Deck 17: The Chemistry of Acids and Bases
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/93
Play
Full screen (f)
Deck 17: The Chemistry of Acids and Bases
1
Which are the Brønsted-Lowry acids in the following equilibrium?
CH3COO-(aq)+ H2O(l) CH3COOH(aq)+ OH-(aq)
CH3COOH(aq)+ OH-(aq)
A) CH3COO- and OH-
B) H2O and OH-
C) H2O,CH3COOH,and OH-
D) CH3COO- and CH3COOH
E) H2O and CH3COOH
CH3COO-(aq)+ H2O(l)
 CH3COOH(aq)+ OH-(aq)
CH3COOH(aq)+ OH-(aq)A) CH3COO- and OH-
B) H2O and OH-
C) H2O,CH3COOH,and OH-
D) CH3COO- and CH3COOH
E) H2O and CH3COOH
H2O and CH3COOH
2
According to the Brønsted-Lowry definition,an acid
A) increases the H3O+ concentration in an aqueous solution.
B) is a strong electrolyte.
C) is a proton acceptor.
D) increases the pH of a solution.
E) is a proton donor.
A) increases the H3O+ concentration in an aqueous solution.
B) is a strong electrolyte.
C) is a proton acceptor.
D) increases the pH of a solution.
E) is a proton donor.
is a proton donor.
3
Which of the following statements is/are consistent with the Brønsted-Lowry concept of acids and bases?
1)A conjugate acid-base pair may differ by only one proton.
2)A Brønsted-Lowry base is defined as a hydroxide ion donor.
3)Brønsted-Lowry acid-base reactions are restricted to aqueous solutions.
A) 1 only
B) 2 only
C) 3 only
D) 1 and 2
E) 1,2,and 3
1)A conjugate acid-base pair may differ by only one proton.
2)A Brønsted-Lowry base is defined as a hydroxide ion donor.
3)Brønsted-Lowry acid-base reactions are restricted to aqueous solutions.
A) 1 only
B) 2 only
C) 3 only
D) 1 and 2
E) 1,2,and 3
1 only
4
Which is NOT an amphiprotic species in water?
A) HClO3
B) HSO3-
C) H3O+
D) HS-
E) HCO3-
A) HClO3
B) HSO3-
C) H3O+
D) HS-
E) HCO3-

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
5
Molecules or ions that can alternately behave as either a Brønsted-Lowry acid or base are called
A) polyanions.
B) hydronium ions.
C) polyprotic acids or bases.
D) conjugate acids or bases.
E) amphiprotic.
A) polyanions.
B) hydronium ions.
C) polyprotic acids or bases.
D) conjugate acids or bases.
E) amphiprotic.

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
6
Which of the following substances is never a Brønsted-Lowry base in an aqueous solution?
A) potassium hydroxide,NaOH(g)
B) sodium hydrogen phosphate,Na2HPO4(s)
C) sodium phosphate,Na3PO4(s)
D) ammonium chloride,NH4Cl(g)
E) sodium bicarbonate,NaHCO3(s)
A) potassium hydroxide,NaOH(g)
B) sodium hydrogen phosphate,Na2HPO4(s)
C) sodium phosphate,Na3PO4(s)
D) ammonium chloride,NH4Cl(g)
E) sodium bicarbonate,NaHCO3(s)

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
7
Which of the following species is amphiprotic in aqueous solution?
A) NH4+
B) H3O+
C) HCl
D) CN-
E) H2O
A) NH4+
B) H3O+
C) HCl
D) CN-
E) H2O

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
8
The autoionization of pure water,as represented by the equation below,is known to be endothermic ( rH > 0.Which of the following correctly states what occurs as the temperature of pure water is raised?
H2O(l)+ H2O(l) H3O+(aq)+ OH-(aq) rH > 0
H3O+(aq)+ OH-(aq) rH > 0
A) Kw decreases,and the hydronium ion concentration decreases.
B) Kw decreases,and the hydronium ion concentration increases.
C) Kw and the hydronium ion concentration do not change.
D) Kw increases,and the hydronium ion concentration decreases.
E) Kw increases,and the hydronium ion concentration increases.
H2O(l)+ H2O(l)
 H3O+(aq)+ OH-(aq) rH > 0
H3O+(aq)+ OH-(aq) rH > 0A) Kw decreases,and the hydronium ion concentration decreases.
B) Kw decreases,and the hydronium ion concentration increases.
C) Kw and the hydronium ion concentration do not change.
D) Kw increases,and the hydronium ion concentration decreases.
E) Kw increases,and the hydronium ion concentration increases.

