base
E) metallic solid

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
67
Which one of the following substances is matched with the kind of solid it forms?
/
A) sulfur dioxide / molecular
B) graphite / covalent
C) calcium bromide / ionic
D) potassium / ionic
E) methane / molecular
/
A) sulfur dioxide / molecular
B) graphite / covalent
C) calcium bromide / ionic
D) potassium / ionic
E) methane / molecular

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
68
Which of the following ionic compounds is expected to have the lowest enthalpy of fusion?
A) RbF
B) RbCl
C) RbBr
D) RbI
A) RbF
B) RbCl
C) RbBr
D) RbI

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
69
Above a substance's ________ temperature,it is not possible to compress the substance into the liquid phase.If enough pressure is applied the substance will become a supercritical fluid.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
70
Which process requires the greatest endothermic change in enthalpy for water?
A) freezing
B) condensation
C) sublimation
D) melting
E) vaporization
A) freezing
B) condensation
C) sublimation
D) melting
E) vaporization

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
71
A sketch of a phase diagram is given below. 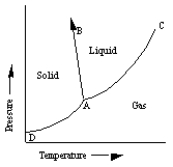 Which statement about this diagram is
Which statement about this diagram is
A) Increasing pressure at constant temperature can melt the solid.
B) Increasing temperature at constant pressure can cause the solid to sublime.
C) Increasing temperature at constant pressure can cause the liquid to vaporize.
D) Increasing pressure at constant temperature can cause deposition of solid from gas.
E) Increasing pressure at constant temperature can cause liquid to freeze.
 Which statement about this diagram is
Which statement about this diagram is A) Increasing pressure at constant temperature can melt the solid.
B) Increasing temperature at constant pressure can cause the solid to sublime.
C) Increasing temperature at constant pressure can cause the liquid to vaporize.
D) Increasing pressure at constant temperature can cause deposition of solid from gas.
E) Increasing pressure at constant temperature can cause liquid to freeze.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
72
A phase diagram of a pure compound has a triple point at 22.0 C and 32 mm Hg,a normal melting point at 22.8 C,and a normal boiling point at 107 C.Which of the following statements regarding this compound is/are CORRECT?
1)The density of the liquid is greater than that of the solid.
2)Sublimation occurs if starting with a solid at a constant temperature of 25 C the pressure is decreased until a phase change occurs.
3)Condensation occurs if the temperature is decreased from 122 C to 75 C at a constant pressure of 1.00 atm.
A) 1 only
B) 2 only
C) 3 only
D) 1 and 2
E) 1,2,and 3
1)The density of the liquid is greater than that of the solid.
2)Sublimation occurs if starting with a solid at a constant temperature of 25 C the pressure is decreased until a phase change occurs.
3)Condensation occurs if the temperature is decreased from 122 C to 75 C at a constant pressure of 1.00 atm.
A) 1 only
B) 2 only
C) 3 only
D) 1 and 2
E) 1,2,and 3

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
73
If a pure substance begins at point C on the phase diagram below and the pressure on the substance is increased until point B is reached,what process occurs? 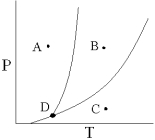
A) fusion
B) vaporization
C) condensation
D) sublimation
E) none of these

A) fusion
B) vaporization
C) condensation
D) sublimation
E) none of these

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
74
The phase diagram for CO2 has a triple point at -56.6 C and 5.19 atm,and a critical point at 31.0 C and 73 atm.The solid and gas phases are in equilibrium at -78.7 C and 1.00 atm.Which of the following statements regarding CO2 is/are CORRECT?
1)Sublimation occurs if the temperature of the solid phase is increased from -79.0 C to 0.0 C at a constant pressure of 2.5 atm.
2)CO2 is a supercritical fluid at 55 C and 75 atm.
3)At pressures greater than its critical pressure (73 atm),CO2 will not exist as a solid at any temperature.
A) 1 only
B) 2 only
C) 3 only
D) 1 and 2
E) 1,2,and 3
1)Sublimation occurs if the temperature of the solid phase is increased from -79.0 C to 0.0 C at a constant pressure of 2.5 atm.
2)CO2 is a supercritical fluid at 55 C and 75 atm.
3)At pressures greater than its critical pressure (73 atm),CO2 will not exist as a solid at any temperature.
A) 1 only
B) 2 only
C) 3 only
D) 1 and 2
E) 1,2,and 3

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
75
Sodium chloride crystallizes in a(n)________ cubic unit cell with chloride ions occupying the lattice points.The sodium ions occupy interstitial regions,with each cation in contact with six chloride ions.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
76
On the phase diagram below,which point corresponds to conditions where solid,liquid,and gas phases all exist? 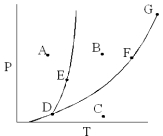
A) B
B) C
C) D
D) E
E) G

A) B
B) C
C) D
D) E
E) G

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
77
Point D on the phase diagram is referred to as the ________ point. 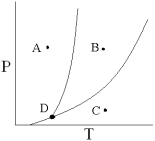
A) triple
B) normal boiling
C) critical
D) normal freezing
E) divergent

A) triple
B) normal boiling
C) critical
D) normal freezing
E) divergent

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck