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
9
Which equation depicts hydrogen phosphate ion behaving as a Brønsted-Lowry acid in water?
A) HPO42-(aq)+ H2O(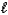 )
)  H2PO4-(aq)+ OH-(aq)
H2PO4-(aq)+ OH-(aq)
B) HPO42-(aq)+ OH-(aq) PO43-(aq)+ H2O(
PO43-(aq)+ H2O( 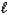 )
)
C) HPO42-(aq)+ H2O(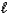 )
)  PO43-(aq)+ H3O+(aq)
PO43-(aq)+ H3O+(aq)
D) 2 HPO42-(aq)+ O2-(aq) PO43-(aq)+ H2O(
PO43-(aq)+ H2O( 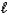 )
)
E) 2 HPO42-(aq)+ H2O( )
)  2 H2O(
2 H2O(  )+ P2O7(s)
)+ P2O7(s)
A) HPO42-(aq)+ H2O(
 )
)  H2PO4-(aq)+ OH-(aq)
H2PO4-(aq)+ OH-(aq)B) HPO42-(aq)+ OH-(aq)
 PO43-(aq)+ H2O(
PO43-(aq)+ H2O( 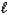 )
)C) HPO42-(aq)+ H2O(
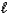 )
)  PO43-(aq)+ H3O+(aq)
PO43-(aq)+ H3O+(aq)D) 2 HPO42-(aq)+ O2-(aq)
 PO43-(aq)+ H2O(
PO43-(aq)+ H2O( 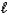 )
)E) 2 HPO42-(aq)+ H2O(
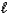 )
) 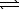 2 H2O(
2 H2O( 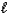 )+ P2O7(s)
)+ P2O7(s)
Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
10
Which equation depicts aqueous hydrogen sulfide behaving as a Brønsted-Lowry acid in water?
A) H2S(aq)+ 2 OH-(aq)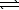 SO2(aq)+ 2 H2(g)
SO2(aq)+ 2 H2(g)
B) H2S(aq)+ H3O+(aq)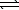 H3S+(aq)+ H2O(
H3S+(aq)+ H2O( 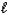 )
)
C) H2S(aq)+ H2O(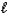 )
) 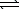 HS-(aq)+ H3O+(aq)
HS-(aq)+ H3O+(aq)
D) HS-(aq)+ H3O+(aq)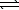 H2S(aq)+ H2O(
H2S(aq)+ H2O(  )
)
E) H2S(aq)+ H2O( )
)  H3S++(aq)+ OH-(aq)
H3S++(aq)+ OH-(aq)
A) H2S(aq)+ 2 OH-(aq)
 SO2(aq)+ 2 H2(g)
SO2(aq)+ 2 H2(g)B) H2S(aq)+ H3O+(aq)
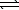 H3S+(aq)+ H2O(
H3S+(aq)+ H2O( 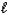 )
)C) H2S(aq)+ H2O(
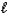 )
) 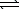 HS-(aq)+ H3O+(aq)
HS-(aq)+ H3O+(aq)D) HS-(aq)+ H3O+(aq)
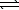 H2S(aq)+ H2O(
H2S(aq)+ H2O( 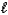 )
)E) H2S(aq)+ H2O(
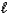 )
) 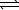 H3S++(aq)+ OH-(aq)
H3S++(aq)+ OH-(aq)
Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
11
What is the conjugate of HPO42-in aqueous solution?
A) not possible
B) PO43-
C) HPO42-
D) H2PO42-
E) H3PO4
A) not possible
B) PO43-
C) HPO42-
D) H2PO42-
E) H3PO4

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
12
Which of the following pairs of species is not a conjugate acid-base pair?
A) HOCl,OCl-
B) HNO2,NO2+
C) O2-,OH-
D) HSO4-,SO42-
E) H2CO3,HCO3-
A) HOCl,OCl-
B) HNO2,NO2+
C) O2-,OH-
D) HSO4-,SO42-
E) H2CO3,HCO3-

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
13
In the following reaction,
HCO3-(aq)+ H2O(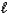 )
)  CO32-(aq)+ H3O+(aq)
CO32-(aq)+ H3O+(aq)
A) H3O+ is an acid and HCO3- is its conjugate base.
B) HCO3- is an acid and CO32- is its conjugate base.
C) HCO3- is an acid and H2O is its conjugate base.
D) H2O is an acid and CO32- is its conjugate base.
E) H3O+ is an acid and CO32- is its conjugate base.
HCO3-(aq)+ H2O(
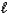 )
)  CO32-(aq)+ H3O+(aq)
CO32-(aq)+ H3O+(aq)A) H3O+ is an acid and HCO3- is its conjugate base.
B) HCO3- is an acid and CO32- is its conjugate base.
C) HCO3- is an acid and H2O is its conjugate base.
D) H2O is an acid and CO32- is its conjugate base.
E) H3O+ is an acid and CO32- is its conjugate base.

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
14
Which of the following substances is never a Brønsted-Lowry acid in an aqueous solution?
A) sodium dihydrogen phosphate,NaH2PO4(s)
B) sodium acetate,NaCH3CO2(s)
C) ammonium nitrate,NH4NO3(s)
D) hydrogen bromide,HCl(g)
E) sodium bicarbonate,NaHCO3(s)
A) sodium dihydrogen phosphate,NaH2PO4(s)
B) sodium acetate,NaCH3CO2(s)
C) ammonium nitrate,NH4NO3(s)
D) hydrogen bromide,HCl(g)
E) sodium bicarbonate,NaHCO3(s)

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
15
What is the conjugate base of [Fe(H2O)6]3+(aq)?
A) H3O+
B) [Fe(H2O)6]2+
C) [Fe(H2O)5H3O]4+
D) [Fe(H2O)5OH]2+
E) [Fe(H2O)5]3+
A) H3O+
B) [Fe(H2O)6]2+
C) [Fe(H2O)5H3O]4+
D) [Fe(H2O)5OH]2+
E) [Fe(H2O)5]3+

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
16
Which of the following equations shows that isoquinoline,C9H7N,behaves as a Brønsted-Lowry base in water?
A) C9H7N(aq)+ H2O(l) C9H7NH+(aq)+ OH-(aq)
C9H7NH+(aq)+ OH-(aq)
B) C9H7N(aq)+ H2O(l) C9H6N-(aq)+ H3O+(aq)
C9H6N-(aq)+ H3O+(aq)
C) C9H7N(aq)+ OH-(aq) C9H6N-(aq)+ H2O(l)
C9H6N-(aq)+ H2O(l)
D) C9H7N(aq)+ H3O+(aq) C9H7NH+(aq)+ H2O(l)
C9H7NH+(aq)+ H2O(l)
E) C9H7NH+(aq)+ H2O(l) C9H7N(aq)+ H3O+(aq)
C9H7N(aq)+ H3O+(aq)
A) C9H7N(aq)+ H2O(l)
 C9H7NH+(aq)+ OH-(aq)
C9H7NH+(aq)+ OH-(aq)B) C9H7N(aq)+ H2O(l)
 C9H6N-(aq)+ H3O+(aq)
C9H6N-(aq)+ H3O+(aq)C) C9H7N(aq)+ OH-(aq)
 C9H6N-(aq)+ H2O(l)
C9H6N-(aq)+ H2O(l)D) C9H7N(aq)+ H3O+(aq)
 C9H7NH+(aq)+ H2O(l)
C9H7NH+(aq)+ H2O(l)E) C9H7NH+(aq)+ H2O(l)
 C9H7N(aq)+ H3O+(aq)
C9H7N(aq)+ H3O+(aq)
Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
17
At 50°C the autoionization constant for pure water,Kw,is 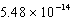 .What is the H3O+ concentration in pure water at 50°C?
.What is the H3O+ concentration in pure water at 50°C?
A)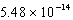 M
M
B) 1.01 10-7 M
C)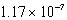 M
M
D)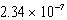 M
M
E) 1.01 10-14 M
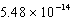 .What is the H3O+ concentration in pure water at 50°C?
.What is the H3O+ concentration in pure water at 50°C?A)
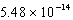 M
MB) 1.01 10-7 M
C)
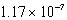 M
MD)
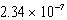 M
ME) 1.01 10-14 M

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
18
Which of the following expressions is not equivalent to pH?
A) -log [H+(aq)]
B)![<strong>Which of the following expressions is not equivalent to pH?</strong> A) -log [H<sup>+</sup>(aq)] B) C) 14.0 - pOH D) E) -log K<sub>w</sub>](https://d2lvgg3v3hfg70.cloudfront.net/TB4499/11ea8937_ab8d_ba2d_a16d_9bd037411557_TB4499_11.jpg)
C) 14.0 - pOH
D)![<strong>Which of the following expressions is not equivalent to pH?</strong> A) -log [H<sup>+</sup>(aq)] B) C) 14.0 - pOH D) E) -log K<sub>w</sub>](https://d2lvgg3v3hfg70.cloudfront.net/TB4499/11ea8937_ab8d_e13e_a16d_43e3f13c08a4_TB4499_11.jpg)
E) -log Kw
A) -log [H+(aq)]
B)
![<strong>Which of the following expressions is not equivalent to pH?</strong> A) -log [H<sup>+</sup>(aq)] B) C) 14.0 - pOH D) E) -log K<sub>w</sub>](https://d2lvgg3v3hfg70.cloudfront.net/TB4499/11ea8937_ab8d_ba2d_a16d_9bd037411557_TB4499_11.jpg)
C) 14.0 - pOH
D)
![<strong>Which of the following expressions is not equivalent to pH?</strong> A) -log [H<sup>+</sup>(aq)] B) C) 14.0 - pOH D) E) -log K<sub>w</sub>](https://d2lvgg3v3hfg70.cloudfront.net/TB4499/11ea8937_ab8d_e13e_a16d_43e3f13c08a4_TB4499_11.jpg)
E) -log Kw

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
19
What volume of water must be added to 14.8 mL of a pH 2.0 solution of HNO3 in order to change the pH to 4.0?
A) 14.8 mL
B) 147 mL
C) 1.47 103 mL
D) 37 mL
E) 85 mL
A) 14.8 mL
B) 147 mL
C) 1.47 103 mL
D) 37 mL
E) 85 mL

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
20
Which of the following is the correct equation for the reaction of ammonia in water?
A) NH3(aq)+ H2O(l) NH4+(aq)+ OH-(aq)
NH4+(aq)+ OH-(aq)
B) NH3(aq)+ H2O(l) NH2-(aq)+ H3O+(aq)
NH2-(aq)+ H3O+(aq)
C) NH3(aq)+ H3O+(aq) NH4+(aq)+ H2O(l)
NH4+(aq)+ H2O(l)
D) NH3(aq)+ OH-(aq) NH2-(aq)+ H2O(l)
NH2-(aq)+ H2O(l)
E) NH3(aq)+ H2O(l) NH2-(aq)+ H3O+(aq)
NH2-(aq)+ H3O+(aq)
A) NH3(aq)+ H2O(l)
 NH4+(aq)+ OH-(aq)
NH4+(aq)+ OH-(aq)B) NH3(aq)+ H2O(l)
 NH2-(aq)+ H3O+(aq)
NH2-(aq)+ H3O+(aq)C) NH3(aq)+ H3O+(aq)
 NH4+(aq)+ H2O(l)
NH4+(aq)+ H2O(l)D) NH3(aq)+ OH-(aq)
 NH2-(aq)+ H2O(l)
NH2-(aq)+ H2O(l)E) NH3(aq)+ H2O(l)
 NH2-(aq)+ H3O+(aq)
NH2-(aq)+ H3O+(aq)
Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
21
What is the pOH of 0.067 M HI(aq)at 25 C? (Kw = 1.01 10-14)?
A) 2.70
B) 12.83
C) 11.30
D) 15.17
E) 1.17
A) 2.70
B) 12.83
C) 11.30
D) 15.17
E) 1.17

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
22
What is the hydroxide-ion concentration in a solution formed by combining 200.mL of 0.15 M HCl with 300.mL of 0.091 M NaOH at 25°C?
HCl(aq)+ NaOH(aq) NaCl(aq)+ H2O(l)
A) 1.7 10-13 M
B) 0.091 M
C) 1.9 10-12 M
D) 0.055 M
E) 1.0 10-7 M
HCl(aq)+ NaOH(aq) NaCl(aq)+ H2O(l)
A) 1.7 10-13 M
B) 0.091 M
C) 1.9 10-12 M
D) 0.055 M
E) 1.0 10-7 M

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
23
The H3O+ concentration of a solution is 2.5 10-6 M.What is the pH of the solution?
A) 6.81
B) 3.77
C) 2.00
D) 5.60
E) 10.60
A) 6.81
B) 3.77
C) 2.00
D) 5.60
E) 10.60

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
24
Hydrofluoric acid has a pKa value of 3.14.What is the value of pKb for fluoride ion?
A) 1.4 10-11
B) 7.2 10-4
C) 3.14
D) 10.86
E) 17.14
A) 1.4 10-11
B) 7.2 10-4
C) 3.14
D) 10.86
E) 17.14

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
25
What is the pH of a solution prepared by dissolving 0.581 L of HCl(g),measured at STP,in enough water such that the total volume of the solution is 2.00 L? (R = 0.0821 L · atm/K·mol)
A) 1.887
B) 12.113
C) 1.586
D) 7.000
E) 12.414
A) 1.887
B) 12.113
C) 1.586
D) 7.000
E) 12.414

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
26
Consider the reaction
CO32-(aq)+ H2O(l) HCO3-(aq)+ OH-(aq).Kb for CO32- is 2.1 10-4 at 25°C.
HCO3-(aq)+ OH-(aq).Kb for CO32- is 2.1 10-4 at 25°C.
What is Ka for the HCO3- ion at 25°C?
A) 4.8 103
B) 4.8 10-11
C) 2.1 10-4
D) 7.2 10-12
E) 9.2 10-8
CO32-(aq)+ H2O(l)
 HCO3-(aq)+ OH-(aq).Kb for CO32- is 2.1 10-4 at 25°C.
HCO3-(aq)+ OH-(aq).Kb for CO32- is 2.1 10-4 at 25°C.What is Ka for the HCO3- ion at 25°C?
A) 4.8 103
B) 4.8 10-11
C) 2.1 10-4
D) 7.2 10-12
E) 9.2 10-8

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
27
Which one of the following aqueous solutions will have a pH of 2.00 at 25 C? (Kw = 1.01 10-14)
A) 0.020 M HNO3
B) 2.0 M NaOH
C) 10.0 M HBr
D) 10.0 M KCl
E) 0.010 M HCl
A) 0.020 M HNO3
B) 2.0 M NaOH
C) 10.0 M HBr
D) 10.0 M KCl
E) 0.010 M HCl

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
28
A solution has a hydroxide-ion concentration of 0.0030 M.What is the pOH of the solution at 25 C? (Kw = 1.01 10-14)
A) 11.48
B) 2.52
C) 7.00
D) 8.19
E) 5.81
A) 11.48
B) 2.52
C) 7.00
D) 8.19
E) 5.81

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
29
What is the H3O+ concentration in 0.0042 M Ba(OH)2(aq)at 25 C? (Kw = 1.01 10-14)?
A) 8.4 10-3 M
B) 4.2 10-3 M
C) 1.2 10-12 M
D) 2.4 10-12 M
E) 1.0 10-7 M
A) 8.4 10-3 M
B) 4.2 10-3 M
C) 1.2 10-12 M
D) 2.4 10-12 M
E) 1.0 10-7 M

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
30
What is the OH- concentration of an aqueous solution with a pH of 9.83? (Kw = 1.01 10-14)
A) 1.3 10-10 M
B) 6.8 10-5 M
C) 6.8 10-1 M
D) 1.5 10-2 M
E) 7.4 109 M
A) 1.3 10-10 M
B) 6.8 10-5 M
C) 6.8 10-1 M
D) 1.5 10-2 M
E) 7.4 109 M

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
31
The pH of a solution at 25°C in which [OH-] = 3.9 10-5 M is ___.(Kw = 1.01 10-14)
A) 4.41
B) 3.90
C) 9.59
D) 4.80
E) none of these
A) 4.41
B) 3.90
C) 9.59
D) 4.80
E) none of these

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
32
What is the pH of a 0.016 M HClO4(aq)at 25 C? (Kw = 1.01 10-14)
A) 15.80
B) 4.14
C) 12.20
D) 1.80
E) 9.86
A) 15.80
B) 4.14
C) 12.20
D) 1.80
E) 9.86

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
33
What is the pH of a solution prepared by dissolving 0.923 g of NaOH(s)in 5.50 L of water? (Kw = 1.01 10-14)
A) 1.637
B) 12.363
C) 7.000
D) 11.623
E) 2.377
A) 1.637
B) 12.363
C) 7.000
D) 11.623
E) 2.377

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
34
The concentration of H3O+ in a solution is 7 10-4 M at 25°C.What is its hydroxide-ion concentration? (Kw = 1.01 10-14)
A) 7 10-4 M
B) 1 10-10 M
C) 2 10-10 M
D) 3 10-10 M
E) 1 10-11 M
A) 7 10-4 M
B) 1 10-10 M
C) 2 10-10 M
D) 3 10-10 M
E) 1 10-11 M

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
35
What is the H3O+ concentration in 0.0047 M NaOH(aq)at 25 C? (Kw = 1.01 10-14)
A) 2.1 10-12 M
B) 4.7 10-3 M
C) 1.0 10-14 M
D) 1.0 10-7 M
E) 4.7 10-17 M
A) 2.1 10-12 M
B) 4.7 10-3 M
C) 1.0 10-14 M
D) 1.0 10-7 M
E) 4.7 10-17 M

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
36
What is the OH- concentration of an aqueous solution with a pH of 2.77? (Kw = 1.01 10-14)
A) 5.9 10-12 M
B) 1.7 10-3 M
C) 5.2 10-2 M
D) 1.1 101 M
E) 5.9 102 M
A) 5.9 10-12 M
B) 1.7 10-3 M
C) 5.2 10-2 M
D) 1.1 101 M
E) 5.9 102 M

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
37
What is the H3O+ concentration of 0.0017 M NaOH(aq)at 25 C? (Kw = 1.01 10-14)
A) 5.9 10-12 M
B) 1.7 10-3 M
C) 1.0 10-14 M
D) 1.0 10-7 M
E) 1.7 10-17 M
A) 5.9 10-12 M
B) 1.7 10-3 M
C) 1.0 10-14 M
D) 1.0 10-7 M
E) 1.7 10-17 M

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
38
What is the hydronium-ion concentration in a solution formed by combining 750 mL of 0.10 M NaOH with 250 mL of 0.30 M HCl?
NaOH(aq)+ HCl(aq) NaCl(aq)+ H2O(l)
A) 0.075 M
B) 1.7 10-13 M
C) 1.0 10-7 M
D) 0.30 M
E) 0.10 M
NaOH(aq)+ HCl(aq) NaCl(aq)+ H2O(l)
A) 0.075 M
B) 1.7 10-13 M
C) 1.0 10-7 M
D) 0.30 M
E) 0.10 M

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
39
An aqueous solution with a pH of 10.60 is diluted from 1.0 L to 2.0 L.What is the pH of the diluted solution?
A) 5.30
B) 9.60
C) 10.30
D) 10.60
E) 10.90
A) 5.30
B) 9.60
C) 10.30
D) 10.60
E) 10.90

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
40
What is the pH of the final solution when 25 mL of 0.021 M HCl has been added to 35 mL of 0.036 M HCl at 25°C?
A) 3.3
B) 1.9
C) 1.5
D) 2.7
E) 3.5
A) 3.3
B) 1.9
C) 1.5
D) 2.7
E) 3.5

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
41
H3PO3 is a diprotic weak acid.What is the balanced equilibrium defined as Kb2 of H3PO3?
A) H3PO3(aq)+ H2O(l) H3O+(aq)+ H2PO3-(aq)
H3O+(aq)+ H2PO3-(aq)
B) H2PO3-(aq)+ H2O(l) H3O+(aq)+ HPO32-(aq)
H3O+(aq)+ HPO32-(aq)
C) HPO32-(aq) + H2O(l) OH-(l)+ H2PO3-(aq)
OH-(l)+ H2PO3-(aq)
D) H2PO3-(aq)+ H2O(l) OH-(l)+ H3PO3(aq)
OH-(l)+ H3PO3(aq)
E) HPO32-(aq)+ H2O(l) OH-(aq)+ H3PO3(aq)
OH-(aq)+ H3PO3(aq)
A) H3PO3(aq)+ H2O(l)
 H3O+(aq)+ H2PO3-(aq)
H3O+(aq)+ H2PO3-(aq)B) H2PO3-(aq)+ H2O(l)
 H3O+(aq)+ HPO32-(aq)
H3O+(aq)+ HPO32-(aq)C) HPO32-(aq) + H2O(l)
 OH-(l)+ H2PO3-(aq)
OH-(l)+ H2PO3-(aq)D) H2PO3-(aq)+ H2O(l)
 OH-(l)+ H3PO3(aq)
OH-(l)+ H3PO3(aq)E) HPO32-(aq)+ H2O(l)
 OH-(aq)+ H3PO3(aq)
OH-(aq)+ H3PO3(aq)
Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
42
The Ka for the monoprotic acid hypochlorous acid is 3.5 10-8.What is Kb for the hypochlorite ion,the conjugate base of hypochlorous acid?
A) 2.9 10-7
B) 3.5 10-8
C) 2.9 107
D) 3.5 106
E) 3.5 10-22
A) 2.9 10-7
B) 3.5 10-8
C) 2.9 107
D) 3.5 106
E) 3.5 10-22

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
43
What is the hydronium-ion concentration of a 0.25 M solution of HCN (Ka = 4.9 10-10)at 25°C?
A) 1.6 10-4 M
B) 3.3 10-6 M
C) 2.1 10-6 M
D) 1.1 10-5 M
E) 4.4 10-5 M
A) 1.6 10-4 M
B) 3.3 10-6 M
C) 2.1 10-6 M
D) 1.1 10-5 M
E) 4.4 10-5 M

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
44
What is the equilibrium pH of an initially 0.64 M solution of the monoprotic acid benzoic acid at 25°C (Ka = 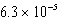 )?
)?
A) 2.20
B) 7.00
C) 1.90
D) 12.10
E) 5.10
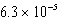 )?
)?A) 2.20
B) 7.00
C) 1.90
D) 12.10
E) 5.10

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
45
Consider the Ka values for the following acids:
Cyanic acid,HOCN,3.5 10-4
Formic acid,HCHO2,1.7 10-4
Lactic acid,HC3H5O3,1.3 10-4
Propionic acid,HC3H5O2,1.3 10-5
Benzoic acid,HC7H5O2,6.3 10-5
Given initially equimolar solutions of each weak acid,which solution will have the highest pH once equilibrium is established?
A) HOCN
B) HC7H5O2
C) HC3H5O2
D) HC3H5O3
E) HCHO2
Cyanic acid,HOCN,3.5 10-4
Formic acid,HCHO2,1.7 10-4
Lactic acid,HC3H5O3,1.3 10-4
Propionic acid,HC3H5O2,1.3 10-5
Benzoic acid,HC7H5O2,6.3 10-5
Given initially equimolar solutions of each weak acid,which solution will have the highest pH once equilibrium is established?
A) HOCN
B) HC7H5O2
C) HC3H5O2
D) HC3H5O3
E) HCHO2

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
46
What is the equilibrium constant for the following reaction,
HCO2H(aq)+ CN-(aq)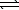 HCO2-(aq)+ HCN(aq)
HCO2-(aq)+ HCN(aq)
And does the reaction favor the formation of reactants or products? The acid dissociation constant,Ka,for HCO2H is 1.8 10-4 and the acid dissociation constant for HCN is 4.0 10-10.
A) K = 1.00.The reaction favors neither the formation of reactants nor products.
B) K = 2.2 10-6.The reaction favors the formation of products.
C) K = 2.2 10-6.The reaction favors the formation of reactants.
D) K = 4.5 105.The reaction favors the formation of products.
E) K = 4.5 105.The reaction favors the formation of reactants.
HCO2H(aq)+ CN-(aq)
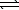 HCO2-(aq)+ HCN(aq)
HCO2-(aq)+ HCN(aq)And does the reaction favor the formation of reactants or products? The acid dissociation constant,Ka,for HCO2H is 1.8 10-4 and the acid dissociation constant for HCN is 4.0 10-10.
A) K = 1.00.The reaction favors neither the formation of reactants nor products.
B) K = 2.2 10-6.The reaction favors the formation of products.
C) K = 2.2 10-6.The reaction favors the formation of reactants.
D) K = 4.5 105.The reaction favors the formation of products.
E) K = 4.5 105.The reaction favors the formation of reactants.

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
47
Which ionic compound forms a pH-neutral aqueous solution at 25 C?
A) KHCO3
B) LiCl
C) KOCl
D) NH4Cl
E) K2S
A) KHCO3
B) LiCl
C) KOCl
D) NH4Cl
E) K2S

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
48
Aqueous solutions of ammonia (NH3)and hydrogen cyanide (HCN)react to produce ammonium cyanide,(NH4CN)according to the following equilibrium reaction.NH3(aq)+ HCN(aq) NH4+(aq)+ CN-(aq)
Given the following equilibrium constants,which statement best describes the reaction once equilibrium is established? (Kw = 1.01 10-14)
NH4+ Ka = 5.6 10-10
HCN Ka = 4.0 10-10
A) The reaction is product favored.
B) The reaction is reactant favored.
C) The reaction is neither product nor reactant favored.
Given the following equilibrium constants,which statement best describes the reaction once equilibrium is established? (Kw = 1.01 10-14)
NH4+ Ka = 5.6 10-10
HCN Ka = 4.0 10-10
A) The reaction is product favored.
B) The reaction is reactant favored.
C) The reaction is neither product nor reactant favored.

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
49
What is Ka at 25°C for the following equilibrium given Kb (CH3NH2)= 4.4 10-4 at 25°C.?
CH3NH3+(aq)+ H2O(l) CH3NH2(aq)+ H3O+(aq)
CH3NH2(aq)+ H3O+(aq)
A) 4.4 10-4
B) 2.3 103
C) 4.4 10-10
D) 4.4 104
E) 2.3 10-11
CH3NH3+(aq)+ H2O(l)
 CH3NH2(aq)+ H3O+(aq)
CH3NH2(aq)+ H3O+(aq)A) 4.4 10-4
B) 2.3 103
C) 4.4 10-10
D) 4.4 104
E) 2.3 10-11

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
50
Consider the Ka values for the following acids:
Cyanic acid,HOCN,3.5 10-4
Formic acid,HCHO2,1.7 10-4
Lactic acid,HC3H5O3,1.3 10-4
Propionic acid,HC3H5O2,1.3 10-5
Benzoic acid,HC7H5O2,6.3 10-5
Which has the strongest conjugate base?
A) HOCN
B) HCHO2
C) HC3H5O3
D) HC3H5O2
E) HC7H5O2
Cyanic acid,HOCN,3.5 10-4
Formic acid,HCHO2,1.7 10-4
Lactic acid,HC3H5O3,1.3 10-4
Propionic acid,HC3H5O2,1.3 10-5
Benzoic acid,HC7H5O2,6.3 10-5
Which has the strongest conjugate base?
A) HOCN
B) HCHO2
C) HC3H5O3
D) HC3H5O2
E) HC7H5O2

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
51
What is the OH- concentration in 0.48 M CH3CO2-(aq)? (Kb of CH3CO2- = 5.6 10-10)
A) 2.7 10-10 M
B) 6.2 10-10 M
C) 1.1 10-5 M
D) 1.6 10-5 M
E) 2.4 10-5 M
A) 2.7 10-10 M
B) 6.2 10-10 M
C) 1.1 10-5 M
D) 1.6 10-5 M
E) 2.4 10-5 M

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
52
The complete reaction of an acid and base is as follows.HClO4(aq)+ LiOH(aq) H2O( 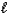 )+ LiClO4(aq)
)+ LiClO4(aq)
What is the equilibrium constant for the net ionic reaction at 25 C?
A) 1.01 10-14
B) 1.01 10-7
C) 1.01 107
D) 1.01 1014
E) more information required
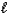 )+ LiClO4(aq)
)+ LiClO4(aq)What is the equilibrium constant for the net ionic reaction at 25 C?
A) 1.01 10-14
B) 1.01 10-7
C) 1.01 107
D) 1.01 1014
E) more information required

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
53
Given the following acid dissociation constants,
Ka (H3PO4)= 7.5 10-3
Ka (NH4+)= 5.6 10-10
Determine the equilibrium constant for the reaction below at 25 C.H3PO4(aq)+ NH3(aq) NH4+(aq)+ H2PO4-(aq)
NH4+(aq)+ H2PO4-(aq)
A) 4.2 10-12
B) 7.5 10-8
C) 4.2 102
D) 1.3 107
E) 2.4 1011
Ka (H3PO4)= 7.5 10-3
Ka (NH4+)= 5.6 10-10
Determine the equilibrium constant for the reaction below at 25 C.H3PO4(aq)+ NH3(aq)
 NH4+(aq)+ H2PO4-(aq)
NH4+(aq)+ H2PO4-(aq)A) 4.2 10-12
B) 7.5 10-8
C) 4.2 102
D) 1.3 107
E) 2.4 1011

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
54
At 25 C,all of the following ions produce an acidic solution,except ____.
A) NH4+
B) HSO3-
C) HPO42-
D) [Fe(H2O)6]3+
E) [Al(H2O)6]3+
A) NH4+
B) HSO3-
C) HPO42-
D) [Fe(H2O)6]3+
E) [Al(H2O)6]3+

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
55
Given the following equilibrium constants,
Ka (HSO4-)= 1.2 10-2
Kb (CH3CO2-)= 5.6 10-10
Kw = 1.00 10-14
Determine the equilibrium constant for the reaction below at 25 C.
HSO4-(aq)+ CH3CO2-(aq)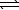 SO42-(aq)+ CH3CO2H(aq)
SO42-(aq)+ CH3CO2H(aq)
A) 6.7 10-12
B) 2.1 10-7
C) 1.5 10-3
D) 6.7 102
E) 2.1 107
Ka (HSO4-)= 1.2 10-2
Kb (CH3CO2-)= 5.6 10-10
Kw = 1.00 10-14
Determine the equilibrium constant for the reaction below at 25 C.
HSO4-(aq)+ CH3CO2-(aq)
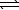 SO42-(aq)+ CH3CO2H(aq)
SO42-(aq)+ CH3CO2H(aq)A) 6.7 10-12
B) 2.1 10-7
C) 1.5 10-3
D) 6.7 102
E) 2.1 107

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
56
At 25 C,all of the following ionic compounds produce a basic aqueous solution,except ____.
A) KSH
B) Na3PO4
C) LiNO2
D) KHSO3
E) KCH3CO2
A) KSH
B) Na3PO4
C) LiNO2
D) KHSO3
E) KCH3CO2

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
57
What is the equilibrium hydronium ion concentration of an initially 5.4 M solution of hypochlorous acid,HOCl,at 25°C (Ka = 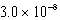 )?
)?
A)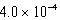 M
M
B)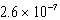 M
M
C)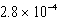 M
M
D)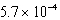 M
M
E)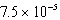 M
M
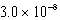 )?
)?A)
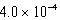 M
MB)
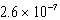 M
MC)
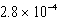 M
MD)
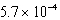 M
ME)
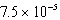 M
M
Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
58
Which acid-base reaction results in an acidic solution?
A) HNO3(aq)+ CsOH(aq) H2O(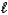 )+ CsNO3(aq)
)+ CsNO3(aq)
B) HI(aq)+ LiOH(aq) H2O(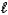 )+ LiI(aq)
)+ LiI(aq)
C) HBr(aq)+ NaOH(aq) H2O( )+ NaBr(aq)
)+ NaBr(aq)
D) H2SO3(aq)+ LiOH(aq) H2O(
H2O(  )+ LiHSO3(aq)
)+ LiHSO3(aq)
E) HF(aq)+ LiOH(aq) H2O(
H2O(  )+ LiF(aq)
)+ LiF(aq)
A) HNO3(aq)+ CsOH(aq) H2O(
 )+ CsNO3(aq)
)+ CsNO3(aq)B) HI(aq)+ LiOH(aq) H2O(
 )+ LiI(aq)
)+ LiI(aq)C) HBr(aq)+ NaOH(aq) H2O(
 )+ NaBr(aq)
)+ NaBr(aq)D) H2SO3(aq)+ LiOH(aq)
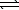 H2O(
H2O( 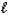 )+ LiHSO3(aq)
)+ LiHSO3(aq)E) HF(aq)+ LiOH(aq)
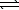 H2O(
H2O( 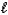 )+ LiF(aq)
)+ LiF(aq)
Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
59
Given that Ka for the weak acid HA is 3.46 10-8,calculate K for the reaction of HA with OH-.HA(aq)+ OH-(aq). A-(aq)+ H2O( 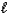 )
)
A) 3.46
B) 3.46 106
C) 3.46 10-22
D) 2.89 1021
E) 2.89 10-7
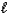 )
)A) 3.46
B) 3.46 106
C) 3.46 10-22
D) 2.89 1021
E) 2.89 10-7

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
60
Given the equilibrium constants for the equilibria,
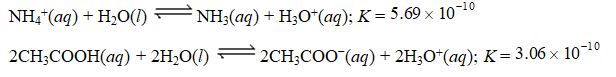
Determine Kc for the following equilibrium.
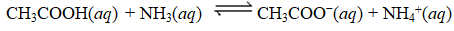
A) 3.08 104
B) 3.25 10-5
C) 9.96 10-15
D) 1.00 1014
E) 1.75 10-5
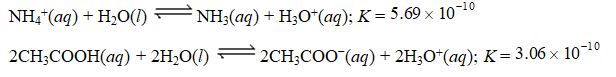
Determine Kc for the following equilibrium.
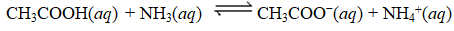
A) 3.08 104
B) 3.25 10-5
C) 9.96 10-15
D) 1.00 1014
E) 1.75 10-5

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
61
What is the hydroxide-ion concentration of a 0.250 M sodium oxalate (Na2C2O4)solution? For oxalic acid (H2C2O4),Ka1 = 5.6 10-2 and Ka2 = 5.1 10-5.(Kw = 1.01 10-14)
A) 7.0 10-6 M
B) 1.0 10-7 M
C) 9.4 10-2 M
D) 3.5 10-3 M
E) 2.1 10-7 M
A) 7.0 10-6 M
B) 1.0 10-7 M
C) 9.4 10-2 M
D) 3.5 10-3 M
E) 2.1 10-7 M

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
62
Calculate the pH of a 0.04 M solution of ascorbic acid (Ka1 = 7.9 10-5; Ka2 is 1.6 10-12).
A) 11.2
B) 2.8
C) 5.5
D) 8.5
E) 11.8
A) 11.2
B) 2.8
C) 5.5
D) 8.5
E) 11.8

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
63
What is the pH of the solution which results from mixing 50.0 mL of 0.30 M HF(aq)and 50.0 mL of 0.30 M NaOH(aq)at 25 C? (Ka of HF = 7.2 10-4)
A) 1.98
B) 5.84
C) 8.16
D) 10.85
E) 12.02
A) 1.98
B) 5.84
C) 8.16
D) 10.85
E) 12.02

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
64
All of the following compounds are acids containing chlorine.Which compound is the weakest acid?
A) HCl
B) HClO
C) HClO2
D) HClO3
E) HClO4
A) HCl
B) HClO
C) HClO2
D) HClO3
E) HClO4

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
65
What is the pH of 0.010 M aqueous hypochlorous acid? (Ka of HOCl = 3.5 10-8)
A) 2.00
B) 4.50
C) 4.73
D) 6.54
E) 7.45
A) 2.00
B) 4.50
C) 4.73
D) 6.54
E) 7.45

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
66
A 0.10 M solution of a weak monoprotic acid has a hydronium-ion concentration of 4.6 10-4 M.What is the acid-ionization constant,Ka,for this acid?
A) 2.1 10-2
B) 3.2 10-3
C) 4.6 10-4
D) 2.1 10-6
E) 5.5 10-5
A) 2.1 10-2
B) 3.2 10-3
C) 4.6 10-4
D) 2.1 10-6
E) 5.5 10-5

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
67
The pH of aqueous 0.10 M pyridine (C5H5N)ion is 9.09.What is the Kb of this base?
A) 8.0 10-10
B) 1.5 10-9
C) 9.0 10-6
D) 1.6 10-5
E) 1.2 10-5
A) 8.0 10-10
B) 1.5 10-9
C) 9.0 10-6
D) 1.6 10-5
E) 1.2 10-5

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
68
Rank the following in order of decreasing acid strength in aqueous solution: HCl,HOCl,HOBr,HOI.
A) HCl > HClO > HBrO > HIO
B) HIO > HBrO > HClO > HCl
C) HCl > HIO > HBrO > HClO
D) HClO > HCl > HBrO > HIO
E) HClO > HBrO > HCl > HIO
A) HCl > HClO > HBrO > HIO
B) HIO > HBrO > HClO > HCl
C) HCl > HIO > HBrO > HClO
D) HClO > HCl > HBrO > HIO
E) HClO > HBrO > HCl > HIO

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
69
Which of the following statements is INCORRECT?
A) H3PO4 is a stronger acid than H2PO4-.
B) HClO3 is a stronger acid than HClO2.
C) HNO3 is a stronger acid than HNO2.
D) [Fe(H2O)6]2+ is a stronger acid than [Fe(H2O)6]3+.
E) HOCl is stronger acid than HOBr.
A) H3PO4 is a stronger acid than H2PO4-.
B) HClO3 is a stronger acid than HClO2.
C) HNO3 is a stronger acid than HNO2.
D) [Fe(H2O)6]2+ is a stronger acid than [Fe(H2O)6]3+.
E) HOCl is stronger acid than HOBr.

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
70
Which of the following species is the strongest acid in an aqueous solution?
A) CH3CH2CO2H
B) CH2ClCO2H
C) CH3CO2H
D) CCl3CO2H
E) CHCl2CO2H
A) CH3CH2CO2H
B) CH2ClCO2H
C) CH3CO2H
D) CCl3CO2H
E) CHCl2CO2H

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
71
What is the equilibrium pH of a 0.835 M solution of H3PO4(aq)? (Ka1 = 7.5 10-3,Ka2 = 6.2 10-8,Ka3 = 4.8 10-13)
A) 1.12
B) 3.64
C) 12.32
D) 6.20
E) 7.21
A) 1.12
B) 3.64
C) 12.32
D) 6.20
E) 7.21

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
72
What is the equilibrium pOH of an initially 5.4 M solution of hypochlorous acid,HOCl,at 25°C (Ka = 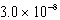 ; Kw = 1.01 10-14)?
; Kw = 1.01 10-14)?
A) 10.60
B) 7.42
C) 10.45
D) 10.76
E) 4.13
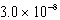 ; Kw = 1.01 10-14)?
; Kw = 1.01 10-14)?A) 10.60
B) 7.42
C) 10.45
D) 10.76
E) 4.13

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
73
The pH of a solution of 2.2 M H2A (Ka1 = 1.0 10-6 and Ka2 is 1.0 10-10)is:
A) 10.00
B) 2.83
C) 11.17
D) 5.66
E) 7.00
A) 10.00
B) 2.83
C) 11.17
D) 5.66
E) 7.00

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
74
What is the hydroxide-ion concentration in a 0.22 M solution of Na2CO3? For carbonic acid,Ka1 = 4.2 10-7 and Ka2 = 4.8 10-11.(Kw = 1.0 10-14)
A) 6.8 10-3 M
B) 2.0 10-4 M
C) 7.2 10-5 M
D) 4.2 10-9 M
E) 3.2 10-6 M
A) 6.8 10-3 M
B) 2.0 10-4 M
C) 7.2 10-5 M
D) 4.2 10-9 M
E) 3.2 10-6 M

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
75
What is the equilibrium concentration of H2C2O4 in a 0.230 M oxalic acid,H2C2O4,solution? For oxalic acid,Ka1 = 5.6 10-2 and Ka2 = 5.1 10-5.
A) 1.4 10-1 M
B) 1.1 10-1 M
C) 2.3 10-1 M
D) 5.1 10-5 M
E) 8.9 10-2 M
A) 1.4 10-1 M
B) 1.1 10-1 M
C) 2.3 10-1 M
D) 5.1 10-5 M
E) 8.9 10-2 M

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
76
Calculate the pH of the following aqueous solution:
0)29 M H2S (pKa1 = 7.00; pKa2 = 12.89)
A) 10.23
B) 6.46
C) 3.77
D) 7.54
E) 7.00
0)29 M H2S (pKa1 = 7.00; pKa2 = 12.89)
A) 10.23
B) 6.46
C) 3.77
D) 7.54
E) 7.00

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
77
Carbonic acid is a diprotic acid,H2CO3,with Ka1 = 4.2 10-7 and Ka2 = 4.8 10-11 at 25°C.The ion product for water is Kw = 1.0 10-14 at 25°C.What is the OH- concentration of a solution that is 0.18 M in Na2CO3?
A) 6.1 10-3 M
B) 2.1 10-4 M
C) 6.5 10-5 M
D) 2.9 10-6 M
E) 2.7 10-4 M
A) 6.1 10-3 M
B) 2.1 10-4 M
C) 6.5 10-5 M
D) 2.9 10-6 M
E) 2.7 10-4 M

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
78
What is the pH of the solution which results from mixing 75 mL of 0.50 M NH3(aq)and 75 mL of 0.50 HCl(aq)at 25 C? (Kb for NH3 = 1.8 10-5)
A) 0.60
B) 2.67
C) 4.74
D) 4.93
E) 9.26
A) 0.60
B) 2.67
C) 4.74
D) 4.93
E) 9.26

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
79
What is the pH of a 0.11 M solution of methylamine (CH3NH2,Kb = 4.4 10-4)at 25oC? (Kw = 1.01 10-14)
A) 5.80
B) 0.96
C) 11.83
D) 13.04
E) 2.17
A) 5.80
B) 0.96
C) 11.83
D) 13.04
E) 2.17

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
80
What is the pH of a 0.28 M solution of sodium propionate,NaC3H5O2,at 25°C? (propionic acid,HC3H5O2,is monoprotic and has a Ka = 1.3 10-5 at 25°C..Kw = 1.01 10-14 )
A) 6.26
B) 4.83
C) 11.10
D) 7.74
E) 9.17
A) 6.26
B) 4.83
C) 11.10
D) 7.74
E) 9.17

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck



